Abstract
Aim
To evaluate the advantages and safety of vitrectomy under air for treating macula-involving rhegmatogenous retinal detachment (RRD).
Methods
Consecutive patients with macula-involving RRD who underwent vitrectomy under air were recruited. Demographic and clinical data were: age, gender, eye, lens status, best corrected visual acuity (BCVA) in logarithm of the minimum angle of resolution (logMAR), axial length, intraocular pressure (IOP). RRD parameters were: RRD extent, retinal breaks number. Surgical data were: cataract surgery, tamponade used. Postoperative parameters were: BCVA, IOP at first, third, sixth month, recurrent RD, incidence of retinal folds, subretinal fluid (SRF) persistence, macular displacement.
Results
Seventy-one eyes (71 patients) were recruited. Cataract surgery was performed in 32 of 45 phakic patients. The tamponade used was: sulfur hexafluoride 18% (41), silicon oil (SO) (26), high-density SO (4). BCVA improved significantly from baseline (1.2 ± 0.4 logMAR) to the last control (0.8 ± 0.7 logMAR) (P = 0.03285). Recurrent RD incidence was 14.1%. Postoperative complications were: retinal folds (2), SRF persistence (3), macular displacement (2).
Conclusion
Vitrectomy under air is a safe alternative technique for treating macula-involving RRD. Vitrectomy under air allows surgeon to remove accurately the vitreous from the peripheral retina and facilitates the removal of SRF reducing the complications related to its postoperative persistence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pars plana vitrectomy (PPV) has been greatly developed significantly in the last few years, and the evolution of the instruments has made it less and less invasive and traumatic, widening the indications and increasing the demand. However, PPV approach for the treatment of macula-involving rhegmatogenous retinal detachment (RRD) is not yet standardized, leaving open different ways of performing it. Which maneuvers are better to perform remains debatable and, often, the surgical management depends on surgeon’s experience. The anatomical success of the retinal re-attachment may be not sufficient to give an adequate visual recovery. This depends not only on the chronicity of the macular detachment but also on some complications related to surgical management. The extent of vitrectomy, the management of subretinal fluid (SRF), the type of intraoperative and final tamponade and the patient’s postoperative position may affect the anatomical and functional success of surgical treatment. Persistence of SRF, retinal folds and macular displacement development are the postoperative complications of surgery that can compromise the functional recovery. The large number of published articles on the surgical variants of the treatment of RRD and on the postoperative complications is the expression of a continuous research to optimize and standardize this surgical approach. Recently, some authors reported the use of air as a medium in which to perform vitrectomy [1,2,3,4,5,6,7,8]. The common use of the air is as a medium for exchanging and removing perfluorocarbon liquid (PFCL) or for injecting the final intravitreal tamponade, like silicon oil (SO) and gas. However, the properties of buoyancy, transparency, the high surface tension and the easy of intraoperative management (rapid injection and removal) lead many surgeons to use the air during vitrectomy. Performing a vitrectomy in the air can be beneficial for many reasons. The air prevents the movement of the retina following the tractions given by vitrectomy, and it provides a wider view of fundus. In this paper, the authors evaluate the advantages and the safety of the vitrectomy under air for the treatment of macula-involving RRD.
Materials and methods
A series of consecutive patients affected by macula-involving RRD who underwent PPV under air, at the department of Ophthalmology of the University of Padova between November 2017 and December 2019, were recruited. At the time of surgery, the surgical procedures were evaluated by institution's ethics committee, and in accordance with the ethical standards of the responsible committee the approval was received. The benefits and potential risks of the treatment were explained, and informed consent was obtained from all patients. The inclusion criteria were macula-involving RRD, absence of metabolic and vascular retinal diseases and negative history of previous surgery for RD repair. All data were collected from clinical charts and electronic database of the department of Ophthalmology of University of Padova. The demographic data were the following: age (years), gender (male/female), eye (right or left), lens status (phakic or pseudophakic), detailed information on previous ocular surgery. The preoperative data collected were the following: best corrected visual acuity (BCVA) measured in Snellen chart and converted to logarithm of the minimum angle of resolution (LogMAR), axial length (AL) measured in millimeters (mm), intraocular pressure (IOP) measured in mmHg. RRD parameters were the following: extent of RRD (expressed as a clock face from 1 to 12 hours) and number of retinal breaks. Postoperative parameters were the following: BCVA (at first, third, sixth month), IOP (at first, third, sixth month), recurrent RD, persistence of SRF, incidence of retinal folds and macular displacement, detected by optical coherence tomography (OCT) and fundus autofluorescence (FAF). Concerning the BCVA, visual acuity line scale adapted for low vision was used. The following conversion was calculated: finger count at 1 m equal to 1.8 logMAR, perception of hand movement equal to 2.3 logMAR, light perception equal to 2.8 logMAR, no light perception equal to 3 logMAR. BCVA and IOP changes were evaluated for patients who underwent one successful surgery, and those who presented a recurrent retinal detachment were excluded from the analysis. All surgical procedures were performed by a single experienced vitreoretinal surgeon (R.F.) using 23 gauge (G) vitrectomy system (Constellation vision system, Alcon Laboratories, Inc.). The variants of surgical technique collected were: the type of tamponade used (silicon oil SO, high-density SO or sulfur hexafluoride SF6 18%), and cataract surgery combined to vitrectomy.
Statistical analysis
Statistical analysis of the data was computed using SPSS software version 22.0 (IBM Corporation, New York, NY, USA). Descriptive statistics are absolute and relative frequencies for qualitative variables and means, standard deviation, median for quantitative variables. Paired t-test was used to evaluate the changes of BCVA and IOP comparing the baseline mean with postoperative means at each time point of follow-up. Statistical significance was set at 0.05 (two-tailed).
Surgical technique
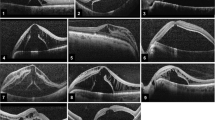
After a core vitrectomy, an early fluid–air exchange was performed to completely fill the vitreous chamber with air, aspirating simultaneously SRF through the retinal break or, if there were more than one break, through the more posterior ones, until the air bubble closed the retinal break and dislocated the residual SRF from the periphery to the macula, already detached (Fig. 1a, b). In this way, vitrectomy was performed on the peripheral retina, attached by the air bubble tamponade, under the effect of a wider visual field viewing due to the different air refraction index of the air (1) compared to fluid/vitreous (balanced salt solution BSS 1.33 versus vitreous 1.337) (Fig. 2a, b). The difference values between air and BSS-vitreous refraction indices also permits a better visualization of the residual vitreous during the vitrectomy, by the turbulence that creates the vitreous cutter port when the vitreous is engaged into it (Fig. 2c). If a condensation of posterior surface of intraocular lens (IOL) in pseudophakic patient with capsulotomy occurred, a bubble-wiping technique was performed [9,10,11]. At the end of base vitreous shaving, perfluorocarbon liquid (PFCL) was injected into the vitreous chamber and a back flush needle was placed in front of the retinal break in order to create a vacuum and to favor the drainage of the SRF pushed by the PFCL from the macula to the periphery (Fig. 1c). The final air–PFCL exchange was performed to remove PFCL. Sulfur hexafluoride (SF6), silicon oil (SO), high-density SO were injected as final intravitreal tamponade. The type of tamponade (SO, high density SO, gas) used was decided on the basis of the complexity of RD (like retinal contraction, location, number of and type of retinal breaks). At the end of surgery, the patient was instructed to maintain a supine position for the immediate postoperative 12 hours. Supplementary video 1 shows the surgical technique of vitrectomy under air.
Macula-involving rhegmatogenous retinal detachment (RRD) with a retinal break and subretinal fluid (SRF) (picture a). Pictures b and c show the steps of the use of the air for management of SRF: early injection of air and displacement of SRF toward the macula (b), injection of perfluorocarbon liquid (PFCL) under air and removal of SRF through the retinal break by back flush needle. Pictures d and e show the conventional use of PFCL with the displacement of SRF toward the peripheral retina for 360° (picture d). The incomplete removal of SRF can cause its displacement toward the macula area and the retinal folds formation (picture e)
The different width of view through the fluid a and air b with the same viewing system. The black arrows indicate laser spots around a retinal break. Picture c shows vitrectomy under air: asterisk indicates the turbulence in the vitreous interface during the vitrectomy. Picture d shows vitrectomy under fluid: asterisk indicates the stretched detached retina and vitreous engaged in the vitrectomy cutter port
Results
Seventy-one clinical chart of 71 consecutive patients (42 males, 29 females) affected by macula-involving RD (32 right and 39 left eyes) who underwent PPV under air were collected. The mean age was 61.94 ± 14.45, ranging from 14 to 90 years. Table 1 summarizes the preoperative data. In 32 of 45 phakic patients, cataract surgery was performed with vitrectomy. The final tamponade was: SF6 in 41 eyes (57.8%), SO in 26 eyes (36.6%) and high density SO in 4 eyes (5.6%). The incidence of recurrent RD after surgery was 14.1% (10 of 71 eyes): 5 in the subgroup of patients with SO tamponade, 2 in subgroup with high density SO tamponade and 3 in the subgroup with SF6 tamponade. In 9 eyes, definitive retinal attachment was obtained after 2 additional surgeries, one of revision of vitrectomy with injection of permanent tamponade (SO or high density SO) and one of tamponade removal after a period between 1 and 3 months. In one case, no further surgery was performed because the patient did not want to undergo additional treatments. Postoperative complications after successful surgery were: two cases of retinal folds, 3 cases of bleb like syndrome and 2 cases of macular displacement (Fig. 4). BCVA mean of 60 patients with a successful retinal reattachment after the first surgery improved significantly from baseline, 1.2 ± 0.4, ranging from 0.7 to 3 logMAR, to each time point follow-up reaching 0.82 ± 0.7, ranging from 0 to 3 logMAR, at 6 postoperative months (P = 0.03285) (Fig. 3). IOP mean increased from 12.5 ± 4.8, ranging from 3 to 31 mmHg at baseline to 21.2 ± 7.1, ranging from 5 to 30 mmHg at the first postoperative month (P = 0.04639); subsequently, it decreased without significant difference compared to preoperative value (Fig. 3).
Discussion
It is common belief that the vitreous should be removed completely when PPV is performed for treating RD. However, the amount of removed vitreous is the result of a compromise between the surgeon skills and the conditions of the vitreous and peripheral retina that varies from case to case. The most realistic goal is not to remove all vitreous, but to remove it as much as possible without causing damage to the retina. For understanding what are the difficulties of vitreous base shaving in the treatment of RRD, the attention must be focused on specific conditions during surgery. The vitreous base shaving is a dangerous procedure due to the fact that the vitreous cutter works almost in contact with the retina. PFCL is injected into the vitreous chamber to reduce the movements of the retina during shaving. In this way, the macula is stabilized and the peripheral retina is stretched limiting its movements during vitrectomy. Despite this, the compartmentalization of SRF, pushed anteriorly in the subretinal space, keeps the retina detached and, during shaving, the movements of the retina, due to turbulence and tractions given by the vitreous cutter port, increase the risk of iatrogenic retinal breaks (Fig. 2d, supplementary video). To perform a shaving of the vitreous base, the surgeon must work with the vitreous cutter extremely close to the peripheral retina. The tractional forces that the vitreous cutter creates on the detached retina cause a movement of the retinal tissue toward the vitreous cutter port. These forces are transformed into retinal stress (Fig. 2d) The stress due to the tractions causes a shortening and elongation of the retina. The ideal condition would be to work with the attached retina. However, even if the retina is still attached and therefore subjected to a force that is opposed to the retinal movement, the stress due to the vitrectomy will act on the internal retinal architecture. The ideal cut of a tissue is when no force is applied to the tissue beyond that of the cut itself. When a tissue is subjected to traction, the cut is composed of additional forces that involve areas of tissue adjacent to the cut and the surgeon is not more able to control the effects of cut on the treated tissue [1]. Vitrectomy under air has the advantage that the air bubble contrasts, with its tamponade force, the tractional forces exerted by vitreous cutter on the retinal surface. This approach stabilizes the retina preventing it from being engaged in the vitreous cutter port and limits the stresses on the internal retinal structure (Fig. 2c, supplementary video).
An important aspect of vitrectomy under air concerns the management of SRF. In the traditional approach, PFCL injection is considered to be an important step. Its function is to stabilize the macula and to displace SRF from the macula to the peripheral retina. When the PFCL fills vitreous chamber up to cover the retinal break, no further drainage of SRF is possible and the residual SRF is displaced anteriorly to form a rim of detached retina. SRF is compartmentalized in the subretinal space anteriorly with a donut-shaped configuration. When PFCL is exchanged with air, the SRF is removed through the retinal break by a back flush needle. However, the complete removal of SRF is not obvious, because when the air bubble closes the retinal break, the residual SRF remains incarcerated and migrates to the macula at the end of the complete air exchange increasing the risk of creating retinal folds (Fig. 1e). The technique described in this paper aims to reduce the risk of residual SRF at the end of surgery, by making an early air exchange, pushing SRF to the macular area, and then by injecting PFCL under air, in order to push SRF to the peripheral retina. The air tension against the peripheral retina and the vacuum created with a back flush needle in front of the retinal break allows the surgeon to drive SRF to the retinal break and to completely remove it without its compartmentalization. It is not possible to be sure of the complete removal of SRF and retinal folds could develop, due to the action of tamponade that pushes the retina against the retinal pigment epithelium (RPE). Therefore, the authors suggest instructing the patient to maintain a specific position in order to avoid the bubble to tamponade the posterior pole: a supine position in case of SO or SF6 tamponades, a prone position in case of high-density SO tamponades. In this way, the presence of fluid in both side of the detached retina (over and under) allows the retina to go flat gradually following the reabsorption of SRF by the RPE pump (Fig. 5). Figure 1d, e shows the traditional approach with PFCL injected under BSS onto the macula and the subsequent donut-shaped compartmentalization of SRF into the peripheral retina with a risk of retinal folds formation after the PFCL air exchange. In this series, the retinal re-attachment was obtained in all eyes.
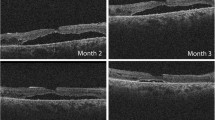
a, b Optical coherence tomography (OCT) scans show the 2 cases of postoperative extrafoveal outer retinal folds. c, d fundus autofluorescence (FAF) shows the two cases of macular displacement (white arrows). d, g, e optical coherence tomography (OCT) scans show the 3 cases of multiple subretinal fluid (SRF) blebs
The incidence of recurrent RD (14.1%, i.e., 10 of 71 eyes) is similar to the surgical failure rate reported in the literature. Recurrence of RD was higher in the subgroup of patients with SO than those with SF6. The authors believe that this difference in outcomes is affected by the complexity of the cases and the longer-term compartmentalization that SO creates compared to the gas in the vitreous chamber. The rate of cases that require additional surgery to repair recurrent RD is extremely variable in the literature. An anatomically successful repair occurs in 95% of people, with 70–90% achieving this in one operation [12,13,14,15,16,17,18,19,20,21]. In our study, we obtained a 14.1% of retinal re-attachment with just one surgery. There are several causes of failure of primary RD surgery. The most important of them is the development of proliferative vitreoretinopathy (PVR) that leads to retinal contraction. This phenomenon occurs in 5–11% of patients [12, 22,23,24,25,26,27,28,29,30,31,32,33]. A higher risk of re-detachment has been reported in PVR ≥ grade C, anterior and inferior PVR [12, 22, 23, 26, 29,30,31]. Another cause of re-detachment is the formation of new breaks or missed breaks [24, 25, 27, 28]. Clinical characteristics of primary RD that increment the risk of recurrence are longer duration of symptoms, involvement of inferior and all four quadrants, persistent and progressive accumulation of SRF [25, 27, 30,31,32].
Last important aspect of vitrectomy under air concerns the quality of visualization of fundus during the surgery. The air has a refractive index lower than BSS. This characteristic of the air allows the surgeon to have a wider field of view in air compared to that in BSS (Fig. 2a, b). In addition, the detection of the vitreous is facilitated during vitrectomy under air by the presence of turbulence on the vitreous/air interface when the vitrectomy cutter engages the vitreous (Fig. 2c). Vitrectomy under air allows to perform shaving more accurately compared to that under BSS, because the air bubble contrasts, with its tamponade force, the tractional forces exerted by vitreous cutter on the retinal surface. This approach stabilizes the retina preventing it from being engaged in the vitreous cutter port during the shaving. A reduced vision under air can occur in pseudophakic patients with opened posterior capsule. If a condensation of posterior surface of IOL with capsulotomy occurs, bubble-wiping technique or a re-exchange air-BSS might be performed. An important advantage of air is that is easy and fast to exchange.
In our series, we noticed the following postoperative complications: 2 cases of retinal folds, 3 cases of SRF persistence and 2 cases of macular displacement.
The mechanisms of retinal folds formation are not fully known. dell’Omo described 3 types of retinal folds: full-thickness and partial retinal folds (outer or inner). The incidence of retinal folds in our series (5.4%) was lower than that reported in the literature [34, 35]. dell’Omo et al. reported 42.4% of incidence of outer retinal folds and 48.8% of inner retinal folds in a series of 33 cases of RRD (22 with macula-involving RRD) [34]. Peiretti E et al. reported 35.71% of incidence of partial-thickness retinal folds in a series of 56 cases of RRD involving macula [36].
In three cases, we detected the presence of multiple SRF blebs after RD repair. In one case, we noticed a complete resolution after one year, and in the other two, we noticed a reduction of the size and the number of blebs at 3 postoperative months. In two cases, SRF blebs did not affect the functional recovery, supporting the previous findings reported in the literature, and in the other one a functional impairment was detected after 6 months [37]. However, it remains unclear whether this complication is a consequence of a persistence of SRF or it is an inflammatory process of RPE.
In two cases (5.4%), we detected a retinal vessel displacement directed downward. The incidence of macular displacement was lower compared to that reported in the literature [38].
In conclusion, vitrectomy under air is a safe alternative technique for treating macula-involving RRD. Although even in our series of cases we found the postoperative complications after successful surgery already reported from other authors, it must be said that the incidence of those is considerably reduced. Probably, there are factors such as greater accuracy in the removal of SRF, a greater collaboration of the patient in maintaining the postoperative position which can affect the final result. Vitrectomy under air facilitates the removal of vitreous from the peripheral retina. The early use of the air and the PFCL injection under air facilitates the removal of SRF reducing the complication related to the postoperative (Fig. 5) persistence of SRF.
References
Charles S (2004) An engineering approach to vitreoretinal surgery. Retina 24(3):435–444
Charles S, Calzada J, Wood B (2007) Vitrectomy under air. In: Williams L, Wilkins (eds) Vitreous Microsurgery. 4th edn. Philadelphia, p 73
Voleti VB, Gee CJ, Devin F (2014) Vitrectomy under air. Retina 32(9):1981–1982
Sigler EJ, Charles S, Calzada JI (2014) Interface vitrectomy. Retina 34(3):616–617
Reibaldi M, Rizzo S, Avitabile T et al (2014) Iatrogenic retinal breaks in 25 gauge vitrectomy under air compared with the standard 25-gauge system for macular diseases. Retina 34(8):1617–1622. https://doi.org/10.1097/IAE.0000000000000112
Erdogan G, Unlu C, Karasu B et al (2016) Comparing peripheral vitrectomy under air and fluid infusion for primary rhegmatogenous retinal detachment. Retina. https://doi.org/10.1097/IAE.0000000000000898
Altan T, Ozbilen KT, Cetin T et al (2017) Results of peripheral vitrectomy under air in rhegmatogenous retinal detachment. Ophthalmic Surg, Lasers and Imaging Retina 48(1):51–54. https://doi.org/10.3928/23258160-20161219-07
Bonfiglio V, Toro MD, Longo A et al (2018) Modified vitrectomy technique for phakic rhegmatogenous retinal detachment with intermediate break. J Ophthalmol 2018:6127932. https://doi.org/10.1155/2018/6127932
Jaffe GJ (1997) Management of condensation on a foldable acrylic intraocular lens after vitrectomy and fluid–air exchange. Am J Ophthalmol 124:692–693
Hainsworth DP, Chen SN, Cox TA, wt al. (1996) Condensation on polymethylmethacrylate, acrylic polymer, and silicone intraocular lenses after fluid–air exchange in rabbits. Ophthalmology 103:1410–1418
Yuda K, Nagashima T, Shimizu T et al (2017) Bubble-wiping technique for clearing the condensation on intraocular lenses. Retina. https://doi.org/10.1097/IAE.0000000000001718
Speicher MA, Fu AD, Martin JP et al (2000) Primary vitrectomy alone for repair of retinal detachments following cataract surgery. Retina 20(5):459–464. https://doi.org/10.1097/00006982-200009000-00005
Schmidt JC, Rodrigues EB, Hoerle S et al (2003) Primary vitrectomy in complicated rhegmatogenous retinal detachment–a survey of 205 eyes. Ophthalmol J Int d’ophtalmologie Int J Ophthalmol Zeitschrift fur Augenheilkd 217(6):387–392. https://doi.org/10.1159/000073067
Brazitikos PD, Androudi S, Christen WG et al (2005) Primary pars plana vitrectomy versus scleral buckle surgery for the treatment of pseudophakic retinal detachment: a randomized clinical trial. Retina 25(8):957–964. https://doi.org/10.1097/00006982-200512000-00001
Heimann H, Bartz-Schmidt KU, Bornfeld N et al (2007) Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment: a prospective randomized multicenter clinical study. Ophthalmology 114(12):2142–2154. https://doi.org/10.1016/j.ophtha.2007.09.013
Steel D, Fraser S (2009) Retinal detachment. BMJ. Clin Evid. 710:1–22
Nagpal M, Chaudhary P, Wachasundar S et al (2018) Management of recurrent rhegmatogenous retinal detachment. Indian J Ophthalmol 66(12):1763–1771. https://doi.org/10.4103/ijo.IJO_1212_18
Schmidt I, Plange N, Rößler G et al (2019) Long-term clinical results of vitrectomy and scleral buckling in treatment of rhegmatogenous retinal detachment. Sci World J. https://doi.org/10.1155/2019/5416806
Znaor L, Medic A, Binder S, Vucinovic A, Marin Lovric J, Puljak L (2019) Pars plana vitrectomy versus scleral buckling for repairing simple rhegmatogenous retinal detachments. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD009562.pub2
Uzel MM, Citirik M, Ilham C, Tekin K (2019) The impact of duration on the recurrence of rhegmatogenous retinal detachment: optimal cutoff value. Int Ophthalmol 39(9):2089–2095. https://doi.org/10.1007/s10792-018-1045-5
Geiger M, Smith JM, Lynch A et al (2020) Predictors factors of macular function after surgery for primary macula-off rhegmatogenous retinal detachment. Int Ophthalmol 40(3):609–616. https://doi.org/10.1007/s10792-019-01219-0
Glaser BM, Cardin A, Biscoe B (1987) Proliferative Vitreoretinopathy: the mechanism of development of vitreoretinal traction. Ophthalmol 94(4):327–332. https://doi.org/10.1016/S0161-6420(87)33443-8
Jonas JB, Knorr HLJ, Rank RM, Budde WM (2001) Retinal redetachment after removal of intraocular silicone oil tamponade. Br J Ophthalmol 85(10):1203–1207. https://doi.org/10.1136/bjo.85.10.1203
Foster R, Meyers S (2002) Recurrent retinal detachment more than 1 year after reattachment. Ophthalmol 109:1821–1827. https://doi.org/10.1016/S0161-6420(02)01182-X
Wickham L, Ho-Yen GO, Bunce C et al (2011) Surgical failure following primary retinal detachment surgery by vitrectomy: risk factors and functional outcomes. Br J Ophthalmol 95(9):1234–1238. https://doi.org/10.1136/bjo.2010.190306
Sigler EJ, Randolph JC, Calzada JI et al (2014) Anatomical and visual outcomes after two-port pars plana vitrectomy reoperation under silicone oil for epimacular membrane or recurrent retinal detachment. Retina 34(10):1939–1944. https://doi.org/10.1097/IAE.0000000000000170
Adelman RA, Parnes AJ, Michalewska Z et al (2014) Clinical variables associated with failure of retinal detachment repair: The European vitreo-retinal society retinal detachment study report number 4. Ophthalmol 121(9):1715–1719. https://doi.org/10.1016/j.ophtha.2014.03.012
Teke MY, Balikoglu-Yilmaz M, Yuksekkaya P et al (2014) Surgical outcomes and incidence of retinal redetachment in cases with complicated retinal detachment after silicone oil removal: Univariate and multiple risk factors analysis. Retina 34(10):1926–1938. https://doi.org/10.1097/IAE.0000000000000204
Itakura H, Kishi S, Li D et al (2014) Vitreous changes in high myopia observed by swept-source optical coherence tomography. Invest Ophthalmol Vis Sci 55(3):1447–1452. https://doi.org/10.1167/iovs.13-13496
Enders P, Schick T, Schaub F et al (2017) Risk of multiple recurring retinal detachment after primary rhegmatogenous retinal detachment repair. Retina 37(5):930–935. https://doi.org/10.1097/IAE.0000000000001302
Ambiya V, Rani PK, Narayanan R et al (2018) Outcomes of recurrent retinal detachment surgery following pars plana vitrectomy for rhegmatogenous retinal detachment. Semin Ophthalmol 33(5):657–663. https://doi.org/10.1080/08820538.2017.1395893
Bilgin AB, Dogan ME, Aysun B et al (2019) Pars plana vitrectomy with or without intraoperative 360° peripheral endolaser for rhegmatogenous retinal detachment treatment. Int Ophthalmol. https://doi.org/10.1007/s10792-018-0986-z
Chatziralli I, Theodossiadis G, Parikakis E et al (2020) Inner retinal layers alterations and microvasculature changes after vitrectomy for rhegmatogenous retinal detachment. Int Ophthalmol. https://doi.org/10.1007/s10792-020-01521-2
dell’Omo R, Tan HS, Schlingemann RO et al (2012) Evolution of outer retinal folds occurring after vitrectomy for retinal detachment repair. Invest Ophthalmol Vis Sci 53(13):7928–7935. https://doi.org/10.1167/iovs.12-10322
Gupta RR, Iaboni DSM, Seamone ME et al (2019) Inner, outer, and full-thickness retinal folds after rhegmatogenous retinal detachment repair: a review. Surv Ophthalmol 64(2):135–161. https://doi.org/10.1016/j.survophthal.2018.10.007
Peiretti E, Nasini F, Buschini E et al (2017) Optical coherence tomography evaluation of patients with macula-off retinal detachment after different postoperative posturing: a randomized pilot study. Acta Ophthalmol 95(5):e379–e384. https://doi.org/10.1111/aos.13397
Otsuka Y, Oishi A, Suda K et al (2019) Multiple subretinal fluid blebs after pars plana vitrectomy for rhegmatogenous retinal detachment repair. Graefes Arch Clin Ophthalml. https://doi.org/10.1007/s00417-018-04231-9
dell’Omo R, Scupola AA, Viggiano D et al (2017) Incidence and influencing retinal displacement in eyes treated for rhegmatogenous retinal detachment with vitrectomy and gas or silicon oil. Invest Ophthalmol Vis Sci. https://doi.org/10.1167/iovs.17-21466
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study, involving human participants, were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent.
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 149207 kb)
Rights and permissions
About this article
Cite this article
Frisina, R., Gius, I., Frascogna, G. et al. A possible strategic role of air during pars plana vitrectomy for macula-involving rhegmatogenous retinal detachment. Int Ophthalmol 41, 421–431 (2021). https://doi.org/10.1007/s10792-020-01591-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-020-01591-2