Abstract
MnCo2O4 nanorods were facilely prepared via microwave hydrothermal method. X-ray diffraction pattern showed pure crystalline spinel phase MnCo2O4 formation for the calcined powder at 400 °C. Fourier transform infrared spectroscopy (FTIR) spectrum of the MnCo2O4 powders showed the strong vibrational modes of Mn–O and Co–O bonds. Raman spectrum showed the structural bonding features and crystalline nature of MnCo2O4. Scanning electron microscopy images exposed a morphology that shows the aggregation of several nanorods to form bundles of nanorods ~ 300–400 nm in diameter and few microns in length. Energy-dispersive spectrometry analysis confirmed the presence of Mn, Co, O elements for the powder calcined at 400 °C. The electrochemical characterization of the MnCo2O4 nanorods with 1 M KOH as the electrolyte exhibited an excellent capacitance of 2394.4 F g−1 at a scan rate of 5 mV s−1 and revealed a highest specific capacitance of 1617.5 F g−1 from the galvanostatic charge/discharge analysis at a current density of 1 A g−1. The cycling stability at different current densities revealed the high rate performances and good reversible capacity retention of the calcined MnCo2O4 nanorods. The cycling life study of MnCo2O4 nanorods demonstrated an excellent cycling stability with 88% of the initial specific capacitance retention at 10 A g−1 after 1000 cycles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The rapid development in energy utilization by the miniaturized portable electronic devices to a hybrid electric vehicle requires extensive improvement in the energy technologies. The most efficient energy storage device is the electrochemical capacitor or supercapacitors because of their high speed of the charge–discharge process, long time durability, cycle stability, enhanced energy and power density compared with batteries [1,2,3,4]. Generally, the supercapacitor can be classified as an electric double layer capacitor (EDLC) and redox- or pseudocapacitor based on the energy storage mechanism [5]. The EDLC works with respect to accumulation of electrical charge by electrostatic separation at electrode/electrolyte interfaces. On the other hand, the pseudocapacitors works based on the fast redox reaction on the surface or around the surface region of the electrodes, where electrosorption/electrodesorption occurs with charge transfer but without any bulk phase transformation upon charging/discharging. As a result, pseudocapacitors deliver a high specific capacitance than the EDLCs [6,7,8]. The binary metal oxides, particularly designated as MCo2O4 (M = Mn, Ni, Cu etc.) are one of the promising electrode materials for pseudocapacitor. These oxides are efficient alternatives to the carbon-based materials due to their advantages such as excellent performances, multiple oxidation states, fast redox reaction, low cost, and eco-friendly compared to other electrode materials. In recent years, MnCo2O4 has found to be a substitute electrode material for supercapacitor due to their high theoretical capacitance (∼ 3619 F g−1) and it demonstrates the superior capacitive performance and high electrochemical performance compared to other binary metal oxides [9,10,11,12,13,14,15]. However, the experimentally achieved capacity of pure MnCo2O4 is lower than the theoretical value, which is due to their low electrical conductivity, high electron transfer resistance and poor electrolyte utilization efficiency active materials [16,17,18].
The morphology of the electrode material finds a significant role in the reduction of the electron transfer resistance and improving the efficiency of active material utilization by the electrolyte ions and this determines the efficiency of the device because the ion diffusion and conductivity are directly depended on the morphology of the electrode material [19]. From the literature survey, it has been identified that the various morphologies of MnCo2O4 synthesized and tested for its feasibility as a supercapacitor electrode. Li et al., has reported that the prepared one-dimensional MnCo2O4 nanowire directly on the nickel foam showed a capacitance of 349.8 F g−1 at 1 A g−1 [20]. Kong et al., has reported that the prepared MnCo2O4 nanoparticles showed a specific capacitance 405 F g−1 at 5 mA cm−2 [21]. Xu et al. synthesized porous MnCo2O4 nanowires, which exhibited a significant specific capacitance of 1342 F g−1 at 1 A g−1 [16]. Venkatachalam et al., has reported the synthesized MnCo2O4 nanorods exhibited 718.75 F g−1 at a current density of 0.5 A g−1 [22]. Krishnan et al., has reported that the prepared MnCo2O4 flakes and it exhibited 600 F g−1 at a current density of 0.5 A g−1 [23]. Hui et al., has reported that successfully prepared chestnut-like MnCo2O4 nanoneedles on nickel and it shows excellent capacitance of 1535 F g−1 at a current density of 1 A g−1 [18]. Thorat et al., has reported that the synthesized MnCo2O4 microspheres exhibited 950 F g−1 at a current density of 2 A g−1 [24]. Thus the literature reports reveal that by controlling the particle size of the electrode material and achieving good morphology with the high surface area is an alternative technique to enhance the pseudocapacitive performance of the binary metal oxide electrodes. In this point of view, various synthetic approaches developed to control the size as well as the morphology of the electrode material, and thereby electrochemical capacitance, ion transport, electrical conductivity, the cyclic reversible performance of the metal oxides can be improved [25, 26]. Recently, microwave hydrothermal (MH) assisted synthesis method is utilized to prepare oxide, hydroxide, and sulfide nanoparticles with various morphologies. The advantages of the MH method are simple, clean, fast reaction time, energy and time-saving process and the morphology of materials are in control, without thermal gradient effects problems [19, 27,28,29,30].
The present work focuses on the facile synthesis of the MnCo2O4 nanostructure material via the MH method. The calcined powder samples are characterized by X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), Raman spectroscopy, Field emission scanning electron microscopy (FE-SEM), and Electrochemical performances and cycle stability at various current densities and longtime cyclic stability with high current density are studied and compared with the reported literature results.
2 Experimental
2.1 Materials and methods
Analar grade precursor chemicals manganese nitrate hexahydrate Mn(NO3)2·6H2O [Merck, 99.9%], cobalt nitrate hexahydrate Co(NO3)2·6H2O [Merck, 99.9%], absolute ethanol (China) and de-ionized (DI) water were used as purchased without further purification. Nickel foam (1.6 mm thickness) was purchased from MTI Corporation (China). The Microwave-hydrothermal synthesis has been carried out in a Microwave reaction system SOLV Multiwave PRO, Anton Paar, Germany.
2.2 Preparation of MnCo2O4
In a typical synthesis, 0.01 mol of manganese nitrate hexahydrate Mn(NO3) 6H2O and 0.02 mol of cobalt nitrate hexahydrate Co(NO3)2·6H2O were dissolved in 250 ml of deionized (DI) water. The solution was stirred for 1 h at room temperature to attain a homogeneous transparent solution. Then the homogeneous clear solution was transferred into the quartz vessels and placed on a rotatable stage for uniform heat treatment at 180 °C for 15 min in a microwave reaction system SOLV, Multiwave PRO Anton-Paar. After the reaction completion, the reaction quartz vessels were cooled to room temperature. Thus obtained brown colour precipitate was carefully transferred, filtered and washed with distilled water and absolute ethanol. The obtained brown colour precipitate was dried in an oven at 60–70 °C for 12 h and followed by calcination at 400 °C for 3 h to form MnCo2O4 nanostructure material.
2.3 Materials characterization
The synthesized samples were characterized with a powder X-ray diffractometer (X’pert PRO MPD, PANalytical) employing Cu Kα radiation (λ = 0.15406 nm), angles ranging from 10° to 80° with step size 0.02. The structural bonding nature of the powders was recorded using Thermo Nicolet FTIR-6700 spectrometers from 4000 to 400 cm−1 for 30 scans for the thin transparent pellets. The transparent pellet was made using 400 °C calcined powders for 3 h and grounded well with pure KBr crystalline powder taken in 1:20 ratio. Raman spectra were recorded in the range 100–2000 nm using RENISHAW inVia confocal Raman microscope, UK, with 785 nm line of an argon ion laser, keeping their maximum output power at 100 mW The morphology and microstructure of the calcined powder at 400 °C for 3 h were characterized using a field emission scanning electron microscope (FE-SEM, JSM-6700F, JEOL Ltd).
2.4 Electrochemical characterization
The working electrode was prepared by mixing the active material MnCo2O4 powder, carbon black as a conducting agent and polyvinylidene difluoride as a binder with the mass ratio 80:10:10 to obtain a homogeneous slurry with N-methyl pyrrolidone. Prior to coating the electrode material the nickel foam was cleaned with 6 M HCl aqueous solution in an ultrasound bath for 25 min and rinsed with DI water and absolute ethanol several times in order to eliminate the NiO layer on the surface. Then the homogeneous slurry was placed and pressed onto Ni foam and dried under vacuum at 80 °C for 24 h. A three-electrode cell consists of the working electrode, platinum as the counter electrode and saturated calomel electrode as a reference electrode were used and 1 M potassium hydroxide (1 M KOH) was used as the electrolyte. The electrochemical behavior of the MnCo2O4 electrodes was characterized by cyclic voltammetry (CV), galvanostatic charge/discharge (GCD) tests, electrochemical impedance spectroscopy (EIS) and cyclic stability using PARSTAT MC Multi-Channel Electrochemical workstation (Ametek, USA). EIS measurement was carried out in the frequency range of 104–1 Hz with a perturbation amplitude of 5 mV at open circuit potential.
3 Results and discussion
3.1 Characterization of MnCo2O4
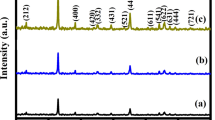
XRD pattern of the MnCo2O4 powder prepared via the MH method and calcined at 400 °C for 3 h is shown in Fig. 1. From Fig. 1, it is observed that the XRD pattern shows diffraction peaks at the 2 theta values of 18.53, 30.52, 36.02, 37.61, 43.75, 54.32, 57.90 and 63.63°, which are assigned to (111), (220), (311), (222), (400), (422), (511) and (440) crystal planes. These crystal planes confirm the spinel MnCo2O4 compound formed at 400 °C. The XRD patterns of the MnCo2O4 are excellently matching with the standard JCPDS card no. 023-1237 [22]. There are no extra peaks other than those attributed to MnCo2O4, which illustrates the phase purity of the prepared MnCo2O4 compound. The broadening of diffraction peaks was observed in the XRD pattern and it indicates the small crystal size. The JCPDS file reveals that manganese and cobalt ions present in their mixed valence states in the MnCo2O4 spinel structure and both of them forms a cubic spinel structure with the + 2 and + 3 valence states. In the spinel, structured tetrahedral sites are occupied by the Mn2+ and Co2+ and the octahedral sites are occupied with the Mn3+ and Co3+ [31]. The crystallite size of the MnCo2O4 powders was calculated using the Scherrer’s equation; t = 0.9λ/βcosθ, where t is the average crystallite size, λ is the wavelength of incident X-rays of Cu Kα radiation, β is the full width half maxima of the diffraction peak and θ is the Bragg diffraction angle [30]. The calculated crystallite si for the MnCo2O4 compound is about 21 nm.
FTIR spectrum of the MnCo2O4 nanopowder calcined at 400 °C for 3 h is shown in Fig. 2. From Fig. 2, it is observed that the strong transmittance bands at 651 and 559 cm−1 are attributed to the stretching vibrational modes of the spinel MnCo2O4 compounds [22]. These two bands are due to the interaction of tetrahedrally coordinated Mn2+, Co2+ and octahedrally coordinated Mn3+, Co2+ ions with the oxygen in the spinel MnCo2O4 structure. The peaks in the spectrum further confirm the presence of phase pure spinel MnCo2O4 and it also validates the pure phase results of XRD analysis.
Raman spectrum of the MnCo2O4 nanopowder calcined at 400 °C for 3 h is shown in Fig. 3. From Fig. 3, it observed that the spectrum shows five Raman bands in the region 100–1000 cm−1 positioned at 193, 479, 512, 609, and 679 cm−1 are related to the Raman-active modes Mn–O, Co–O of cubic spinel oxides MnCo2O4 [32]. The Raman modes confirm the formation of spinel MnCo2O4. Also, the absence of Mn–OH and Co–OH bands or other related bands in the spectrum confirms the formation of the spinel oxide MnCo2O4 [32, 33].
The SEM micrograph images of the MnCo2O4 nanopowder calcined at 400 °C for 3 h is shown in Fig. 4. From Fig. 4, it is observed that the SEM micrographs scanned at different magnifications expose the presence the aggregation of several nanorods to form bundles of nanorods of ~ 300–400 nm in diameters and few microns in length. These aggregated bundles of nanorods like structure can facilitate the maximum access of electrolyte ion from bulk solution. In order to identify the elemental composition presence, the elemental analysis was performed using energy-dispersive spectrometry (EDS) and it is shown in Fig. 5. The EDS spectrum confirms the presence of Mn, Co and O. The percentage composition of Mn, Co and O of the nanorods surface clearly confirmed the atomic ratios of Mn, Co, and O present in 1:2:4 ratio.
3.2 Electrochemical analysis
The CV curves of the MnCo2O4 electrode at different scan rates from 5 to 100 mV s−1 using 1 M KOH as the electrolyte in the potential range of 0–0.6 V are shown in Fig. 6. In Fig. 6, the CV curves show the presence of redox peak which is due to the reversible transition between the oxidation states of cobalt (Co2+ and Co3+) and manganese ions (Mn2+ and Mn3+). The anodic peak at around 0.35 V and the cathodic peak at around 0.1 V may correspond to the oxidation and the reduction process, respectively. The CV curve shows that the capacitance arises mainly due to reversible redox reactions of the electrode materials [22].
The reversible redox reaction mechanism may be described by the following equation
The CV curves do not contain separate cathodic and anodic peaks for cobalt and manganese ions and it reveals that the cobalt and manganese ions were identical in their electrochemical reactivity [34]. The shapes of the CV curves do not change at different scan rates, 5–100 mV s−1, but the CV curves peak positions widely shift with increased scan rate. The oxidation peaks gradually move to higher potentials, whereas the reduction peaks shift to lower potentials in the opposite directions. As a result, it is identified that at higher scan rates the electronic and ionic movements are rapid and a fast redox reaction occurs at the interface of electroactive material/electrolyte [35, 36]. The capacitance arises from the material can be determined from the relationship between peak current with the square root of the scan rate. As a result, it confirms that either the capacitance arises from the surface redox reactions or from the bulk diffusion. The peak current versus the square root of the scan rate of the MnCo2O4 nanorods bundles is shown in Fig. 7. It shows a linear relationship of peak current with square root scan rate, which indicates the electrode reaction is diffusion-controlled. Moreover, it shows that the electrochemical process is controlled by the diffusion of OH− ions [37]. The specific capacitances are calculated for the electrodes using the CV data and GCD data in Eqs. (1) and (2), respectively,
where Cs is the specific capacitance, Q is the average charge of the electrode (Coulomb), ∆V is the potential window (V), m is the loaded mass of the active material (g), I is the discharge current (A) and Δt is the discharge time (s) [38, 39]. The calculated Cs for the MnCo2O4 nanorods bundles are 2394, 1671, 1121, 847, 657, 576, 458 and 408 F g−1 at scan rates of 5, 10, 20, 30, 40, 50, 75 and 100 mV s−1, respectively. From the above-calculated value it is observed that the calculated specific capacitance decreases with increase in the scan rates, this is due to the faster mobility of the electrolyte ions and as a result not able to entirely participate in the electrochemical reaction with the active material at higher scan rates [40]. Figure 8 shows the GCD curves at various current densities with a potentials window between 0 and 0.4 V for MnCo2O4. From Fig. 8, it is observed that the quasi-symmetric charge/discharge curve propose that the pseudocapacitive behavior of the material and were calculated the specific capacitances using the Eq. (2). Figure 9 shows the decrease in the specific capacitance 1617, 1505, 1395, 1390, 1250, and 900 F g−1 with respect to the different discharge current densities 1, 2, 3, 4, 5, and 10 A g−1 for the MnCo2O4. From Fig. 9, it is observed that an increase in the current density results in the capacitance decrease, which may be attributed to a reduction in diffusion of OH− ion into the active sites of electrode material [41]. The electrochemical reaction demonstrated the highest specific capacitance of 1617 F g−1 at a current density 1 A g−1.
The GCD curves at a current density of 20, 30, 50 and 100 A g−1 are shown in Fig. 10. From Fig. 10 it is observed that the shape of quasi-symmetric charge/discharge curve does not change even at a high current (100 A g−1) and the electrode retains the capacitance of 275 F g−1 (17%). The above result proves the excellent stability of the electrode materials and it is due to the morphology of bundles of MnCo2O4 nanorods bundles like structural features, such a high current structural stability is extremely essential for the applicability of the MnCo2O4 materials for the supercapacitor. Thus, in the present work, the obtained specific capacitance of the MnCo2O4 nanorod bundles synthesized via the MH method is comparatively higher than the literature reports. The comparison of few literature reports such as Li et al., reported a high specific capacitance of 349.8 F g−1 for MnCo2O4 nanowire arrays on nickel foam by hydrothermal method at a current density of 1 A g−1 [20]. Venkatachalam et al., reported the high specific capacitance of 507.5 F g−1 for the MnCo2O4 nanostructured electrode at a current density of 1 A g−1 [22]. Table 1 represents the comparison of the specific capacitances reported in the literature with the present work.
The GCD measurement was carried out at different current densities 5, 10, 20, 30, 50 and 100 A g−1 for 120 cycles are shown in Fig. 11. From Fig. 11 it is observed that the MnCo2O4 nanorods bundle-like structure electrode material exhibit the high reversible capacity, high capacitance retention performance and excellent cycling stability as the cycling current was varied from low (5 A g−1) to high (100 A g−1) current and reversed back to a current at 5 A g−1. Thus, the high rate performances and good reversible capacity retention at different current densities demonstrate the structural stability of the calcined MnCo2O4 nanorods bundles, which is capable of delivering required high energy at various load levels and confirm to be a promising electrode material for supercapacitors application [42].
The cycling performance is significant for the electrode materials in supercapacitor applications [44]. The cyclic stability test of the electrodes was carried out using GCD measurement at a current density of 10 A g−1 for 1000 cycles. Figure 12 shows the specific capacitance and the capacitance retentions with respect to a number of cycles and the inset fig. shows the first ten charge/discharge cycles of MnCo2O4 nanorods. From Fig. 12, it is observed that about 88% of initial capacitance was retained even after 1000 cycles at a high current density of 10 A g−1, which demonstrates the good cyclic stability of the MnCo2O4 material as the electrode even at high current.
EIS of the electrodes are measured in an open circuit potential at a frequency range from 10 kHz to 1 Hz after 1st and 1000th cycles at a current density of 10 A g−1 is shown in Fig. 13. From Fig. 13, it is observed that the presence of a depressed semi-circle at the higher frequency region is attributed to the combination of parallel circuits of capacitance and the ionic charge transfer resistance. The presence inclined straight line at the low-frequency region represents the Warburg resistance (Zw). It is also observed that this is due to the frequency dependent ion-diffusion or transport in the electrolyte to the electrode surface [44]. From the figure, it is noted that the slight increase in the radius of the depressed semicircle at high-frequency region after the 1000th cycle compared with the 1st cycle reveals the small decrease in the interfacial charge transfer conductivity of electrodes. At low-frequency region, Warburg resistance slightly increased after 1000 cycles and the slope was not altered, which suggest the mobility of the ions in the electrolyte to the electrode surface was not relatively altered. The internal drop in the specific capacitance can be attributed to the slight decrease in the interfacial charge transfer conductivity, which causes a decrease in the retention of the initial capacitance for MnCo2O4 during the cycle performance. The overall results suggested that the MH synthesized MnCo2O4 nanorods bundles would be a promising electrode material for the supercapacitor applications.
The variations of the energy density as a function of the power density for the MnCo2O4 electrodes are shown in Fig. 14. The energy density and power density were derived from charge/discharge curves at various current densities and these can be calculated from the following equations [30].
where P is the power density (W kg−1), E is the energy density (Wh kg−1), Cs is the specific capacitance based on the mass of the electroactive material (F g−1), Q is the total charge delivered (C), ΔV is the potential window of discharge (V), and t is the discharge time (s). From Fig. 14, it is observed that the energy density decreases with an increase in the power density. The maximum energy density obtained for the electrodes 35.9 and 27 Wh kg−1 at the power density of 200 and 1000 W kg−1. The obtained energy density is higher than the literature reports such as C@MnCo2O4 (25.5 Wh kg−1 at 856 W kg−1) [48], NiMn2O4 (3.6 Wh kg−1 at 224.76 W kg−1) [47], C@NiMn2O4 (13.23 Wh kg−1 at 224.76 W kg−1) [49], MnMoO4 (15.5 Wh kg−1 at 224.9 W kg−1) [27], NiMn2O4 (29.64 Wh kg−1 at 200.27 W kg−1) [50], ZnCo2O4 (25 Wh kg−1 at 255 W kg−1) [51]. The high energy density without any significant loss of the power density demonstrated that the MnCo2O4 nanorods bundle-like structure is vital to consider as an efficient supercapacitor electrode materials.
4 Conclusion
MnCo2O4 nanorods have been successfully synthesized via the MH methods. MnCo2O4 electrode exhibited an excellent specific capacitance of 1617.5 F g−1 at the current density of 1 A g−1. The cycling stability had revealed a good reversibility, with a cycling efficiency of 88% after 1000 cycles at a high current density of 10 A g−1. The high rate performances and good reversible capacity retention at different current densities demonstrated the structural stability of the calcined MnCo2O4. Thus, the facile MH approach to synthesis MnCo2O4 nanorod may be a valuable method to obtain a promising electrode with a high electrochemical performance for the supercapacitor applications.
References
H. Wang, Y. Yang, Y. Liang, J.T. Robinson, Y. Li, A. Jackson, Y. Cui, H. Dai, Graphene wrapped sulfur particles as a rechargeable lithium–sulfur battery cathode material with high capacity and cycling stability. Nano Lett. 11, 2644–2647 (2011)
F. Shi, L. Li, X.L. Wang, C.D. Gu, J.P. Tu, Metal oxide/hydroxide-based materials for supercapacitors. RSC Adv. 4, 41910–41921 (2014)
Y. Yang, T. Liu, L. Zhang, S. Zhao, W. Zeng, S. Hussain, C. Deng, H. Pan, X. Peng, Facile synthesis of nickel doped walnut-like MnO2 nanoflowers and their application in a supercapacitor. J Mater Sci. Mater. Electron. 27, 6202–6207 (2016)
R. Ramkumar, M. Minakshi, Sundaram, Electrochemical synthesis of polyaniline cross-linked NiMoO4 nanofibre dendrites for energy storage devices. New J. Chem. 40, 7456–7464 (2016)
B. Li, Q. Sun, R. Yang, D. Li, Z. Li, Simple preparation of graphene-decorated NiCo2O4 hollow nanospheres with enhanced performance for supercapacitor. J. Mater. Sci. Mater. Electron. 29, 7681–7691 (2018)
S. Biswas, L.T. Drzal, Multilayered nano-architecture of variable sized graphene nanosheets for enhanced supercapacitor electrode performance. ACS Appl. Mater. Interfaces 2, 2293–2300 (2010)
G.S. Gund, D.P. Dubal, S.S. Shinde, C.D. Lokhande, One step hydrothermal synthesis of micro-belts like β-Ni (OH)2 thin films for supercapacitors. Ceram. Int. 39, 7255–7261 (2013)
K. Adib, M.R. Nasrabadi, Z. Rezvani, S.M. Pourmortazavi, F. Ahmadi, H.R. Naderi, M.R. Ganjali, Facile chemical synthesis of cobalt tungstates nanoparticles as high performance supercapacitor. J Mater Sci. Mater. Electron. 27, 4541–4550 (2016)
H. Wang, Y. Yang, Y. Liang, G. Zheng, Y. Li, Y. Cui, H. Dai, Rechargeable Li–O2 batteries with a covalently coupled MnCo2O4–graphene hybrid as an oxygen cathode catalyst. Energy Environ. Sci. 5, 7931–7935 (2012)
Y. Liang, H. Wang, J. Zhou, Y. Li, J. Wang, T. Regier, H. Dai, Covalent hybrid of spinel manganese-cobalt oxide and graphene as advanced oxygen reduction electrocatalysts. J. Am. Chem. Soc. 134, 3517–3523 (2012)
J. Li, S. Xiong, X. Li, Y. Qian, Spinel Mn1.5Co1.5O4 core–shell microspheres as Li-ion battery anode materials with a long cycle life and high capacity. J. Mater. Chem. 22, 23254–23259 (2012)
H. Chen, J. Jiang, L. Zhang, T. Qi, D. Xia, H. Wan, Facilely synthesized porous NiCo2O4 flowerlike nanostructure for high-rate supercapacitors. J. Power Sources 248, 28–36 (2014)
Q. Zhou, J. Xing, Y. Gao, X. Lv, Y. He, Z. Guo, Y. Li, Ordered assembly of NiCo2O4 multiple hierarchical structures for high-performance pseudocapacitors. Appl. Mater. Interfaces 6, 11394–11402 (2014)
A. Pendashteh, S.E. Moosavifard, M.S. Rahmanifar, Y. Wang, M.F. El-Kady, R.B. Kaner, M.F. Mousavi, Highly ordered mesoporous CuCo2O4 nanowires, a promising solution for high-performance supercapacitors. Chem. Mater. 27, 3919–3926 (2015)
S.G. Krishnan, M.V. Reddy, M. Harilal, B. Vidyadharan, I.I. Misnon, M.H.A. Rahim, J. Ismail, R. Jose, Characterization of MgCo2O4 as an electrode for high performance supercapacitors. Electrochim. Acta 161, 312–321 (2015)
Y. Xu, X. Wang, C. An, Y. Wang, L. Jiao, H. Yuan, Facile synthesis route of porous MnCo2O4 and CoMn2O4 nanowires and their excellent electrochemical properties in supercapacitors. J. Mater. Chem. A 2, 16480–16488 (2014)
S. Sahoo, K.K. Naik, C.S. Rout, Electrodeposition of spinel MnCo2O4 nanosheets for supercapacitor applications. Nanotechnology 26, 455401 (2015)
K.N. Hui, K.S. Hui, Z. Tang, V.V. Jadhav, Q.X. Xia, Hierarchical chestnut-like MnCo2O4 nanoneedles grown on nickel foam as binder-free electrode for high energy density asymmetric supercapacitors. J. Power Sources 330, 195–203 (2016)
R. Krishnapriya, S. Praneetha, A. Vadivel, Murugan, Energy-efficient, microwave-assisted hydro/solvothermal synthesis of hierarchical flowers and rice grain-like ZnO nanocrystals as photoanodes for high performance dye-sensitized solar cells. CrystEngComm 17, 8353–8367 (2015)
L. Li, Y.Q. Zhang, X.Y. Liu, S.J. Shi, X.Y. Zhao, H. Zhang, X. Ge, G.F. Cai, C.D. Gu, X.L. Wang, J.P. Tu, One-dimension MnCo2O4 nanowire arrays for electrochemical energy storage. Electrochim. Acta 116, 467–474 (2014)
B. Kong, C. Lu, M.C. Liu, Y.C. Luo, L. Kang, X. Li, F.C. Walsh, The specific capacitance of sol–gel synthesised spinel MnCo2O4 in an alkaline electrolyte. Electrochim. Acta 115, 22–27 (2014)
V. Venkatachalam, A. Alsalme, A. Alghamdi, R. Jayavel, High performance electrochemical capacitor based on MnCo2O4 nanostructured electrode. J. Electroanal. Chem. 756, 94–100 (2015)
S.G. Krishnan, M.H.A. Rahim, R. Jose, Synthesis and characterization of MnCo2O4 cuboidal microcrystals as a high performance psuedocapacitor electrode. J. Alloys Compd. 656, 707–71365 (2016)
G.M. Thorat, H.S. Jadhav, J. Gil Seo, Bi-functionality of mesostructured MnCo2O4 microspheres for supercapacitor and methanol electro-oxidation. Ceram. Int. 43, 2670–2679 (2017)
Y. Dong, Y. Wang, Y. Xu, C. Chen, Y. Wang, L. Jiao, H. Yuan, Facile synthesis of hierarchical nanocage MnCo2O4 for high performance supercapacitor. Electrochim. Acta 225, 39–46 (2017)
M.C. Liu, L.B. Kong, C. Lu, X.J. Ma, X.M. Li, Y.C. Luob, L. Kang, Design and synthesis of CoMoO4–NiMoO4.H2O bundles with improved electrochemical properties for supercapacitors. J. Mater. Chem. A 1, 1380–1387 (2013)
I. Bilecka, M. Niederberger, Microwave chemistry for inorganic nanomaterials synthesis. Nanoscale 2, 1358–1374 (2010)
M. Baghbanzadeh, S.D. Škapin, Z.C. Orel, C.O. Kappe, A critical assessment of the specific role of microwave irradiation in the synthesis of ZnO micro- and nanostructured materials. Chem. Eur. J. 18, 5724–5731 (2012)
Y. Yang, Y. Liang, J. Hu, J. Zou, Q. Tang, Z. Wan, Y. Lu, Rapid microwave-assisted hydrothermal synthesis of hierarchical micro/nanostructured TiO2 with tunable nanomorphologies. J Mater Sci. Mater. Electron. 27, 11606–11612 (2016)
S. Jayasubramaniyan, S. Balasundari, P.A. Rayjada, N. Satyanarayana, P. Muralidharan, Microwave hydrothermal synthesis of α-MnMoO4 nanorods for high electrochemical performance supercapacitors. RSC Adv. 8, 22559–22568 (2018)
S. Ma, L. Sun, L. Cong, X. Gao, C. Yao, X. Guo, L. Tai, P. Mei, Y. Zeng, H. Xie, R. Wang, Multiporous MnCo2O4 microspheres as efficient bifunctional catalyst for non aqueous Li–O2 batteries. J. Phys. Chem. C 117, 25890–25897 (2013)
N. Padmanatha, S. Selladurai, Mesoporous MnCo2O4 spinel oxide nanostructure synthesized by solvothermal technique for supercapacitor. Ionics 20, 479–487 (2014)
J. Pal, P. Chauhan, Study of physical properties of cobalt oxide (Co3O4) nanocrystals. Mater. Charact. 61, 575–579 (2010)
R. Tholkappiyan, A.N. Naveen, S. Sumithra, K. Vishista, Investigation on spinel MnCo2O4 electrode material prepared via controlled and uncontrolled synthesis route for supercapacitor application. J. Mater. Sci. 50, 5833–5843 (2015)
D. Cai, B. Liu, D. Wang, Y. Liu, L. Wang, H. Li, Y. Wang, C. Wang, Q. Li, T. Wang, Enhanced performance of supercapacitors with ultrathin mesoporous NiMoO4 nanosheets. Electrochim. Acta 125, 294–301 (2014)
I. Shakir, M. Shahid, H.W. Yang, D.J. Kang, Structural and electrochemical characterization of alpha-MoO3 nanorod-based electrochemical energy storage devices. Electrochim. Acta 56, 376–380 (2010)
X. Cao, J. Wei, Y. Luo, Z. Zhou, Y. Zhang, Spherical nickel hydroxide composite electrode. Int. J. Hydrog. Energy 25, 643–647 (2000)
P. Vinothbabu, P. Elumalai, Tunable supercapacitor performance of potentiodynamically deposited urea-doped cobalt hydroxide. RSC Adv. 4, 31219–31225 (2014)
K. Liu, Z. Hu, R. Xue, J. Zhang, J. Zhu, Electropolymerization of high stable poly(3,4-ethylenedioxythiophene) in ionic liquids and its potential applications in electrochemical capacitor. J. Power Sources 179, 858–862 (2008)
S. Saranya, R.K. Selvan, N. Priyadharsini, Synthesis and characterization of polyaniline/MnWO4 nanocomposites as electrodes for pseudocapacitors. Appl. Surf. Sci. 258, 4881–4887 (2012)
P. Justin, G.R. Rao, CoS spheres for high-rate electrochemical capacitive energy storage application. Int. J. Hydrog. Energy 35, 9709–9715 (2010)
H. Chen, A. Liu, J. Mu, C. Wu, X. Zhang, Template-free synthesis of novel flower-like MnCo2O4 hollow microspheres for application in supercapacitors. Ceram. Int. 42, 2416–2424 (2016)
W. Li, K. Xu, G. Song, X. Zhou, R. Zou, J. Yang, Z. Chena, J. Hu, Facile synthesis of porous MnCo2O4.5 hierarchical architectures for high-rate supercapacitors. CrystEngComm 16, 2335–2339 (2014)
Y. Li, X. Peng, J. Xiang, J. Yang, Synthesis of MnCo2O4.5/graphene composite as electrode material for supercapacitors. Int. J. Electrochem. Sci. 12, 10763–10772 (2017)
H. Che, Y. Wang, Y. Mao, Novel flower-like MnCo2O4 microstructure self-assembled by ultrathin nanoflakes on the microspheres for high-performance supercapacitors. J. Alloys Compd. 680, 586–594 (2016)
S.G. Krishnan, M. Hasbi, A. Rahim, R. Jose, Synthesis and characterization of MnCo2O4 cuboidal microcrystals as a high performance psuedocapacitor electrode. J. Alloys Compd. 656, 707–713 (2016)
M. Li, W. Yang, J. Li, M. Feng, W. Li, H. Li, Y. Yu, Porous layered stacked MnCo2O4 cubes with enhanced electrochemical capacitive performance. Nanoscale 10, 2218–2225 (2018)
L. Li, F. He, S. Gai, S. Zhang, P. Gao, M. Zhang, Y. Chen, P. Yang, Hollow structured and flower-like C@MnCo2O4 composite for high electrochemical performance in a supercapacitor. CrystEngComm 16, 9873–9881 (2014)
M.R. Kim, R.M. NaiduKalla, S. Kim, M.-R. Kim, I. Kim, NiMn2O4 nanosheet-decorated hierarchically porous polyaromatic carbon spheres for high-performance supercapacitors. ChemElectroChem 4, 1–9 (2017)
H. Wei, J. Wang, L. Yu, Y. Zhang, D. Hou, T. Li, Facile synthesis of NiMn2O4 nanosheet arrays grown on nickel foam as novel electrode materials for high-performance supercapacitors. Ceram. Int. 42, 14963–14969 (2016)
G. Zhou, J. Zhu, Y. Chen, L. Mei, X. Duan, G. Zhang, L. Chen, T. Wang, B. Lu, Simple method for the preparation of highly porous ZnCo2O4 nanotubes with enhanced electrochemical property for supercapacitor. Electrochim. Acta 123, 450–455 (2014)
Acknowledgements
Authors are grateful to PFRC and BRNS, DAE, Govt. of India, for utilizing the instruments purchase under the various funded project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jayasubramaniyan, S., Balasundari, S., Rayjada, P.A. et al. Enhanced electrochemical performance of MnCo2O4 nanorods synthesized via microwave hydrothermal method for supercapacitor applications. J Mater Sci: Mater Electron 29, 21194–21204 (2018). https://doi.org/10.1007/s10854-018-0269-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-0269-5