Abstract
Electrochemical removal of sulfide ions was achieved in salt water using graphite anodes in an autoclave under high temperatures and pressures, simulating geothermal fluids. The reaction products were characterized using microscopy and X-ray photoelectron spectroscopy (XPS). At low temperatures the reaction rate is quite small. It decreases rapidly with time down to a negligibly small value, which increases only slightly with temperature. The reaction produces elemental sulfur, which was seen under the microscope and identified using XPS. It passivates the electrode and hence diminishes its activity. Above about 115 °C, much higher removal rates can be sustained for much longer times, while the increase of temperature has a much stronger effect on the reaction rate. Under this condition, elemental sulfur was no longer detected among the reaction products, while the electrode retained its activity for continuous operation. The XPS spectra at high temperatures reveal the presence of oxygen bearing sulfur species, such as sulfates. The melting of sulfur (at 115 °C) has a much stronger effect on the efficiency of the process than the transition of orthorhombic to monoclinic sulfur (at 95 °C). A Clausius-Clapeyron’s analysis reveals that the melting point of sulfur inside the autoclave is nearly equal to its normal melting point.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Hydrogen sulfide is listed by the US Environmental Protection Agency (EPA) as an extremely hazardous substance [1]. It is a dangerous toxic material that jeopardizes the health of humans and promotes the corrosion of metallic materials [2, 3]. It contaminates massive volumes of natural geothermal fluids that are encountered in the drilling of wells for the production of oil and for the utilization of geothermal energy [3–5]. It exists in many other natural water bodies [6, 7] and contaminates many waste water streams resulting from various industries [8–11].
Control of hydrogen sulfide in oil production involves precipitation and oxidation using scavengers, such as zinc carbonate, sodium chromate, ferric oxide, etc. at doses of some kilograms per barrel, depending on the sulphide level and other oil well fluid properties [3, 12–16]. Depending on the volume of production, a particular oil field may require injection of some hundreds of tons of these scavengers. This entails great cost and exerts a heavy toll on the environment.
The removal of H2S from gas streams is achieved using the Claus process [17]. This is a gas phase catalytic process that is performed at high temperature. Various electrochemical versions of the Claus process were proposed to oxidize H2S from gas streams in molten electrolytes at temperatures up to 900 °C [18–20]. A similar electrochemical process, using a molten salt electrolyte, has been proposed for the removal of SO x from flue gas streams [21]. The removal of H2S from sour gas streams was achieved by scrubbing the gas in 1 M NaOH and electrolyzing the resulting solution at 80 °C and 1 atm. [22, 23]. These processes are not suitable for the removal of sulfide ions from geothermal fluids.
Direct electrochemical oxidation of such fluids provides an attractive environmentally friendly process that converts the polluting sulfide ions into more innocuous products, using electrons rather than chemicals. The process leads to the formation of elemental sulfur at temperatures up to 80 °C [22–24]. The electrode reaction can be represented by:
Naturally occurring geothermal fluids deep underground exist under much higher temperatures and pressures [3–5]. Consequently, one needs to test the direct electrochemical oxidation of sulfide ions under higher temperatures to make a closer simulation of the natural environment where the polluting sulfide ions exist. Natural geothermal fluids under this condition are also particularly amenable to electrochemical treatment as they exhibit high electrical conductivity.
This paper presents the results of some experiments that were performed under conditions which simulate geothermal fluids over a range of temperatures that exceeds the normal melting point of sulfur (115 °C). Since this temperature is above the normal boiling point of water, it is necessary to perform the measurements in a closed system (an autoclave). In such a system, the pressure increases above 1 atm as the temperature increases above 100 °C. This increase in pressure might affect the thermodynamics of the various transitions of sulfur, i.e. from orthorhombic to monoclinic to liquid sulfur. This issue is also addressed in this paper.
2 Experimental details
Working electrodes were in the form of rods of pyrolytic graphite (0.64 cm diameter). These were machined from a 0.7 cm thick slab of pyrolytic graphite. The cross sectional area of the rod (0.32 cm2) served as the working electrode while the side walls of the rod were insulated with Teflon. The electrodes were polished successively down to 0.05 μm alumina giving a mirror finish. All measurements were performed in a supporting electrolyte of 3.5% NaCl containing 0.01 M sodium sulfide. The test solutions were prepared from deionized water and analytical grade chemicals. Measurements were performed using a Gamry PC4/750 Potentiostat. The potential was scanned from cathodic towards anodic potentials against an Ag/AgCl reference electrode, E = 0.197 V (SHE). The graphite electrodes were examined using AXIO Imager A1 M optical microscope, and an X-ray photoelectron spectrometer (XPS), FISON Instruments, Model ESCA-lab 200 (VG instruments).
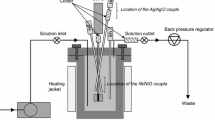
Experiments were performed in a Teflon cell housed in an autoclave made from 316 L stainless steel. The head of the autoclave has ports for a thermocouple and contacts to the working, counter and reference electrodes. To ensure gas-tight sealing of the head onto the autoclave, the head was machined to accommodate two o-rings. The electrolytic cell was fabricated from a block of PTFE to fit tightly in the main body of the autoclave. The cell is also composed of a main body which contains the salt solution and a cover. After the cell was assembled and the autoclave sealed, the system was placed in a specially designed electric furnace equipped with a temperature regulator. Attainment of constant temperature required about 2 h. After the measurements were performed and the heating turned off, the system required many more hours to cool down before it could be disassembled.
3 Results
Figure 1 illustrates some current-potential curves for the electrochemical oxidation of sulfide ions at various temperatures, from a solution containing 0.01 M HS− at a scanning rate of 1 mV s−1. The anodic current increases gradually with potential towards a well defined limiting current, which remains constant for several hundred mV. This limiting current is a measure of the highest possible rate of the reaction. The shape of these curves is characteristic of electrochemical reactions under control by charge transfer and material transport processes.
Potentiostatic current transients were measured under various conditions. Figure 2 is an illustrative example obtained at 25 °C and a potential of 0.8 V (Ag/AgCl). The current in the presence of sulfide ions is more than two orders of magnitude greater than that in the supporting electrolyte. This indicates that the currents measured in Fig. 1 are indeed resulting from the oxidation of the sulfide ions.
Figure 3 displays the effect of temperature on the potentiostatic current transients obtained under a potential of 0.8 V (Ag/AgCl). As the temperature increases, the current increases. However, the more significant increase in current occurs around 100 °C. An increase of about 55 °C in temperature from 25 to 80 °C has a much smaller effect on the current than an increase of only 20 °C from 100 to 120 °C. The currents obtained at temperatures up to 80 °C decrease rapidly with time to negligibly small values which remain nearly constant. This is an indication of the passivation of the surface by elemental sulfur. The presence of elemental sulfur on the electrode surface has been confirmed below, using microscopy and XPS. On the other hand, the currents obtained at and above 100 °C are much larger and more sustainable. This behavior is associated with the absence of elemental sulfur on the electrode surface, as shown below.
Figure 4 illustrates the effect of temperature on the currents measured at the end of the tests shown in Fig. 3. The relationship is quite nonlinear. Two regions are clearly seen in Fig. 4. In Region 1 the current increases with temperature only slightly. This is followed by rapid increase of current with temperature (Region 2). The intersection of the two straight lines shown in Fig. 4 occurs at about 115 °C, which coincides with the normal melting point of sulfur.
Effect of temperature on the currents obtained from Fig. 3
3.1 Reaction products
The electrode surfaces were inspected microscopically and subjected to XPS after performing the electrolyses at different temperatures. Figures 5a–c show some micrographs of the graphite surfaces after tests similar to those described in Fig. 3. Note the presence of sulfur in the images shown in Fig. 5a and b, at 80 and 100 °C, respectively. On the other hand, no sulfur is seen in the image of Fig. 5c, at 150 °C. Several XPS spectra are shown in Fig. 6a–c for electrodes treated at different temperatures. The well developed peaks at about 164.8 and 161.8 eV correspond to elemental sulfur and sulfide ions, respectively [25, 26]. On the other hand, the peak at about 168.5 eV is often associated with oxygen bearing species, such as sulfate ions. Note the presence of the peak at 164.8 eV on the electrode surfaces treated at 25 and 100 °C, indicating the presence of elemental sulfur, which is seen in the images presented in Fig. 5a and b. On the other hand, the electrode treated at 175 °C is totally void from elemental sulfur. The presence of a sulfide peak at a binding energy of 161.8 eV after this treatment reveals that the sulfide ions have not been completely removed. The formation of elemental sulfur at low temperatures was shown to occur via reaction 1 [24]. On the other hand, the formation of sulfate ions proceeds according to reaction 5, described below.
3.2 Removal rate and efficiency
The rate of removal of sulfide ions (∂m/∂t in mol s−1) is directly proportional to the currents (i in A) measured in Fig. 3, through Faraday’s law:
where i is in A, n is the number of electrons transferred in the reaction and F is the Faraday’s constant. A simple calculation illustrates the effect of temperature on the rate of removal of sulfide ions. The currents supported by a fairly small electrode (0.32 cm2 area) are about 0.001 and 0.003 A at 120 and 150 °C, respectively. The electrochemical oxidation of HS− at such temperatures is shown below to produce oxygen bearing sulfur species, such as sulfates SO4 −. This reaction is associated with a value of n = 9 (see Eq. 5). Using the Faraday’s constant and this value of n in Eq. 2, one obtains removal rates of about 1 × 10−9 and 3 × 10−9 mol s−1 at 120 and 175 °C, respectively. The amount of removed sulfide ions is directly affected by the area of the electrode and the duration of the process. A flat electrode of a modest size (10 cm × 10 cm) can remove sulfide ions at rates of 2.25 and 6.75 mmol h−1 at 120 and 175 °C, respectively. Much higher electroactive areas can be utilized for this purpose using porous electrodes [27].
The charge passed during the electrolysis process can be obtained from the area under each curve in Fig. 3. The curves measured at 120 and 175 °C corresponding to charges of about 12 and 40 C, respectively. An electrode of larger area would pass proportionately higher number of coulombs under otherwise the same conditions. Assuming all the sulfide ions in the electrolyte are oxidized to sulfate ions, the total charge would be about 200 C. This indicates that the efficiency of the removal process is about 6% at 120 °C and 20% at 175 °C after electrolysis for 3 h using an electrode of only 0.32 cm2 area. An electrode of much larger area would support much higher currents and hence much higher removal rates. With an electrode of much larger area, one expects that the current will decay more rapidly with time because of the rapid depletion of the sulfide ions from the electrolyte. Measurements with much larger and/or porous electrodes are planed to achieve this objective.
4 Discussion
4.1 Sulfur species
Anodic oxidation of sulfide ions can yield a variety of products e.g. elemental sulfur, polysulfide, thiosulfates and sulfates [28–30], i.e.
Polysulfides may also undergo oxidation to elemental sulfur, i.e.
Both elemental sulfur and the various polysulfides undergo oxidation to oxyanions, e.g. thiosulfate and sulfate, e.g.
On the basis of thermodynamic predictions, many of the above reactions can occur simultaneously. For example at a potential of E = 0.35 V (SHE), all the above reactions can, in principle, occur simultaneously under standard conditions. On the other hand, at 0.20 V, only reactions 1, 4, 6 and 7 can proceed simultaneously. Although reaction 7 is thermodynamically feasible, elemental sulfur was found to be stable at lower temperatures under strongly oxidizing potentials [24]. This indicates that reaction 7 does not proceed to any great extent at the lower temperatures due to kinetic limitations. Determination of the selectivity of each of the above reactions and the simultaneous determination of the concentration of each of the above species are major tasks that are yet to be achieved.
Stefansson et al. [31, 32] gave a brief summary of sulfur chemistry in hydrothermal waters. They indicated that the oxidation of H2S proceeds through a series of complex reactions that involve meta stable sulfur species with oxidation states from −2 to +6, such as sulfur (So), poly sulfides (S n S2−), thiosulfate (S2O3 2−), polythionates (S x O6 2−) and sulfite (SO3 2−).
4.2 Transition temperatures
The thermodynamics of the sulfur system have important bearings on the interpretation of the present results, which are covering a range of temperature from 25 to 175 °C. The equilibrium between orthorhombic (o) and monoclinic (m) sulfur is given by:
The transition temperature of this equilibrium is about 95 °C at one atmosphere. This temperature occurs shortly after the beginning of the region of rapid increase of current with temperature, as shown in Fig. 4. However, the rate of increase of current with temperature around 95 °C is much less than that around (115 °C) which is the normal melting point of sulfur (see Fig. 4). The present results suggest that the orthorhombic to monoclinic phase change (Eq. 3) is not significant in terms of the rate and efficiency of sulfide removal, as shown in Fig. 4. On the other hand, the melting of sulfur is represented by:
Our results show that above 115 °C, the current increases quite rapidly as the temperature increases. This might be attributed to the melting of sulfur, or to a possible change in the reaction product. Had elemental sulfur remained to be the predominant reaction product above 115 °C, it would melt away and go into the electrolyte. However, elemental sulfur was not detected.
Above 100 °C the pressure inside the autoclave is above 1 atm. The increase in pressure affects the melting point of sulfur. An estimate of the rate of change of the melting point of sulfur with pressure can be obtained using the Clausius-Clapeyron’s equation, i.e.
where ΔV is the change in molar volume (between liquid and monoclinic sulfur) and ΔH f is the molar enthalpy of fusion of monoclinic sulfur. The densities of monoclinic and liquid sulfur are 2.0 and 1.82 g cm−3, respectively while ΔH f ≈ 1.72 kJ mole−1. Using the above values and Eq. 10, we obtain a value of dT/dp ≈ 3.5 × 10−2 k atm−1. This rate of change requires a pressure increase of about 30 atm to produce an increase of the melting point of monoclinic sulfur of only one degree. Since the pressure in the autoclave was less than 10 atm, the melting point of monoclinic sulfur under the condition of our measurements is not much different than its normal melting point.
5 Conclusions
This work proved the feasibility of direct electrochemical desulfurization of geothermal fluids under high temperature. Above the melting point of sulfur (115 °C), high sustainable rates of desulfurization can be achieved, while the reaction produces oxygen bearing sulfur species such as sulfates. At lower temperatures, the reaction produces elemental sulfur which was seen under the microscope and identified using XPS. It passivates the electrode and hence diminished its activity. It has also been shown that the melting point of sulfur (at 115 °C) has more profound effects on the rate and efficiency of sulfide removal than the transition of monoclinic to orthorhombic sulfur (at 95 °C).
The efficiency of the process was shown to be strongly affected by the temperature, in measurements above the melting point of sulfur. This is contrary to the findings at temperatures much below 115 °C. The present work identified some areas that deserve more measurements and analyses. Some of these areas are briefly described below:
-
1.
Determination of the selectivity of the various anodic oxidation reactions of the sulfide ion, under different conditions of temperature, potential, electrode material and sulfide concentration.
-
2.
Simultaneous determination of the concentrations of the various sulfur species that result from sulfide oxidation.
-
3.
The present measurements were performed on pyrolytic graphite electrodes of quite a small area (0.32 cm2). This resulted in fairly small rates and efficiencies of removal. Much higher rates and efficiencies of removal can be achieved using flat electrodes with much larger surface areas or porous electrodes. These tasks are planned for future work.
References
U.S. Environmental Protection Agency (1988) Extremely hazardous substance: superfund chemical profiles, CAS registry number 7783-06-4, vol 1. Noys Data Corporation, New Jersey
Tuttle RN, Kane RD (eds) (1981) H2S corrosion in oil and gas production. National Association of Corrosion Engineers, Houston, Texas
Garverick L (ed) (1994) Corrosion in the petrochemical industry. ASM International, Metals Park, Ohio, p 259
Kagel A (2008) The state of geothermal technology, part II: surface technology. The Department of Energy, Washington, DC, p 5
Clauser C (2006) Geothermal energy, In Heinloth K (ed), Landolt-Börnstein, group VIII: Advanced materials and technologies, vol 3: energy technologies, subvol. C: renewable energies, Springer Verlag, Heidelberg-Berlin, 493–604, p 98
Xu Y, Shonnen MAA, Nordstram DK, Cunningham KM, Ball JW (2000) J Volcanol Geotherm Res 97:407
Cutter GA, Walsh RS, de Echols CS (1999) Deep-Sea Res II 46:991
Rajalo G, Petrovskaya T (1996) Environ Technol 17:605
Rao NN, Somasekhar KM, Kaul SN, Szpyrkowicz L (2001) J Chem Technol Biotechnol 76:1124
Szpyrkowicz L, Kaul SN, Neti RN (2005) J Appl Electrochem 35:381
Behm M, Simonsson D (1997) J Appl Electrochem 27:507
Garrett RL, Clark RK, Carney LL, Granthm CK (1997) Chemical scavengers for sulfides in water-based drilling fluids, SPE Reprint Series, 44:170
Singh AK, Kohil BS, Wendt RP (1989) World Oil, 209:99, 77
Al-Humaidan AY, Nasr-El-Din HA (1999) Optimization of hydrogen sulfide scavengers used during well stimulation, In: Proceedings of the SPE international symposium on oilfield chemistry
Schorling P Chr, Brauchle M (2001) Application of hydrogen sulfide scavengers in the oil and gas field, Erdoel Ergas Kohle/EKEP, 117:78
Scott P (1994) Oil Gas J 92:72
Weil ED, Sandler SR, (1991) Sulfur Compounds, In: Mary Howe-Grant (ed) Kirk-Othmer Encyclopedia of Chemical Technology, vol 23, 4th edn. Wiley, New York
Tatapundi P, Fenton JM (1995) Electrolytic processes for pollution treatment and pollution prevention. In: Gerischer H, Tobias CW (eds) Advances in electrochemical science and engineering, VCH, Weinheim, V 4 364
Lloyd CL, Gilbert II (1994) J. Electrochem. Soc. 141:2642
Pujare NU, Tsai KJ, Sammells AF (1989) J Electrochem Soc 136:3662
Schmidt DS, Winnick J (1998) AIchEJ 44:323
Anani A, Mao Z, White RE, Srinivasan S, Appleby AJ (1990) J Electrochem Soc 137:2703
Mao Z, Anani A, White RE, Srinivasan S, Appleby AJ (1991) J Electrochem Soc 138:1299
Ateya BG, AlKharafi FM, Alazab AS, Abdullah AM (2007) J Appl Electrochem 37:395
Briggs D, Seah MP (1990) Practical surface analysis. Wiley, New York, p 605
Gerson AR, Bredow T (2000) Surf Interface Anal 29:145
Ateya BG, AlKharafi FM, Abdullah RM, Alazab AS (2005) J Appl Electrochem 35:297
Zhdanow SI (1982) In Bard AJ (ed) Encyclopedia of the electrochemistry of the elements 6, Marcel Dekker Inc, New York
Bard AJ, Parson R, Jordan J (ed) (1985) Standard potentials in aqueous solutions, Marcel Dekker Inc., New York, p 94
Valensi G, van Muylder J, Pourbaix M (1974) In: Pourbaix M (ed) Atlas of electrochemical equilibria in aqueous media. NACE, Texas, p 545
Stefánsson A, Gunnarsson I (2005) Sulphur geochemistry of hydrothermal waters I. Report of preliminary study. Institute of Earth Sciences Report, RH-17
Kaasalainen H, Stefansson A (2008) The chemistry of sulfur in volcanic geothermal fluids Geochemica Et Cosmochimica Acta 72–12: A443–A443
Acknowledgments
The authors gratefully acknowledge the support of this work by the Research Administration of Kuwait University, under Grant numbers SC04/04 and GS01/01. They also acknowledge the help of the unit of Electron Microscopy for the SEM measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ateya, B.G., Al Kharafi, F.M., El-Shamy, A.M. et al. Electrochemical desulfurization of geothermal fluids under high temperature and pressure. J Appl Electrochem 39, 383–389 (2009). https://doi.org/10.1007/s10800-008-9683-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-008-9683-3