Abstract
LiFePO4 nanopowders (50 nm) were prepared through a simple hydrothermal process. Poly vinylidene fluoride (PVDF): LiFePO4 complex membranes as solid polymer electrolytes were characterized. X-ray diffraction (XRD) and differential scanning calorimetric (DSC) studies show a decrease in crystalline size and crystallinity of the polymer PVDF with increasing LiFePO4 concentration. Scanning electron micrographs show smaller spherical domains in the dry film with increased LiFePO4 nanopowder content. Impedance measurements suggest that the ionic conductivity of (PVDF + LiFePO4) increases with increased temperature and lithium powder concentration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Solid polymer electrolyte materials (SPE) are receiving attention, particularly in the field of solid-state ionics owing to their potential applications in advanced ionic devices [1–5] such as high performance batteries, efficient energy conversion by fuel cells, sensors, super capacitors, electro-chromic windows and analog memory devices. SPE have several advantageous over liquid electrolytes, such as good mechanical properties, ease of fabrication as thin films in desirable size and ability to form good electrode–electrolyte contact. PEO-based polymer electrolytes using alkali salts, plasticizers and inorganic fillers have been extensively studied [6–15]. Polymers such as poly (acrylonitrile) (PAN), poly (methyl methacrylate) (PMMA), poly (vinyl chloride) (PVC) and poly (vinylidene fluoride) (PVDF) have been examined as gel-type polymer electrolytes in energy-storage devices [16–23]. Presently, the majority of lithium batteries use some form of gelated PVDF film as a separator between cathode and liquid electrolyte. Modifications of PVDF-based polymer electrolytes were developed by blending with PMMA, PVC and PVAAc [24–28]. The physical properties of polymer electrolytes are largely affected by the molecular arrangement and chemical dynamics of the polymer chains. An understanding of the interplay between molecular structure and the ion transport mechanism is critical to the growth of new polymer electrolyte materials.
In spite of the industrial importance and wide application of gelated PVDF, the ion conduction mechanism remains unresolved. PVDF, which has a strong electron withdrawing function and a unique arrangement, delivers a high dielectric constant (ε = 8.4). This is effective in dissociating lithium salts to generate a large quantity of charge carriers for conduction. It is highly possible that, apart from the diffusion established by a plasticizer such as ethylene carbonate (EC), propylene carbonate (PC), conduction through the swollen PVDF matrix is also highly credible. The objective of this research is to study the morphology, structure, miscibility, ionic conductivity and electrochemical performance of a PVDF electrolyte membrane with lithium nanopowders incorporated during film formation.
2 Experimental
LiFePO4 nanopowders were prepared using a rheological phase reaction method followed by a self-assembling process. LiOH.H2O, FeC2O4.2H2O and NH4H2PO4 with molar ratio 1.5:1:1 were dissolved in water and stirred for 4 h; the rheological phase slurry was introduced to the Teflon-lined Parr reactor and kept at 180°C for 24 h. The final solution was cooled naturally and filtered three times. The powder was dried at 80°C for 8 h.
Polymer electrolyte films of LiFePO4 complexed PVDF were prepared using a solution-cast technique. N-methyl 1-2 pyrrolidone (NMP) was used as a solvent. The compositions of the polymer complexes were the following: (100-x) PVDF + xLiFePO4, where x (wt%) = 0,5,10 and 15. The mixtures of salt solutions were stirred for 24 h at 60°C for homogenous mixing and cast in polypropylene dishes. The dish solutions were kept at 50°C for a week in an oven. Thick films of 100–200 μm were collected from the dishes and these were vacuum dried thoroughly at 10−3 mbar to remove solvent traces. The XRD patterns of the films were determined using a HZG4/B-PC X-ray diffractrometer with CoKα radiation and a graphite monochrometer. The IR spectra were determined using a 60-SXB FTIR spectrophotometer over the range 400–4000 cm−1. For the DSC measurements, a Netzsch STA 409 PC, operating in dynamic mode (heating rate = 10 Kmin−1), was employed. Samples of ≈ 5 mg weight were placed in sealed aluminium pans. The morphology of the samples was characterized using a JSM-5610LV scanning electron microscope (SEM).
A home made conductivity cell was used to measure the ionic conductivity of the polymer electrolytes as a function of temperature. The ac impedance measurements of the polymer electrolytes were performed using an Agilent 4294A precision impedance analyzer in the range 40–300 kHz and temperature range 298–368 K. The system was thermally equilibrated at each selected temperature for 15 min. The bulk resistance (Rp) determined from the equivalent circuit analysis by using frequency response analyzer (FRA) software. The conductivity values (σ) were calculated from the equation σ = (1/Rp)(t/A), where t is the thickness and A is the sample area.
3 Results and discussion
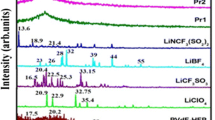
XRD patterns of the pure PVDF and (PVDF + LiFePO4) films are shown in Fig. 1. Sharp diffraction peaks appear at 2θ∼18–21° and demonstrate the crystalline forms of PVDF. A small number of differences are observed between the pure PVDF and LiFePO4 nanoparticle PVDF complexed films. The clear and sharp crystalline diffraction peaks in pure PVDF, however, become less prominent in the presence of the nanoparticles. The decrease in intensity of the diffraction peaks with dopant concentration suggests a decrease in both crystalline size and the degree of PVDF crystallinity. Even though PVDF is not an ionomer, the highly depolarized CF bond is capable of forming a weak acid-based complexation with lithium and thus retards the order of crystalline PVDF.
Figure 1e shows the XRD patterns of synthesized LiFePO4 nanoparticles. All peaks can be indexed as pure and well-crystallized LiFePO4 phase with an ordered olivine structure and a space group of Pnma. The calculated lattice parameters from the XRD data are a = 1.0342 nm, b = 0.6005 nm, and c = 0.4693 nm, which are comparable to the standard data JCPDS 83-2092. The XRD result demonstrates that the rheological self-assembling route can be used to synthesize single-phased LiFePO4 product with no unwanted impurity phases, such as Li3PO −4 and Fe3+-related compounds [29]. Conventional solid state methods, although carried out under carefully controlled conditions, have shown the unwanted presence of the impurity phases such as Fe2O3 and Li3Fe2 (PO4)3 [30]. Another advantage is that the sample can be prepared by this process without sintering. This makes it simple to prepare LiFePO4 olivine materials. In comparison with the previous solid state processes, the rheological self-assembling process reduces the synthesis temperature of LiFePO4. The soft solution environment may remarkably accelerate the reaction kinetics of the formation of LiFePO4.
IR spectra of pure PVDF and (PVDF + LiFePO4) electrolytes of different compositions are shown in Fig. 2a–d. The vibrational peaks of complexed polymer electrolytes are shifted towards higher wave numbers with increasing LiFePO4 concentration. Fig. 2e shows IR spectra of LiFePO4 nanoparticles which show the peaks at wavenumbers 3349, 1628, 1371, 1070, 945, 635, 547 and 498 cm−1. The characteristic absorption band of LiFePO4 at 1371 cm−1 is associated with the vibration of the P–O–Fe bond in LiFePO4, whereas the bands situated at 1070 and 945 cm−1 correspond to the characteristic absorption of the [PO4]3−. The LiFePO4 absorption band between 635 and 547 cm−1 is associated with the vibration of P–O bond in LiFePO4. Particularly, the characteristic Li-O absorption band bond at 498 cm−1 is weak, which indicates the weak interaction between Li and O atoms, which enables Li + ions to insert/extract easily in the ordered olivine LiFePO4 structure [31]. The absence of absorption peaks of pure LiFePO4 nanoparticles in the complexed systems and peak shifts with LiFePO4 concentration indicate the miscibility of the dopant in the prepared electrolyte systems. In addition to the PVDF and LiFePO4 vibrational peaks, some other peaks were observed at the frequencies 1699 and 1450 cm−1 and were assigned to CH3 asymmetric stretching and bending vibrations of PVDF [22]. The appearance of new peaks along with changes in existing peaks in IR spectra indicate directly the complexation of PVDF with LiFePO4.
Differential scanning calorimetry also demonstrated a loss in the long-range order of PVDF in the presence of LiFePO4. DSC curves of pure PVDF and (PVDF + LiFePO4) electrolytes are shown in Fig. 3. Except for pure PVDF, a re-crystallized peak is apparent for all compositions of LiFePO4 complexed PVDF electrolytes during the endothermic reaction. This peak shifts towards lower temperature with increasing dopant content. This behaviour suggests that the additional PVDF crystallization occurs in freshly-prepared (PVDF + LiFePO4) electrolytes upon heating at elevated temperature, possibly by driving the salt out of the amorphous PVDF domain. The heat of fusion (ΔHf) and the melting temperature (Tm) of polymer PVDF decreases with increasing dopant content. The relative crystallinity (χ) has been determined, by assuming that pure PVDF is 100%, using a formula χ = ΔHf / ΔH 0 f (where ΔH 0f is the fusion heat of pure PVDF and ΔHf is related to the dopant in the polymer). The crystallinity (χ), melting temperature (Tm) and crystallization temperature (Tc) are listed in Table 1. Polymer electrolytes show lower χ in the presence of lithium ionic nanoparticles.
The TG plots of the (PVDF + LiFePO4) polymer electrolyte system are shown in Fig. 4. The thermal decomposition temperature was determined from the differential curve, and is about 140°C for all (PVDF + LiFePO4) electrolytes. The absence of weight loss prior to polymer melting indicates no impurity, such as solvent or water, is present. Absence of impurity confirms that the vacuum drying is efficient in the present case. The degradation temperature decreases continuously with the increased dopant concentration in the polymer and is directly related to an increase in the amorphous fraction of the electrolyte. The thermal stability of the polymer is reduced with the addition of LiFePO4 due to the growth of the amorphous fraction, which is a characteristic of polymers. A higher fraction of residue is found, at above 500°C, with increased dopant concentration.
SEM photographs of PVDF and (PVDF + LiFePO4) films are shown in Fig. 5a–c. The surface structure of pure PVDF shows connecting big spheres (see Fig. 5a). As PVDF is polymerized with a high dielectric constant, the surface tension is also high. Sol formation is necessary in the process of drying solvent from the miscible solution. The size of the spheres and the morphology in the PVDF film are thus highly dependent on the preparation conditions such as the solvent and the temperature. Addition of LiFePO4 to PVDF, the acid-base interaction, not only changes the crystallinity but also reduces the surface potential and the equilibrium sol dimension becomes smaller. As seen in Fig. 5b, the spheres are converted into a cauliflower shape with improved smoothness. In Fig. 5c, all the spheres are merged together and develop a uniform surface, due to the increasingly amorphous nature of the polymer.
A series of impedance measurements at different temperatures were obtained from 25–95°C. The impedance plots for pure PVDF electrolyte at different temperatures are presented in Fig. 6. Most curves are semicircular in shape with an oblique line. For each curve, the value of Rp was obtained from the intercept point at the high frequency end. The conductivity of the polymer electrolyte was calculated from the measured resistance (Rp), the area and the thickness of the polymer film.
The temperature dependence of conductivity is presented in Fig. 7. Conductivity data at room temperature are noted in Table 1. Interestingly, the conductivity of the complexed PVDF electrolytes increases continuously with LiFePO4 content for all temperatures. This behaviour is different from that of PEO solid polymer electrolyte systems, where a maximum conductivity is identified at a particular compositional ratio [32–36]. In this case, incomplete dissociation and the formation of triplets at higher salt content are responsible for the reduced conductivity. The ion- paring mechanism appears to be absent in the PVDF polymer electrolytes studied, which may be due to the high dielectric constant of the medium.
The plot of logσ versus 1000/T follows an Arrhenius-type thermally-activated process. The conductivity relationship can be expressed as σ = σ 0 exp(-E a /kT), where σ 0 is the pre-exponential factor, E a is the activation energy and k is the Boltzmann constant. Activation energies of (PVDF + LiFePO4) solid polymer electrolytes were evaluated by a linear fitting method; and the results are listed in Table 1. The activation energy decreases with increasing Li ion concentration in the PVDF polymer.
Druger et al. [37, 38] have attributed the change in conductivity with temperature in solid polymer complexed systems to segmental (i.e. polymer chain) motion, which results in an increase in the free volume of the system. Thus, the segmental motion either permits the ions to hop from one site to another or provides a pathway for ions to move. In other words, the segmental movement of the polymer facilitates the transitional ionic motion. From this, it is clear that the ionic motion is due to ionic transitional motion/hopping facilitated by the dynamic segmental motion of the polymer. As the amorphous region increases, however, the polymer chain acquires faster internal modes in which bond rotations produce segmental motion to favor inter- and intra-chain ion hopping and, thus, the degree of conductivity becomes high.
4 Conclusions
The interaction between Li+ ions and fluorine in the polymer effectively disrupts the crystallinity of PVDF. The ionic conductivity increases continuously with increased salt concentration. The increased conductivity is due to a decrease in the degree of crystallinity and increase in the amorphous nature of the polymer. The activation energies decrease with increasing dopant concentration.
References
Armand MB (1986) Ann Rev Mater Sci 16:245
Ratner MA, Shriver DF (1988) Chem Rev 88:109
Mac Callum JR, Vincent CA (eds.), (1987) Polymer Electrolyte Reviews, Elsevier, Amsterdam
Owen JR, Laskar AL, Chandra S (1989) Superionic Solids and Solid State Electrolytes-Recent Trends, Academic Press, New York
Murata K (1995) Electrochim Acta 40:2177
Reitman EA, Kaplan ML, Kava RJ (1985) Solid State Ionics 17:67
Sorensen PR, Jacobson T (1982) Electrochim Acta 27:1675
Fautex D, Robitaille C (1986) J Electrochem Soc 133:307
Oleksiak AL, Inerowicz HD (1999) J Power Sources 81–82:813
Mclin M, Angell CA (1992) Solid State Ionics 56:1027
Lee YL, Crist B (1986) J Appl Phys 60:2683
Hashmi SA, Chandra A, Chandra S (1992) In: BVR Chowdari, et al. (eds.), Solid State Ionics: Materials and Applications, World Scientific, Singapore :p 567
Sreepathi Rao S, Jaipal Reddy M, Laxmi Narsaiah E, Subba Rao UV (1995) Mater Sci Eng B 33:173
Sreekanth T, Jaipal Reddy M, Subramanyam S, Subba Rao UV (1999) Mater Sci Eng B 64:107
Sreekanth T, Jaipal Reddy M, Ramalingaiah S, Subba Rao UV (1999) J Power Sources 79:105
Peramunage D, Pasquariello DM, Abraham KM (1995) J Electrochem Soc 42:1789
Hong H, Liquan C, Xujie H, Rongjan X (1992) Electrochim Acta 37:1671
Bohke O, Frand C, Razrazi M, Rousselt C, Truche C (1993) Solid State Ionics 66:97
Boudin F, Andrieu X, Jehoulet C, Olsen II (1999) J Power Sources 81–82:804
Saito Y, Kataoka H, Quartarone E, Mustarelli P (2002) J Phys Chem B 106:7200
Saito Y, Kataoka H, Stephan AM (2001) Macromolecules 34:6995
Rajendran S, Kannan R Mahendran O (2001) Mater Lett 49:172
Subba Reddy ChV, Zhu Quan-Yao, Mai Li-Qiang, Chen Wen J. Solid State.Elect.Chem : In press
Moussaif N, Jerome R (1999) Polymer 40:3919
Jin Z, Pramoda KP, Goh SH, Xu G (2002) Mater Res Bull 37:271
Bauduin G, Boutevin B, Gramain P, Malinova A (1999) Eur Polym J.35:285
Rajendran S, Mahendran O Kannan R (2002) Fuel 81:1077
Stephan AM, Kumar TP, Renganathan NG, Pitchumani S, Thirunakaran R, Muniyandi N (2000) J Power Sources 89:80
Yamada, Chung SC, Hinokuma K (2001) J Electrochem Soc 148:A224
Padhi. K, Nanjundaswamy KS, Goodenough JB (1997) J Electrochem Soc 144:1189
Chen W, Mai LQ, Xu Q, Zhu QY, Yang HP (2003) Mater Sci Eng B 100:221
Chu PP, Jen HP, Lo FR, Lang CL (2000) Macromolecules 32:4738
Zahurak SM, Kaplan ML, Rietman EA, Murphy DW, Cava RJ (1988) Macromolecules 21:654
Ratner MA, Nitzman A (1989) Faraday Discuss Chem Soc 88:19
Dupon R, Papke BL, Ratner MA, Whitmore DH, Shriver DF (1982) J Am Chem Soc 104:6247
Reddy MJ, Chu PP (2002) J Power Sources 109:340
Druger SD, Nitzam A, Ratner MA (1983) J Chem Phys 79:3133
Druger SD, Nitzam A, Ratner MA (1985) Phy Rev B 31:3939
Acknowledgements
One of the authors (Ch.V.S. Reddy) thanks Wuhan University of Technology for the award his Post Doctoral Fellowship and he also thanks Ajou University for the award of his Post Doctoral Fellowship from the BK 21 project. The authors thank Dr. Rajamohan R Kalluru, Mississippi Ethanol LLC, USA for fruitful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Subba Reddy, C.V., Chen, M., Jin, W. et al. Characterization of (PVDF + LiFePO4) solid polymer electrolyte. J Appl Electrochem 37, 637–642 (2007). https://doi.org/10.1007/s10800-007-9294-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-007-9294-4