Abstract
Sodium chlorate is produced in undivided electrolysis cells. Hydrogen is evolved on the cathodes, usually made of steel, while chloride ions are oxidised to chlorine on the anodes, usually DSA®s. Parasitic cathodic reactions, lowering the cathodic current efficiency (CE), are the reduction of hypochlorite and chlorate ions. These reactions are suppressed by the addition of Cr(VI) to the electrolyte. In this work the effects that time of the electrolysis, chromate concentration and interruption of the electrolysis process have on CE has been investigated. New steel, as well as steel samples cut from cathodes used in a chlorate plant, were used as cathode material. Laboratory experiments in a divided cell were made to determine the rate of hydrogen production, and thereby indirectly CE, at varying operating conditions. It was found that the chromate concentration is important for the CE in the range 0.5–6 g l−1 Na2Cr2O7. The CE was higher on new steel than on the used steel, which had a more corroded and inhomogeneous surface. When starting the electrolysis the CE was initially low, at a value depending on the operating conditions, but increased with time of polarisation. The time to reach an approximate steady CE was generally in the order of hours. Electrolysis shut downs in the presence of hypochlorite (≤ 3 g l−1 NaClO) resulted in corrosion of iron and a low CE when restarting the process. After one such corrosion shut down the new steel showed as low CE as the used steel. When restarting the electrolysis after a shut down without hypochlorite the CE was higher than before the shut down. Current densities of a simulated bipolar plate during a shut down were measured to 50–150 A m−2, resulting from oxidation of steel and reduction of oxy chlorides on the catalytic DSA® electrode.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Sodium chlorate, NaClO3, is a bulk chemical mainly used for production of chlorine dioxide, ClO2, a bleaching agent in the pulp and paper industry. During the last decade chlorine dioxide has replaced chlorine for environmental reasons, which has increased the demand for sodium chlorate. Since the price of electrical energy is constantly increasing it has become even more important to find ways to improve the current efficiency in order to minimise costs. Furthermore, the variation of electricity tariffs during the hours of the day forces the producers to impose plants to current load variations, and even shut downs, when prices are too high. This means that shut downs and start-ups are becoming more frequent, which may have a negative impact on the electrodes.
Sodium chlorate is commercially produced in a process where the main electrode reactions are hydrogen and chlorine formation (reactions 1 and 2) similar to those in the chlor-alkali process. As chlorate cells are undivided, chlorine is hydrolysed and disproportionates to form hypochlorite and hypochlorous acid (reactions 3 and 4). Chlorate is formed in a chemical reaction (reaction 5). The rate of reaction 5 is at an optimum at a pH of 6–7 and the electrolyte is therefore set in this pH range.
Chromate is added to the electrolyte primarily to increase the current efficiency (CE). It also acts as a pH buffer, affects the production of the by-product oxygen, [1] and [2], and alters the potential of the DSA® anode, [3]. In addition chromate may have a retarding effect on the corrosion of steel cathodes, [1]. During cathodic polarisation Cr(VI) is reduced to Cr(III) and forms a thin film of chromium(III) hydroxide on the cathode, [4]. This film hinders the unwanted side reactions 6 and 7, while hydrogen evolution can still take place, though with changed kinetics [5]. The thickness and growth rate of the film depends on factors such as the cathode material, the electrode potential and the chromate concentration, [6]. As low chromate concentrations as 0.1 g l−1 Cr2O 2−7 has been shown to efficiently hinder reduction of Fe(CN) 3−6 on smooth platinum [2], however the concentration in chlorate electrolyte is generally 3–8 g l−1 Na2Cr2O7, [7]. A parasitic reaction that can have some importance during start-up of the electrolysis process is reduction of iron oxides on steel cathodes.
DSA®s or platinum–iridium coated titanium are currently used as anodes, whereas steel or titanium are the preferred cathode materials. Steel corrodes in the aggressive chlorate electrolyte when not under cathodic protection. The corrosion not only reduces the lifetime of the cathodes but also contaminates the electrolyte with metal particles that may cause short circuits in the narrow electrode gaps. A positive effect of the corrosion is that the cathode surface is renewed with removal of cathodic deposits. However, the beneficial chromate film may also dissolve or react chemically during a shut down.
In the absence of chromate the rate of electrochemical chlorate reduction depends strongly on the electrocatalytic properties of the electrode material, whereas electrochemical hypochlorite reduction is mass transport controlled. Chlorate is present at a high electrolyte concentration, typically 500–650 g l−1, whereas the hypochlorite concentration is about 1–6 g l−1 HClO and ClO−, calculated as NaClO3 and NaClO respectively. Thermally prepared iron oxides have been found to catalyse chlorate reduction, whereas smooth Co, Ni, Mo, Ti, Hg and C were inactive for chlorate reduction, [7]. Relatively smooth iron cathodes, mildly corroded in chlorate electrolyte, have shown a high catalytic activity for chlorate reduction in the absence of chromate. The current efficiency for hydrogen evolution increased with time of polarisation, [5]. Current efficiency measurements on RuO2 in chlorate electrolyte with no chromate at 3 kA m−2 and 70 °C showed a very high activity for chlorate reduction – the CE for hydrogen evolution was less than 5%. Lowering the temperature of the cell favoured the selectivity for hydrogen evolution and increased CE, [8].
Most studies of the chromium hydroxide film in the chlorate process have been made on inert, smooth electrodes such as polished platinum and gold. In this work we approached practical chlorate process conditions by studying the current efficiency on steel cathodes, some of which had a corroded and inhomogeneous surface from operation in a chlorate plant. The primary questions addressed in this work are:
-
How high a chromate concentration is required to reach a high current efficiency on corroded steel cathodes?
-
How long does a production shut down affect current efficiency and how long does it take to reach the same level as before the shut down?
-
How do the conditions during the shut down affect the CE when restarting the electrolysis?
2 Experimental
Measurements of current efficiencies were made using a divided cell. The technique to measure CE, outlined below, required a divided cell to separate formed chlorine gas, oxy chlorides and oxygen from the cathode compartment. Measurements of current densities and electrode potentials of a simulated bipolar electrode were made in an undivided cell that better represented the industrial case.
2.1 Current efficiency measurements
A divided cell was used, where the rate of hydrogen gas evolving from the cathode compartment was measured. The two compartments, separated by a diaphragm from Gore Scandinavia AB, each had a volume of 300 ml. Gas evolved from the cathode compartment was passed through a washing bottle with water at room temperature and then fed to a gas burette. The electrolyte contained 550 g l−1 NaClO3 (Eka Chemicals) and 110 g l−1 NaCl (p.a. grade) at 70 °C. Additions of chromate were made from a concentrated stock solution made from Na2Cr2O7•2H2O (p.a. grade) to reach concentrations between 0.5–6 g l−1 Na2Cr2O7 in the electrolyte. In some experiments NaClO was added to the electrode compartments from a concentrated solution from BDH Laboratory Supplies, analysed by arsenite titration. Additions of 25 ml NaClO-solution gave an initial electrolyte concentration of about 3 g l−1, decreasing with time as chlorate formed according to reaction 5. The electrolyte pH was adjusted using NaOH and HCl to 6.5 ± 0.05 prior to polarisation, but was not controlled during electrolysis. Thus the pH of the catholyte bulk increased during electrolysis, but it is assumed that the pH at the cathode surface remained approximately constant and strongly alkaline. Temperature was controlled at 70 °C by placing the cell in a thermostatic water bath.
The time needed to collect 25 ml gas was measured and converted to a CE from the relation CE (%) =\({I_{\rm H_{2}}}\) /I tot*100, where \({I_{\rm H_{2}}}\) was calculated from the actual hydrogen production rate measured and I tot was the total current applied. A current density of 3 kA m−2 was used in all experiments. The gas measurements were calibrated using a 5 m NaCl electrolyte and a platinum cathode, where 100% CE for hydrogen evolution was assumed.
New and used steel electrodes were investigated, all of a low carbon steel grade, with an iron content over 99%. The used electrodes had been operated in a chlorate plant and washed with water only, thus having traces of chromium, calcium etc. deposits on the corroded surface. Only one side (6 cm2) of the samples was in contact with electrolyte, the other was covered by epoxy resin. A new electrode sample was used for each experimental curve. As counter electrode a DSA® from Permascand AB was used, immersed in the anodic compartment.
The series of trials with shut downs at different chromate concentrations were made as follows: after one hour of electrolysis the current was switched off, pH in the catholyte adjusted to 6.5 and the cathode allowed to corrode for 30 min. Then 40 min of electrolysis followed, and after switching off the current 25 ml hypochlorite solution was added to the catholyte compartment, pH adjusted to 6.5 and the cathode allowed to corrode for 30 min. After elapsed time another period of 40 min of polarisation followed, whereafter the catholyte pH was adjusted and the cathode was allowed to corrode for 60 min. The trials ended with a final period of 40 min of polarisation. During all periods of electrolysis the CE was measured. The electrical connections during the shut down were made in two different ways: either the electrodes (DSA® and steel) were not connected during all shut downs, or they were electrically connected (short circuited) during all shut downs. When the electrodes were short circuited a current was allowed to run from the former cathode to the former anode, simulating a bipolar electrode (though in this case in different cell compartments).
2.2 Measurements of potentials and currents on a simulated bipolar electrode during a shut down
A jacketed one-compartment cell with an electrolyte volume of 0.5 l was used, connected to a water bath for temperature control. The electrolyte and the electrodes were similar to those described above, though in these experiments the exposed electrode areas were 3 cm2. The electrodes were immersed in the electrolyte with a distance of 1 cm between them. A Luggin capillary, centred between the electrodes, was connected to a Ag/AgCl reference electrode (K201 from Radiometer) at room temperature. This arrangement gave an iR drop of less than 20 mV at measured current densities during shut downs. A PAR 273A potentiostat in combination with a Nicolet Integra 20 oscilloscope was used to control and monitor the currents and potentials. After 30 min electrolysis at a current density of 3 kA m−2 the cell was switched off. Hypochlorite solution, 25 ml, was added to the electrolyte and pH was adjusted to 6.5. To polarise the electrodes prior to the measurements another 5 min of electrolysis followed. An electrolyte sample was taken and the hypochlorite concentration analysed with arsenite titration to give 1 g l−1 NaClO. The potential between anode and cathode was then set to 0 V, simulating a bipolar electrode and the electrode potentials and the current in the cell were monitored on the oscilloscope. In this single compartment cell low concentrations of hypochlorite built up during electrolysis and were thus also present during the shut downs with no hypochlorite addition. During the shut downs the hypochlorite concentration gradually decreased.
3 Results and discussion
3.1 Current efficiency measurements
The current efficiency for a new steel cathode at varying chromate concentrations is shown in Figure 1. It is clear that the efficiency increased with time of polarisation as the chromium(III) hydroxide film built up and the iron oxides, active for chlorate reduction, were reduced. Apart from chlorate reduction, the current loss may be due to reduction of iron oxides. Hypochlorite was not present in the cathodic compartment in these experiments. An efficiency of 98–100% was reached for the highest chromate concentration, 5 g l−1 Na2Cr2O7, after 20 min of polarisation. For 1 and 3 g l−1 Na2Cr2O7 the current efficiency reached 98% after about 1 h, whereas lower efficiencies were found for 0.5 g l−1 Na2Cr2O7.
Data for a used cathode, Figure 2, show lower CE than the new cathode. After 2 h of polarisation the CE was about 95% for 3–6 g l−1 Na2Cr2O7. It is also likely that the CE values in this work (see for example Figure 2) would continue to slowly increase with continued polarisation. Chromate was most important in the lower concentration range in Figure 2 but increasing from 3 to 5 and 6 g l−1 had a smaller effect.
Current efficiency for hydrogen evolution on used steel cathodes in chlorate electrolyte with different chromate concentrations at the current density 3 kA m−2. No hypochlorite added. Temperature 70 °C, initial pH 6.5. ○ 0.5 g l−1 Na2Cr2O7, Δ 1 g l−1 Na2Cr2O7, × 3 g l−1 Na2Cr2O7, - 5 g l−1 Na2Cr2O7, • 6 g l−1 Na2Cr2O7.
Lower CE values for used electrodes than for new may be explained by the longer time needed to form a protective chromium hydroxide film on the more complex surface of the corroded used electrodes. The larger amount of iron oxides, active for chlorate reduction, will also affect CE but will decrease with time of polarisation.
Figure 3 shows results from two trials with shut downs for new electrodes when the chromate concentration was 3 g l−1, with unconnected and short circuited electrodes. It is seen that a 30 min shut down, with unconnected electrodes and no hypochlorite added, did not adversely affect CE as about 100% CE was noted both prior to and after shut down 1. Shut down 2, where hypochlorite was added, as well as number 3 gave a low CE when restarting the electrolysis, and 40 min of polarisation was not sufficient to regain 100% efficiency. The drop in CE after a short circuited shut down was significantly larger than when the electrodes were not connected. When the electrodes were short circuited, which can be compared to a bipolar cell or when a current is allowed to go through the turned off rectifier, the corrosion attack on the cathodes was much more severe and could be seen by eye. A comparison with results from similar experiments for used electrodes, Figures 4 and 5, indicate that new electrodes behave like used electrodes. They become “used” after a shut down in the presence of hypochlorite, or after a shut down with short circuited electrodes.
Current efficiency for hydrogen evolution on new steel cathodes in chlorate electrolyte with a chromate concentration of 3 g l−1 at the current density 3 kA m−2. Shut down 1: 30 min. Shut down 2: 30 min with 25 ml of NaClO added. Shut down 3: 60 min. Temperature 70 °C, pH when each shut down started was 6.5.
Current efficiency for hydrogen evolution on used steel cathodes in chlorate electrolyte with different chromate concentrations at the current density 3 kA m−2. Shut down 1: 30 min. Shut down 2: 30 min with 25 ml of NaClO added. Shut down 3: 60 min. Temperature 70 °C, pH when each shut down started was 6.5. Unconnected electrodes during shut down. ○ 0.5 g l−1 Na2Cr2O7, × 3 g l−1 Na2Cr2O7, • 6 g l−1 Na2Cr2O7.
Figures 4 and 5 show trials with used electrodes, where the chromate concentration was varied. The electrodes represented in Figure 4 were not connected during the shut downs. It is clearly seen that after the first shut down, where there was no hypochlorite present in the catholyte, the CE increased for all chromate concentrations. This can be explained by a growth of the chromium hydroxide film in a corrosion reaction during the shut down, where iron is oxidised while Cr(VI) is reduced to Cr(III), forming a protective film. Similar reactions, and also formation of mixed iron–chromium oxides have been discussed regarding iron passivation in alkaline solution, [9].
Current efficiency for hydrogen evolution on used steel cathodes in chlorate electrolyte with different chromate concentrations at the current density 3 kA m−2. Shut down 1: 30 min. Shut down 2: 30 min with 25 ml of NaClO added. Shut down 3: 60 min. Temperature 70 °C, pH when each shut down started was 6.5. Short circuited electrodes during shut down. ○ 0.5 g l−1 Na2Cr2O7, × 3 g l−1 Na2Cr2O7, • 6 g l−1 Na2Cr2O7.
Chromate had a clear positive effect on the CE in Figure 4. At the highest chromate concentration, 6 g l−1 Na2Cr2O7, the CE also remained at a high level of 95–98% after shut downs 2 and 3. For 0.5 g l−1 Na2Cr2O7 and 3 g l−1 Na2Cr2O7 the approximate steady state values were about 10% lower and initial values after shut downs 2 and 3 were even lower, particularly for 0.5 g l−1.
Results in Figure 5, where the electrodes were short circuited during the shut downs, show no CE increase after shut down 1. Instead the initial CE decreased after all shut downs and the decrease was smaller the higher the chromate concentration. The CE values reached for 6 g l−1 Na2Cr2O7 are lower than those in Figure 4, likely due to the more severe corrosion for short circuited electrodes needing a longer time of cathodic polarisation to reduce the iron oxides and to form a protective chromium hydroxide film. From the trend of the CE values after the shut downs, it seems likely that the CE would continue to increase with increased time of polarisation – no steady state level was reached after 40 min electrolysis.
3.2 Measurements of potentials and currents on a simulated bipolar electrode during a shut down
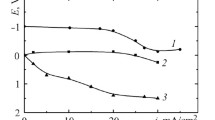
Figure 6 shows the current during a shut down with short circuited electrodes, where the steel electrode acted as anode and cathodic reactions dominated on the DSA®. The curve trends are similar – directly after the shut down the currents were high, but decreased quickly to lower values after which they slowly declined in an approximately linear manner. Both hypochlorite and chromate concentrations affected the current during the shut down – a low chromate concentration and a high hypochlorite concentration both resulted in a high corrosion current (trials with low hypochlorite concentration are not shown in Figure 6).
As explained above, in connection with the trials in the divided cell, the corrosion rate was much more severe during a shut down with short circuited electrodes than when the electrodes were not connected. A reverse current during the shut down with short circuited electrodes can explain this, where hypochlorite and chlorate are reduced on the DSA® and iron oxidised on the steel electrode. The electrode potentials during the shut downs were about −0.1 to −0.15 V vs. Ag/AgCl, after an initial lower potential during the first minute of the shut down (the iR drop was calculated to less than 20 mV and has not been corrected for). This is within a potential region where a chromium(III) hydroxide film may start to form on the DSA®, hindering hypochlorite and chlorate reduction. Thus the effect of chromate concentration on the currents in Figure 6 may be due to phenomena on the DSA® – at higher chromate concentration a protective film is more easily formed, hindering the cathodic reactions and thereby hindering the counter reaction – oxidation of steel. Chromate may also affect the steel corrosion rate; a high chromate concentration slowing the oxidation process down.
4 Conclusions and practical implications
-
The chromate concentration had an effect on the current efficiency in the range studied (0.5– 6 g l−1 Na2Cr2O7). The higher concentration the greater values of CE, though the effect was largest when increasing from 0.5 to 3 g l−1 Na2Cr2O7.
-
The time to reach an approximately steady CE on a corroded steel surface was generally in the order of hours.
-
New electrodes showed higher current efficiencies than used electrodes. During a shut down in the presence of hypochlorite or with short circuited electrodes, the new electrode corroded and showed current efficiencies like a used electrode when the electrolysis was restarted.
-
A shut down in a hypochlorite-free electrolyte with unconnected electrodes improved the CE. The chromium(III) hydroxide film probably grew during the shut down as Cr(VI) was reduced to Cr(III) while iron was oxidised.
-
A shut down with short circuited electrodes lowered the CE and the drop, as well as the galvanic current, was larger at low chromate concentration.
-
After a shut down with hypochlorite in the catholyte the CE was lower than before the shut down for chromate concentrations lower than 6 g l−1. The drop in CE was more pronounced at lower chromate concentrations. Hypochlorite oxidised the protective film and the steel electrode and the increased amount of iron oxides on the surface enhanced chlorate reduction.
-
The net reverse current on a simulated bipolar electrode during an electrolysis shut down was 50–150 A m−2, higher at low chromate concentration and high hypochlorite concentration.
A practical implication of this work is that in order to reach a high current efficiency, steel corrosion should be minimised during the production shut downs. Therefore the hypochlorite concentrations during the shut downs should be as low as possible. Monopolar technology allows cathodic protection to be used during the shut downs by passing a current of about 15–75 A m−2, [10], thereby suppressing steel corrosion. The chromate concentration should be relatively high (probably at least 6 g l−1 Na2Cr2O7) for a high current efficiency, though in specifying the optimum chromate concentration for a specific plant other effects of chromate concentration must be taken into account.
Extended measurements of current densities and electrode potentials during shut downs at different conditions, combined with analyses of corrosion products, would give valuable information to take this work further.
References
Viswanathan K., Tilak B.V. (1984) J. Electrochem. Soc. 131:1551
Tilak B.V., Viswanathan K., Rader C. (1981) J. Electrochem Soc. 128:1228
A. Cornell, B. Håkansson and G Lindbergh, J. Electrochem. Soc. 150 (2003) D6.
Lindbergh G., Simonsson D. (1990) J. Electrochem. Soc. 137:3094
Cornell A., Lindbergh G., Simonsson D. (1992) Electrochim. Acta 37:1873
Ahlberg Tidblad A., Mårtensson J. (1997) Electrochim. Acta 42:389
Tilak B.V., Tari K., Hoover C.L. (1988) J. Electrochem. Soc. 135:1386
Cornell A., Simonsson D. (1993) J. Electrochem. Soc. 140:3123
Calvo E.J., Palotta C.D., Hild S., Garcia E. (1988) . J. Electrochem. Soc. 135:314
J.E. Colman and B.V. Tilak, in J.J. McKetta and M. Dekker (Eds), ‘Encyclopedia of Chemical Process and Design’, (Inc., N.Y., 1995) pp. 126.
Acknowledgements
Eka Chemicals AB and Permascand AB are gratefully acknowledged for valuable discussions and for financing this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wulff, J., Cornell, A. Cathodic current efficiency in the chlorate process. J Appl Electrochem 37, 181–186 (2007). https://doi.org/10.1007/s10800-006-9263-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-006-9263-3