Abstract
Purpose
To examine the macular microstructure in macular edema (ME) due to retinal vein occlusion (RVO) in terms of ischemic or nonischemic type to determine whether and how ischemia affects macular microstructure.
Methods
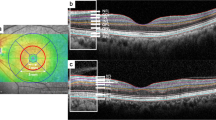
This retrospective, nonrandomized study included 75 newly diagnosed RVO cases (group 1: 55 nonischemic RVO cases and group 2: 20 ischemic RVO cases) with evidence of center-involving ME without any treatment. Quantitative measures on spectral-domain optical coherence tomography images were performed. Central subfield thickness (CST) was collected in the central 1 mm from the thickness map. The following items were evaluated in a 3-mm-wide area (perifoveal ETDRS circle) centered on the fovea: disorganization of the retinal inner layers (DRIL), disrupted external limiting membrane (ELM) and ellipsoid zone disruption (EZD). The microstructural changes were measured manually.
Results
Baseline characteristics, such as age, sex, study eye, and RVO risk factors, were similar between the groups (P > 0.05). CST was 554.15 ± 191.45 µm for group 1 and 769.90 ± 290.00 µm for group 2 (P: 0.001). The extent of DRIL was 1864.09 ± 941.70 µm and 2447.25 ± 492.59 µm for groups 1 and 2, respectively (P: 0.010). The disrupted ELM length was 1700 (0–3000) µm for group 1 and 2725 (300–3000) µm for group 2 (P: 0.027). The EZD length was 1453.09 ± 870.38 µm for group 1 and 1846.00 ± 926.54 µm for group 2 (P: 0.093).
Conclusions
Ischemic RVOs cause greater macular edema and greater disruption in the macular microstructure compared to nonischemic RVOs, especially in terms of DRIL and ELM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Retinal vein occlusions (RVOs) are the second most common primary vascular diseases of the retina after diabetic retinopathy. RVOs may occur as central RVOs (CRVOs) or branch RVOs (BRVOs). BRVO is approximately 3 to 4 times more prevalent than CRVO [1]. RVO may be classified as ischemic or nonischemic, each of which has a different prognosis [2].
Macular edema (ME) is one of the most common complications and a major cause of visual decrease in eyes with RVO. The upregulation of vascular endothelial growth factor (VEGF) and the breakdown of the blood–retinal barrier cause ME. Moreover, cytokine release, hypoxia, increased hydrostatic pressure, and vascular stasis may contribute to ME formation [3].
The diagnosis of ME is best performed with spectral-domain optical coherence tomography (SD-OCT). SD-OCT is widely used to identify individual retinal layers, and it can provide a detailed view of the retinal microstructure. Although many studies have suggested that sizes of macular microstructure changes are predictive and prognostic, they only evaluated an area of 1000 µm or 500 µm, and mostly at the posttreatment follow-up examination [4,5,6,7,8].
In the present study, we aimed to examine a 3000-µm-wide area of the macular microstructure in macular edema due to ischemic versus nonischemic RVO to determine whether and how ischemia affects the macular microstructure.
Materials and methods
We retrospectively reviewed the charts of 116 consecutive patients with ME due to RVO (84 BRVO cases and 32 CRVO cases) between May 2009 and July 2018 in the Retina Department of Ophthalmology at Inonu University. Approval from the institutional ethics review board was obtained, and the study was conducted in accordance with the tenets of the Declaration of Helsinki.
Information on age, gender, study eye, medical history of risk factors, and vein occlusion types was collected. Data from the charts of the study participants, including best-corrected visual acuity (BCVA), slit-lamp biomicroscopy, fundus examination, SD-OCT, and fluorescein angiography, were reviewed to determine patient inclusion. BCVA was recorded in Snellen units and converted to the logarithm of the minimum angle of resolution for statistical analysis.
The inclusion criteria were patients with newly diagnosed RVO with evidence of center-involving ME without any treatment. The exclusion criteria were significant media opacity, ocular comorbidities (e.g., retinal artery occlusion, uveitis, ME from any other cause, and vitreous traction), and eyes with poor OCT images to measure the boundaries. The patients were assigned into two groups (group 1: nonischemic, group 2: ischemic) based on the fluorescein angiography findings (FFA). Ischemic CRVO was defined as having more than ten disks of ischemic area in the FFA, and ischemic BRVO was defined as having more than five disks in the FFA [9,10,11].
All images were obtained at the time of first diagnosis before any treatment. Each OCT scan was evaluated by the same grader. Image analysis was conducted as previously described [12]. Briefly, the scan passing through the foveal center and the three B-scans immediately above and below it, accounting for a total of seven scans, were included in the analysis. Central subfield thickness (CST) was defined as the average thickness in the central 1-mm-diameter circle of the ETDRS grid and collected from the thickness map. Foveal depression loss (FDL) was evaluated in the central 1 mm diameter circle of the ETDRS grid. The following items were evaluated in a 3-mm-wide area (perifoveal ETDRS circle) centered on the fovea: maximum extent of the disorganization of the retinal inner layers (DRIL), external limiting membrane (ELM) disruption, ellipsoid zone disruption (EZD), visibility of the cone outer segment tips (COST), subretinal fluid (SRF) height, small hyperreflective foci (sHF), and maximum intraretinal cyst size (CS) (vertical height of the largest cyst through all sections). Quantitative measures on the OCT were performed manually by the caliper function for structural lines (DRIL, ELM, EZD…) (Fig. 1).
Statistical analysis
Statistical analyses were performed using IBM SPSS (version 22.0; IBM Corporation, Somers, NY, USA) statistical software. The normality of the data was assessed using the Shapiro–Wilk test. Continuous variables are presented as the means and standard deviations (±), categorical variables as percentages (%), and continuous variables as the medians (min–max) if nonnormally distributed. Continuous variables were compared using independent t tests. The Mann–Whitney U test was used if the continuous variables were nonnormally distributed. We examined the relationship of the categorical outcomes using the Chi-squared test or Fisher’s exact test. The correlations between the OCT parameters were determined by Pearson’s correlation test or Spearman correlation tests for a simple regression analysis. Regression analysis was performed to determine the effect of microstructural changes on visual acuity. A P value of less than 0.05 was considered statistically significant.
Results
Seventy-five eyes of 75 patients fulfilled the inclusion and exclusion criteria and were enrolled in the study.
Table 1 depicts the demographic information of the subjects. Briefly, group 1 had 55 eyes, and group 2 had 20 eyes. The mean age was 62.44 ± 11.10 years for group 1 and 63.65 ± 9.47 years for group 2. Male accounted for 50.9% of group 1 and 65.0% of group 2. Approximately 47.3% of group 1 and 55% of group 2 were right eyes (P: 554). All patients had at least one classic risk factor for RVO, such as hypertension, diabetes, smoking, glaucoma, and hyperlipidemia (P > 0.05). The baseline characteristics, such as age, sex, study eye, and RVO risk factors, were similar between the groups. Vein occlusion types included 41 BRVO (74.5%) and 14 CRVO (25.5%) for group 1 and 12 BRVO (60.0%) and 8 CRVO (40.0%) for group 2 (P: 0.221). BCVA was 0.85 ± 0.53 for group 1 and 1.34 ± 0.54 for group 2 (P: 0.001).
The CST was 554.15 ± 191.45 µm for group 1 and 769.90 ± 290.00 µm for group 2. Ischemic RVO caused significantly greater ME (P: 0.001). The extent of DRIL was 1864.09 ± 941.70 µm and 2447.25 ± 492.59 µm for groups 1 and 2, respectively, and the difference was statistically significant (P: 0.010).
The disrupted ELM length was 1700 (0–3000) µm for group 1 and 2725 (300–3000) µm for group 2. Ischemic RVO caused significantly greater ELM disruption (P: 0.027). The EZD length was 1453.09 ± 870.38 µm for group 1 and 1846.00 ± 926.54 µm for group 2 (P: 0.093). The COST was 1544.18 ± 870.92 µm for group 1 and 1154.00 ± 926.54 µm for group 2 (P: 0.096).
The median SRF height was 60 (0–327) µm for group 1 and 0 (0–696) µm for group 2 (P: 0.141). The number of sHF was 12 (2–27) for group 1 and 10 (3–26) for group 2 (P: 0.728). The CS was 250 (0–1700) µm for group 1 and 356 (90–690) µm for group 2. A statistically significant difference was found (P: 0.022). The FDL was present in the majority of patients in group 1 (74.5%) and group 2 (90.0%) (P: 0.208) (Table 2).
In terms of the relationship between the binary variables, a positive correlation was observed between CST and EZD (Pearson’s r: 0.641, P < 0.01) and a negative correlation between CST and COST (Pearson’s r: − 0.640, P < 0.01). A negative correlation was found between COST and EZD (Pearson’s r: − 1.00, P < 0.01). A positive correlation was observed between DRIL and ELM (Spearman’s r: 0.479, P < 0.01), between CS and ELM (Spearman’s r: 0.400, P < 0.01), and between HF and ELM (Spearman’s r: 0.259, P < 0.05). A negative correlation was found between CS and SRF (Spearman’s r: − 0.242, P < 0.05).
In regression analysis, none of the OCT measurements were found to affect BCVA for group 1 (P > 0.05 for all). In group 2, DRIL and EZD were found to affect BCVA (P: 0.013, R2: 0.295; P: 0.006 R2: 0.348, respectively). The other OCT measurements were found to not affect BCVA for group 2 (P > 0.05 for all).
Discussion
We examined a-3000-µm-wide zone in the macular area, as ME usually extends at the 3000 µm-wide zone in RVOs. We presented the results of our preliminary study on this subject in the European Vitreoretinal Society (EVRS) meeting in 2018 [13]. In that preliminary study, we did not quantitatively measure the units but evaluated them as present or absent. Thus, we found a difference in ELM but not in DRILL, SRF, and HF in the preliminary study results. In the present study, we performed quantitative measurements at 3000 µm and achieved very striking results.
Our results suggest that peripheral ischemia in RVO causes more disruption of the macular structure and promotes greater ME. Prasad et al. [14] reported an association between untreated nonperfusion in ultra-widefield fluorescein angiography (UWFFA) and ME in branch RVO and hemi-RVO. Additionally, Singer et al. [15] speculated that the degree of peripheral nonperfusion in UWFFA correlated with the severity of ME in eyes with CRVO. This finding was attributed to higher levels of circulating VEGF and other cytokines in eyes with greater amounts of capillary nonperfusion [16].
Whereas many studies have argued an inverse correlation between the amount of CST and visual prognosis, Ota et al. [17] suggested that the preserved thickness and integrity of the foveal photoreceptor layer are necessary. ME disrupts the synaptic flow between layers. Therefore, dysfunction in the edematous retina cannot be attributed to only the loss of photoreceptors. Disruptions in synaptic conduction due to defective retinal layers should also be considered.
Our results indicate that the macular microstructure is important to the amount of ME. Therefore, ischemic vein occlusions may lead to more disruptions of the macular microstructure and a greater amount of ME. Many studies have emphasized that both inner and outer retinal structural integrity could be affected in RVOs [7, 18,19,20]. The indicators of disruption of the macular microstructure in OCT are DRIL, ELM, and EZD. Sun et al. [12] speculated that DRIL could represent the disorganization or destruction of cells within the inner retinal layers (bipolar, amacrine, or horizontal). Mimouni et al. [4] found that DRIL represents a compromise to the inner retinal circulation, which seems to be partly reversible. They also reported that DRIL in the 1 mm foveal area is associated with the baseline BCVA and that persistent DRIL could represent irreversible damage to the inner retinal layers [4]. Moreover, the recovery of DRIL could indicate the resolution of initial retinal edema and reperfusion [7]. In agreement with these views, we found a significant increase in DRIL and disrupted ELM in ischemic vein occlusion in comparison with the nonischemic form. In addition, we found a positive correlation between DRIL and disrupted ELM, which could be due to the greater ischemic hazard to the retinal layers aside from the greater VEGF in ischemic vein occlusions. Ischemic death of the amacrine, horizontal, bipolar, and ganglion cells and loss of their synaptic connections could cause DRIL in macular deturgescence [7]. The ELM acts as a barrier for macromolecules, and it is regarded as the zonula adherens between the Müller cells and photoreceptors [21,22,23]. The ELM serves as an important barrier to free protein diffusion across the retina from the inner retinal layers to the subretinal space and vice versa [24]. When ELM is disrupted, it fails to block the migration and deposits of extravasated lipoproteins in the retinal layers [5]. Our correlation results between ELM and HF support this report. One study claimed that a thicker CST, which represents the severity of ME, could lead to a disruption of ELM integrity [25]. Conversely, we consider that in the case of a large amount of ME, the disruption of ELM could be the cause of the edema, not the result. In addition to the proven property of VEGF, the direct effect of ischemic status on the macular microstructure should be considered regardless of VEGF. Moreover, microscopic imaging studies on rodents have demonstrated the breakdown of the outer blood–retinal barrier induced by ischemia [26]. ME, which is the result of the breakdown of the inner blood–retinal barrier, may also be associated with outer blood–retinal barrier breakdown, especially in ischemic vein occlusions. Studies on and treatment options for ME due to vein occlusions mostly focused on the disruption of the inner blood–retinal barrier. Studies on the external blood–retinal barrier may provide important information on ME due to vein occlusions.
In terms of EZD, although it was still high in ischemic form, the difference was not significant in our results. Some authors reported that ELM status was more highly correlated with visual acuity than with EZ [18, 21, 22]. Conversely, some authors have demonstrated that the integrity of the EZ was more highly associated with visual acuity than with the ELM [27,28,29]. In connection with these views, we consider ELM to be more sensitive to ischemia than EZ based on our results. The study participants were relatively at an early stage in their condition, and thus, the insignificance in the EZD could be due to the resistance to the deterioration or reorganization capability of EZ in the early term. Statistical insignificance may also be due to the difference in the number of patients between the groups. It has been claimed that the differences in changes in EZ according to disease periods are due to refractive characteristics [30]. However, we consider it differently because our correlation results show a positive correlation between CST and EZD, as we mentioned above. Studies comparing the early and late term using precision tools because of the still-insufficient resolution of the SD-OCT devices may explain the EZ changes in more detail.
We observed no difference in HF, which is another blood–retinal barrier breakdown indicator, between the two groups. In one study, HFs were found to associated with ELM, which is a barrier, and a defective ELM caused more HF formation [8]. Accordingly, we found a positive association between ELM and HF in our correlation results. Chatziralli et al. [31] speculated that both the inflammatory response and hyperpermeability with subsequent lipoprotein extravasation play a role in the pathogenesis of HF in ME. As our patients were treatment-naive, they were considered to be in the relatively early term. It could be find a significant difference between groups in HF in later periods because a large amount of HF indicates long-standing inflammation [32, 33].
No significant difference was found in the subretinal fluid measures in our results. Tsujikawa et al. [34] reported that a breakdown of the barrier function of the ELM leads to movement of the intraretinal fluid into the subretinal space. Therefore, if the ELM damage is excessive, the subretinal fluid should be expected to be higher, but we did not find any association between ELM and SRF. Although our statistical comparison results were not significant, we considered the ischemic patients to have larger fluid volumes based on the SRF ranges. Therefore, other causes (e.g., RPE dysfunction leading to SRF) aside from ELM should be considered. Preserved foveal depression was attributed to the less leakage from the retinal capillaries and better absorption of leakage by the retinal pigment epithelium [35]. Although no significant difference was found, the loss of foveal depression was higher in the ischemic group in our results. The statistical insignificance could have been caused by the difference in the number of patients between the groups.
Retinal Müller glial (RMG) cells are structural backbones of the retinal architecture [36]. RMG degeneration causes the formation of cysts. In our results, we found significantly larger cyst diameters in the ischemic group. Moreover, disrupted ELM was positively and SRF was negatively correlated with CS in the correlation results. This finding suggests that RMG degeneration is greater in the ischemic group. Some studies have claimed that greater edema has a better response to treatment [15]. However, we consider the macular microstructure to be more predictive of the treatment response and prognosis because of the effect of ischemic status on the macular microstructure. In other words, more microstructure damage, more edema and poor prognosis can be expected due to ischemia.
In our regression analysis on the effect of microstructural changes on visual acuity, only DRIL and EZD were found to affect BCVA, but only in the ischemic group. It may reflect severe break of signal production and connection in ischemic conditions. There are many studies investigating the effect of macular microstructure on visual acuity in retinal vein occlusion. Different studies have highlighted different structures [4, 7, 37]. However, none of these studies classified retinal vein occlusions according to ischemia status when investigating visual acuity.
Many studies have been conducted on this issue. Although they have indicated that defect sizes are predictive of macular microstructure changes, they only evaluated an area of 1000 µm or 1500 µm and mostly at the posttreatment follow-up examination [4,5,6,7,8]. Thus, we examined a 3000-µm-wide macular area upon the patients’ first presentation of OCT measures to provide real quantitative information. This is one of the strengths of our study. Another strength of our study is that we grouped ME according to ischemia status and then examined it quantitatively, whereas other studies on this subject generally have not. For example, Parodi et al. [38] distinguished CRVO between ischemic and nonischemic based on the macular structure, but they did not measure the defects quantitatively.
The device capabilities should not be overlooked when evaluating all the results. A thickened retina or cystoid ME usually reduces the signal intensity of the OCT device. Shin et al. [18] considered that the reduced backscattering from the retinal layer is the result of a shadowing effect instead of a disruption of these structures. Measurements with devices free of these problems can give more reliable results.
This study has several limitations. The study is retrospective, has a relatively small cohort, different sample sizes and is nonrandomized in nature. OCT interpretations were performed by a single physician, and blinding was not possible. We did not perform vertical or radial scans, and consequently, our results were based only on the horizontal axis measurements. We did not separate the groups central or branch manner, because of the limited prevalence of CRVO. We did not perform a subtype analysis in each group. We did not perform a correlation study between the FAZ status and the macular microstructure on OCT.
In conclusion, ischemic RVOs cause greater edema and greater disruption of the macular microstructure, especially in terms of DRIL and ELM. We recommend an OCT examination of a central 3000 µm-wide zone of the macula microstructure for evaluating ME due to RVO because of more detailed and objective information. Large cohort studies examining a central 3000 µm-wide macular area in the follow-up and treatment responses are required to prioritize this information.
References
Rogers S, McIntosh RL, Cheung N, Lim L, Wang JJ, Mitchell P, Kowalski JW, Nguyen H, Wong TY, International Eye Disease Consortium (2010) The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology 17:313–319
Hayreh SS (1983) Classification of central retinal vein occlusion. Ophthalmology 90:458–474
Munk MR, Sacu S, Huf W, Sulzbacher F, Mittermüller TJ, Eibenberger K, Rezar S, Bolz M, Kiss CG, Simader C, Schmidt-Erfurth U (2014) Differential diagnosis of macular edema of different pathophysiologic origins by spectral domain optical coherence tomography. Retina 34(11):2218–2232
Mimouni M, Segev O, Dori D, Geffen N, Flores V, Segal O (2017) Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with macular edema secondary to vein occlusion. Am J Ophthalmol 182:160–167
Tang F, Qin X, Lu J, Song P, Li M, Ma X (2019) Optical cohorence tomography predictors of short-term visual acuity in eyes with macular edema secondary to retinal vein occlusion treated with intravitreal conbercept. Retina. https://doi.org/10.1097/IAE.0000000000002444
Liu H, Li S, Zhang Z, Shen J (2018) Predicting the visual acuity for retinal vein occlusion after ranibizumab therapy with an original ranking for macular microstructure. Exp Ther Med 15(1):890–896
Chan EW, Eldeeb M, Sun V, Thomas D, Omar A, Kapusta MA, Galic IJ, Chen JC (2019) Disorganization of retinal inner layers and ellipsoid zone disruption predict visual outcomes in central retinal vein occlusion. Ophthalmol Retina 3(1):83–92
Kang JW, Lee H, Chung H, Kim HC (2014) Correlation between optical coherence tomographic hyperreflective foci and visual outcomes after intravitreal bevacizumab for macular edema in branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol 252(9):1413–1421
Balci Ö, Öngör E (2004) Retinal Vein Occlusions. Turk J Ophthalmol 34:445–455
The Central Vein Occlusion Study Group N report (1995) A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. Ophthalmology 102:1434–1444
The Branch Vein Occlusion Study Group (1984) Argon laser photocoagulation for macular edema in branch vein occlusion. Am J Ophthalmol 98:271–282
Sun JK, Lin MM, Lammer J, Prager S, Sarangi R, Silva PS, Aiello LP (2014) Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol 132:1309–1316
Polat N (2018) Optical coherence tomographic comparison of naive macular edema due to ischemic and non-ischemic retinal vein occlusion. In: Retinal Vein Occlusion session-2018 EVRS meeting (Prague- CZECH REPUBLIC). https://www.evrs.eu/optical-coherence-tomographic-comparison-of-naive-macular-edema-due-to-ischemic-and-non-ischemic-retinal-vein-occlusion
Prasad PS, Oliver SC, Coffee RE, Hubschman JP, Schwartz SD (2010) Ultra wide-field angiographic characteristics of branch retinal and hemicentral retinal vein occlusion. Ophthalmology 117:780–784
Singer M, Tan CS, Bell D, Sadda SR (2014) Area of peripheral retinal nonperfusion and treatment response in branch and central retinal vein occlusion. Retina 34:1736–1742
Thomas AS, Thomas MK, Finn AP, Fekrat S (2019) Use of ischemic index on widefield flourescein angiography to characterize a retinal vein occlusion as ischemic or nonischemic. Retina 39(6):1033–1038
Ota M, Tsujikawa A, Murakami T, Yamaike N, Sakamoto A, Kotera Y, Miyamoto K, Kita M, Yoshimura N (2008) Foveal photoreceptor layer in eyes with persistent cystoid macular edema associated with branch retinal vein occlusion. Am J Ophthalmol 145(2):273–280
Shin HJ, Chung H, Kim HC (2011) Association between integrity of foveal photoreceptor layer and visual outcome in retinal vein occlusion. Acta Ophthalmol 89:e35–e40
Ota M, Tsujikawa A, Kita M, Miyamoto K, Sakamoto A, Yamaike N, Kotera Y, Yoshimura N (2008) Integrity of foveal photoreceptor layer in central retinal vein occlusion. Retina 28:1502–1508
Ko J, Kwon OW, Byeon SH (2014) Optical coherence tomography predicts visual outcome in acute central retinal vein occlusion. Retina 34:1132–1141
Wolf-Schnurrbusch UEK, Ghanem R, Rothenbuehler SP, Enzmann V, Framme C, Wolf S (2011) Predictors of short-term visual outcome after anti-VEGF therapy of macular edema due to central retinal vein occlusion. Invest Ophthalmol Vis Sci 52:3334–3337
Oishi A, Hata M, Shimozono M, Mandai M, Nishida A, Kurimoto Y (2010) The significance of external limiting membrane status for visual acuity in age-related macular degeneration. Am J Ophthalmol 150:27–32
Roberts P, Mittermueller TJ, Montuoro A, Sulzbacher F, Munk M, Sacu S, Schmidt-Erfurth U (2014) A quantitative approach to identify morphological features relevant for visual function in ranibizumab therapy of neovascular AMD. Invest Ophthalmol Vis Sci 55:6623–6630
Daruich A, Matet A, Moulin A, Kowalczuk L, Nicolas M, Sellam A, Rothschild PR, Omri S, Gélizé E, Jonet L et al (2018) Mechanisms of macular edema: beyond the surface. Prog Retin Eye Res 63:20–68
Hasegawa T, Ueda T, Okamoto M, Ogata N (2014) Presence of foveal bulge in optical coherence tomographic images in eyes with macular edema associated with branch retinal vein occlusion. Am J Ophthalmol 157(2):390–396
Xu HZ, Le YZ (2011) Significance of outer blood-retina barrier breakdown in diabetes and ischemia. Invest Ophthalmol Vis Sci 52:2160–2164
Sakamoto A, Tsujikawa A, Ota M, Yamaike N, Kotera Y, Miyamoto K, Kita M, Yoshimura N (2009) Evaluation of potential visual acuity in eyes with macular oedema secondary to retinal vein occlusion. Clin Experiment Ophthalmol 37:208–216
Ota M, Tsujikawa A, Murakami T, Kita M, Miyamoto K, Sakamoto A, Yamaike N, Yoshimura N (2007) Association between integrity of foveal photoreceptor layer and visual acuity in branch retinal vein occlusion. Br J Ophthalmol 91:1644–1649
Kang HM, Chung EJ, Kim YM, Koh HJ (2013) Spectral-domain optical coherence tomography (SD-OCT) patterns and response to intravitreal bevacizumab therapy in macular edema associated with branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol 251:501–508
Tao LW, Wu Z, Guymer RH, Luu CD (2016) Ellipsoid zone on optical coherence tomography: a review. Clin Exp Ophthalmol 44:422–430
Chatziralli IP, Sergentanis TN, Sivaprasad S (2016) Hyperreflective foci as an independent visual outcome predictor in macular edema due to retinal vascular diseases treated with intravitreal dexamethasone or ranibizumab. Retina 36:2319–2328
Segal O, Barayev E, Nemet AY, Geffen N, Vainer I, Mimouni M (2016) Prognostic value of hyperreflective foci in neovascular age-related macular degeneration treated with bevacizumab. Retina 36:2175–2182
Framme C, Wolf S, Wolf-Schnurrbusch U (2010) Small dense particles in the retina observable by spectral-domain optical coherence tomography in age-related macular degeneration. Invest Ophthalmol Vis Sci 51:5965–5969
Tsujikawa A, Sakamoto A, Ota M, Kotera Y, Oh H, Miyamoto K, Kita M, Yoshimura N (2010) Serous retinal detachment associated with retinal vein occlusion. Am J Ophthalmol 149(2):291–301
Kitagawa S, Yasuda S, Ito Y, Ueno S, Iwase T, Terasaki H (2018) Better prognosis for eyes with preserved foveal depression after intravitreal ranibizumab for macular edema secondary to central retinal vein occlusion. Retina 38(7):1354–1360
Franze K, Grosche J, Skatchkov SN, Schinkinger S, Foja C, Schild D, Uckermann O, Travis K, Reichenbach A, Guck J (2007) Muller cells are living optical fibers in the vertebrate retina. Proc Natl Acad Sci 104:8287–8292
Fujihara-Mino A, Mitamura Y, Inomoto N, Sano H, Akaiwa K, Semba K (2016) Optical coherence tomography parameters predictive of visual outcome after anti-VEGF therapy for retinal vein occlusion. Clin Ophthalmol 10:1305–1313
Battaglia Parodi M, Iacono P, Scaramuzzi M, Bandello F (2017) Outer retinal layer changes after dexamethasone implant for central retinal vein occlusion. Retina 37(10):1888–1895
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Polat, N., Yoloğlu, S. Optical coherence tomographic comparison of naive macular edema due to ischemic and nonischemic retinal vein occlusion. Int Ophthalmol 40, 2085–2093 (2020). https://doi.org/10.1007/s10792-020-01385-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-020-01385-6