We study the corrosion behavior of electrode materials in the alkaline-water electrolysis within a broad range of electrolyte concentrations and for electrodes of different nature. It is discovered that the use of steel materials with low contents of chromium and vanadium impurities (0.1–0.3%) increases the corrosion resistance of the electrodes and weakens the dissolution of iron of steel anodes. The operation of anodes made of aluminum- and zinc-based alloys is accompanied by their dissolution. This fact makes it possible (due to the depolarization of the anodic process) to exclude the oxygen release by the mechanism of hydrogen depolarization, substantially simplify the technology of electrochemical synthesis of hydrogen, and to reduce the losses of materials and energy for the electrolysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In the contemporary hydrogen power-generating industry, an important role is played by the electrode materials used in the alkaline-water electrolysis. In industrial electrolyzers, carbon steels play the role of cathodes, whereas nickel-plated carbon steels serve as anodes. Under the conditions of electrolysis on anodes, parallel with the oxygen release in alkaline solutions, we observe a partial dissolution of iron accompanied by the formation of ferrite ions according to the reaction
In the process of electrolysis, \( {\mathrm{HFeO}}_2^{-} \) ions diffuse through diaphragms into cathodic chambers, are reduced on the cathodes in the form of dendritic oxides of iron, and affect the surface state of the cathodes, which changes the electrochemical parameters of hydrogen release (working current density and the overvoltage of hydrogen release). According to the Pourbaix diagram [1, 2], the equilibrium potential of the transition of iron into ferrite ions is given by the formula
For pH 14, within the range of concentrations of \( {\mathrm{HFeO}}_2^{-}\kern1em {10}^{-4}-{10}^{-5} \) mole/dm3, the equilibrium potential becomes equal to
As a result of the anodic polarization, the actual anode and cathode potentials become as high as (1–1.5) V and (– 0.7–(– 1)) V, respectively.
In addition, the corrosion fracture of steel electrodes playing the roles of anodes and cathodes occurs in the process of operation of electrolyzers as a result of contact with the materials of leads and structural components made of various metals, such as copper, tin, nickel, and zinc. Therefore, the crucial aspect in the investigation of the electrolysis of water is connected with the analysis of the electrochemical behavior of the anode and cathode materials in which the processes of formation of ferrites and corrosion processes are absent. In this case, copper, tin, and zinc can be introduced in the anodic and cathodic chambers. First of all, the indicated group of materials contains iron alloys doped with chromium, nickel, vanadium, and molybdenum and, e.g., 12Kh1MF (chromiumvanadium) steel.
The analysis of the physicochemical characteristics of 12Kh1MF steel and AMTs (Al–Mn) alloys shows that the chromium-vanadium steel exhibits high corrosion resistance in aggressive alkaline-chloride and alkaline-sulfate electrolytes in broad ranges of their concentrations and temperatures. The phase composition of this steel includes α -iron containing chromium (Fe, Cr)23C6, vanadium, and molybdenum (VMo)C carbides [3].
The role of materials guaranteeing the depolarization of the anodic reaction due to high negative potentials is played by aluminum and zinc alloys (AMTs and TsAM). In alkaline solutions on these materials, the potentials of the anodic processes are more negative than the potentials of oxygen release, which enables us to perform the electrolysis of water at voltages of 0.1–1 V without oxygen release. The investigation of these reactions proves to be quire promising for the practical electrolysis [4].
Experimental Procedure
We studied the electrochemical and corrosion behaviors of 12Kh1MF steel and AMTs alloy (96.35–99% Al and 1–1.5% Mn) under the conditions of electrolysis of water in alkaline-chloride and alkaline-sulfate solutions extensively used in the electrochemical processes.
The investigations were carried out on specimens made of 12Kh1MF steel, mechanically treated up to the sixth or seventh class of purity, degreased, and chemically polished in concentrated phosphate acid at t = 140–150°C.
The corrosion tests were carried out by the weight method with evaluation of the corrosion rate according to the 10-point scale of corrosion resistance in NaOH solutions (20 g/dm3) with admixtures of NaCl and Na2SO4 (1 and 20 g/dm3, respectively). The tests were performed at a temperature of 18–20° for 720 h. For the sake of comparison, we measured the corrosion rate of carbon steel in a reference solution for the water-alkaline electrolysis.
The electrochemical studies were performed on similar electrodes with the use of an RS-485 programmed impulsive current source in membrane and membraneless electrolyzers under static and dynamic conditions of electrolysis with recording the potentials, current strength, amount of electricity used for the electrolysis, and the volume of released hydrogen. On the anodes made of AMTs aluminum alloy, we determined the parameters of anodic reactions in the presence of the negative differential effect. These parameters included the total amount of hydrogen released due to the chemical and electrochemical dissolution of aluminum.
Results and Discussion
In Table 1, we present the results of corrosion tests showing that the corrosion processes on 12Kh1MF steel are more rapid in NaOH solutions with admixtures of sodium chloride then in the solutions with sodium-sulfate admixtures.
The maximum corrosion rate was detected on a carbon steel used in the industrial electrolysis of water. Its decrease on 12Kh1MF steel is explained by the action of chromium and vanadium admixtures in steel participating in the formation of oxide and carbide compounds of chromium, vanadium, and molybdenum. In contact with electrolytes, these compounds strongly decrease the rate of corrosion accompanied by the hydrogen depolarization due to their chemical resistance in alkaline solutions [4, 5].
The corrosion processes on the AMTs alloy for pH > 12 run with high rates as a result of the chemical interaction of aluminum with the electrolyte and are accompanied by the hydrogen release and the formation of aluminate compounds and manganese oxide by the following reactions:
on the AMTs alloy:
on manganese:
The results of investigations of the corrosion rate of AMTs alloy in a solution of NaOH (20 g/dm3) with NaCl (1 g/dm3) at temperatures of 20–25° demonstrate that it is equal to i = 2.8·10–3A/cm2 and that the corrosion process runs, as a rule, with hydrogen depolarization.
The determined anodic and cathodic behaviors of the analyzed materials in alkaline electrolytes with admixtures of chlorides and sulfates confirm the indicated characteristic of the electrode materials.
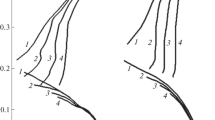
In Fig. 1, we display the anodic galvanostatic dependences for the electrodes made of AMTs alloy and 20 and 12Kh1MF steels in alkaline-chloride solutions. They show that, within the interval of current densities 5–30 mA/cm2 , the anodes made of AMTs alloy (curve 1) are in the active state with negative values of potentials E = (– 1)–(– 0.1) V.
Due to the negative differential effect, hydrogen is released on the anode and aluminum ions pass into the solution. In the presence of Cl– and \( {\mathrm{SO}}_4^{2-} \) ions in the electrolyte, the protective film fails under the conditions of anodic polarization. The indicated ions either lead to the transformation of the film into the soluble state (as a result of the chemical reactions) or, as a result of adsorption on the metal surface, promote the process of anodic dissolution (act electrochemically). Similar processes also run in alkaline-sulfate and alkaline-chloride solutions. For the same current densities, the electrodes made of chromium-vanadium steel (curve 2) are characterized by insignificant variations of the potentials, while the potentials of steel electrodes shift to the positive region up to E = 1.5 V. For current densities higher than 10 mA/cm2 , oxygen is released on these anodes. The presented dependences on the semilogarithmic coordinates have no linear section, which reveals the complicated mechanism of the anodic reaction caused not only by the ionization of electrodes but also by the formation of compounds and diffusion processes running in the near-anode layer [6].
In the cathodic polarization dependences within the range of current densities 1–30 mA/cm2 , the potentials of 20 steel and AMTs alloy changed to E = – 1.5–1.82 V as compared with the stationary values, whereas the potentials of 12Kh1MF steel and nickel are lower by 150–200 mV. The given current density corresponds to the stable potential of the electrode. The surfaces of cathodes remain invariable (with the exception of the AMTs alloy).
On the semilogarithmic coordinates, we observe linear sections in the curves of cathodic polarization of these electrodes (Fig. 2). These sections reveal the electrochemical nature of the overvoltage of cathodic reaction η caused by the reduction of water [6]. On the chromium-vanadium steel, the overvoltage of hydrogen release is lower than for the carbon steel, which is explained by the catalytic action of vanadium and molybdenum carbides present on the surfaces of cathodes [7].
The presented experimental results reveal the advantages of cathodes made of the chromium-vanadium steel in the alkaline electrolysis of water. They are connected with its high corrosion resistance and the low overvoltage of hydrogen release [8].
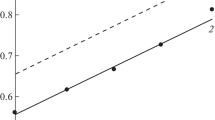
In Fig. 3, we present the results of long-term tests of the electrode materials made of AMTs alloys and 20 and 12Kh1MF steels in alkaline solutions with admixtures of chlorides and sulfates.
The highest voltage of electrolysis for a current density of 30 mA/cm2 was observed on electrodes made of carbon steel (Fig. 3, curve 2). On the electrodes made of 12Kh1MF steel, it was lower by 0.3–0.5 V (curve 4). If we use AMTs alloy as the anode and 12Kh1MF steel as the cathode, then the voltage of electrolysis decreases to 0.5–0.7 V (curves 5 and 6) due to the negative values of the anode potentials in alkaline solutions. Instead of the oxygen release as a result of depolarization of the anodic reaction, we observe the dissolution of aluminum accompanied by the hydrogen release. Due to the use of aluminum anodes, it is possible to realize the electrolysis of water in membraneless electrolyzers in which hydrogen is released on the cathodes and anodes, which enables us to significantly decrease the energy losses.
Conclusions
On the basis of the results of investigations of the corrosion behaviors of electrode materials in the process of alkaline electrolysis of water, we determined the corrosion rates of carbon and 12Kh1MF chromiumvanadium steels in alkaline solutions with admixtures of chlorides and phosphates and evaluated the electrochemical parameters of the anodic and cathodic reactions on carbon and chromium-vanadium steels and AMTs alloy under the conditions of electrolysis of water. We revealed a decrease in the overvoltage of hydrogen release on the cathodes made of 12Kh1MF steel and the anode process in the case where the AMTs alloy is used as the anode. We computed the rate and the depth index of corrosion of 20 and 12Kh1MF steels in the alkaline media containing sulfate or chloride ions. The 12Kh1MF steel has a higher corrosion resistance in this medium than 20 steel. The AMTs alloy chemically reacts with the compounds of electrolyte with the formation of hydrogen. The detected variations of the voltage of electrolysis are caused by the electrochemical behavior of the electrode materials, which enable one to decrease the losses of materials and energy for the electrolysis.
References
O. M. Stepanenko, L. G. Reiter, V. M. Ledovs’kykh, and S. V. Ivanov, General and Inorganic Chemistry [in Ukrainian], Ped. Presa, Kiev (2000), Part 2.
A Handbook of a Chemist [in Russian], Khimiya, Moscow (1964), Vol. 3.
A. N. Smirnov, “Investigation of the microstructure and the phase composition of 12Kh1MF steel after long-term operation,” Vestn. Kuzbass. Gos. Tekh. Univ., No. 2, 67–72 (2004).
B. I. Bairachnyi, G. G. Tul’s’kyi, V. V. Shtefan, and I. A. Tokareva, Industrial Electrochemistry: Chemical Current Sources, Electrolysis of the Melts, Electrosynthesis of Chemical Substances [in Ukrainian], Pidruchnyk NTU “KhPI”, Kharkiv (2016).
N. P. Zhuk, A Course of the Theory of Corrosion and Protection of Metals [in Russian], Metallurgiya, Moscow (1976).
H. Nykyforchyn, E. Lunarska, O. Tsyrulnyk, et al., “Environmentally assisted “in-bulk” steel degradation of long-term service gas trunkline,” Eng. Failure Anal., 17, 624–632 (2010).
L. F. Kozin and S. V. Volkov, Contemporary Power Engineering and Ecology: Problems and Prospects [in Russian], Naukova Dumka, Kiev (2006).
I. V. Gorynin and B. T. Timofeev, “Aging of materials of the equipment of nuclear power plants after designed service life,” Fiz.-Khim. Mekh. Mat., 42, No. 2, 13–27 (2006); English translation : Mater. Sci., 42, No. 2, 155–169 (2006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Fizyko-Khimichna Mekhanika Materialiv, Vol. 53, No. 3, pp. 32–36, May–June, 2017.
Rights and permissions
About this article
Cite this article
Bairachnyi, B.I., Zhelavs’kyi, S.G., Maizelis, A.O. et al. Corrosion Behavior of Electrode Materials in the Production of Hydrogen. Mater Sci 53, 324–329 (2017). https://doi.org/10.1007/s11003-017-0078-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11003-017-0078-7