Abstract
Purpose
To investigate the effect of posterior ocular hemodynamics on the retinal nerve fiber layer (RNFL), choroid thickness (CT) and central macular thickness (CMT) in patients with obstructive sleep apnea syndrome (OSAS) and to reveal the association with glaucomatous optic neuropathy.
Methods
The research was planned as a prospective, randomized study. The ophthalmic, retinal and posterior ciliary artery pulsatile index (PI) and resistive index (RI) were measured by colored Doppler sonography. RNFL thickness, CMT and CT were then measured by spectral-domain optical coherence tomography.
Results
Sixty subjects were divided into four groups—mild, moderate and severe OSAS and a control group. There were 16 subjects in the control group, 14 in the mild OSAS group, 15 in the moderate OSAS group and 15 in the severe OSAS group. Ophthalmic artery and central retinal artery PI and RI values of the OSAS patients did not show statistically significant difference than those of the control group, but posterior ciliary artery (PCA) PI and RI values were significantly higher. In addition, mean, superior and inferior RNFL thickness values were significantly lower than those in the control group. Moreover, the glaucoma prevalence of the OSAS patients in this study was 6.8% and all of these patients were in the severe OSAS group.
Conclusion
PI and RI values of the PCA, which supplies the optic nerve, show a linear increase as the apnea hypoxia index values in OSAS. As the grade of OSAS improves, this situation leads to a more serious ischemic optic neuropathy. Furthermore, the prevalence of glaucoma in this study is found to be higher in the severe OSAS group.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea syndrome (OSAS) involves recurring partial or complete obstruction of the upper airways during sleep [1, 2]. It is quite common in general society, ranging from 3 to 7%. Changes in various blood parameters caused by hypercapnia and hypoxia lead to a decrease in blood pH and increased blood NO, VEGF and endothelin levels and result in humoral and vascular changes, such as vascular endothelial dysfunction, vascular remodeling and hypertension [3,4,5]. OSAS is also associated with various ocular disorders, such as ischemic optic neuropathy, glaucoma and floppy iris syndrome [6,7,8,9,10,11,12,13,14,15]. Choroidal tissue possesses a diffuse vascularity, and although it is more exposed to systemic autonomic changes than other posterior ocular tissues, it easily adapts to these conditions [16]. In addition, endothelial levels are more dominant than NO in choroidal blood flow [17, 18]. For these reasons, choroidal evaluation by OCT may be a useful method, not only as a marker of changes in ocular tissue, but also as an early indicator of systemic vascular changes.
Materials and methods
This study was performed between December, 2016, and March, 2017, in line with the principles of the Declaration of Helsinki. Approval was granted by the local ethical committee. Written and verbal consent was obtained from the participants.
Forty-four patients diagnosed with OSAS formed the OSAS group, and 16 subjects with a low OSAS risk profile formed the control group. Subjects aged over 18 years old with Snellen’s best corrected vision acuity equal or greater than 20/25, diagnosed as OSAS for the first time, without a history of previous treatment for OSAS and with no other systemic disease were included in this study. Subjects with a history of previous eye surgery, intravitreal injection or any other ocular disease (such as ocular trauma, secondary glaucoma, uveitis) with a spherical equivalent greater than ± 2 diopters (D) and an axial length (AL) greater than 24.99 mm were excluded.
All patients were monitored using a polysomnography system (GRASS, AS40, Natus Medical, Ontario, Canada) at the Department of Pulmonary Diseases Sleep Laboratory. Subjects with a clinical suspicion of OSAS underwent standard nocturnal polysomnography. Subjects were monitored for at least 6 h from 10 pm in a silent, individual sleep laboratory. The AHI was assessed for each patient based on American Academy of Sleep Medicine standards. Subjects with AHI < 5 were classified as the control (C) group, those with AHI between 5 and 15 as the mild (M) OSAS group, those with AHI between 15 and 30 as the moderate (Mo) OSAS group and those with AHI ≥ 30 as the severe (S) OSAS group [19].
The mean and standard deviation values were calculated for parametric variables. Kolmogorov–Smirnov test yielded normal distribution for all groups (p > 0.05). Variance homogeneity was determined by Levene’s test. Parametric variables were analyzed by one-way analysis of variance (ANOVA) test. Scheffé’s test was used for post hoc comparisons of significant values. Sex differences among all groups were analyzed by Chi-square test. In addition, ANCOVA statistical test was performed to control the effect of intraocular pressure on mean RNFL decrease. In all analyses, the level of significance was set at 0.05. All statistical analyses were performed using SPSS for Windows version 22.0 (IBM Corp., Armonk, NY, USA).
Ophthalmological examination
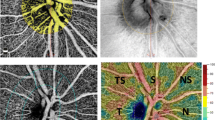
All subjects received a comprehensive ophthalmological examination including Snellen’s best corrected visual acuity, biomicroscopic anterior segment examination, dilated fundus examination, pachymetry, Goldman applanation tonometry, perimetry (Octopus 500) EZ perimeter (Interzeag AG, Schlieren, Switzerland) and peripapillary RNFL, macula and CT screening. CMT, RNFL and CT measurements were performed by advanced depth imaging Cirrus HD spectral OCT (Carl Zeiss Meditec, Dublin, CA) [19]. The pupils were not dilated for routine imaging. However, pupillary dilation was performed on subjects with very small pupil dimensions in order to obtain proper quality images when necessary. Images with a signal power equal or greater than 7 were evaluated. CT measurements using OCT were performed at 10 am in order to reduce the possibility of diurnal choroid fluctuation-related changes [20]. Central subfoveal choroid thickness (CSCT), temporal choroid thickness (TCT; 0.5, 1, and 1.5 mm from the fovea) and nasal choroid thickness (NCT; 0.5, 1, and 1.5 mm from the fovea) measurements were performed by two technicians (RY and AS) blinded to the patient data. Measurements that show difference by more than 10% between the two technicians were excluded from the study. Peripapillary RNFL thickness values were assessed in the mean, superior, temporal, nasal and inferior quadrants by using an optic disk 200 × 200 scan protocol.
Orbital color Doppler ultrasonography for retrobulbar blood flow was performed on all patients at the Department of Radiology (XDclear LOGIQ E9, GE Vingmed, Norway). A 9-MHz linear surface probe was used, and the measurements were performed between 12 and 13 pm. The patients were asked to lie down in a supine position. They were asked to shut their eyes and look toward the ceiling. Pulsatile index (PI) and resistive index (RI) were calculated from the OA, CRA and PCA (RI = peak systolic velocity (PSV) − end diastolic velocity/PSV).
Results
Sixty subjects, 16 forming the control group, 14 the mild OSAS group, 15 the moderate OSAS group and 15 the severe OSAS group, were included in the study. The patients with OSAS were subdivided on the basis of AHI values.
Participants’ age, sex and clinical data are shown in detail in Table 1. There was no statistically significant difference between the groups in terms of age, sex, pachymetry, visual acuity (VA), spherical equivalent (SE) values. However, body mass index (BMI), IOP and AHI values showed statistically significant difference among all groups. (p < 0.05).
Three of the patients in the severe OSAS group had a decrease in RNFL thickness values and, moreover, had IOP values over 21 mm-Hg. These patients’ visual field loss patterns were compatible with glaucoma. OCT and colored Doppler USG measurements of posterior ocular structures in the OSAS and control groups are shown in detail in Tables 2 and 3. RNFL thickness values decreased significantly in the severe OSAS group compared to the other groups, in terms of mean values and the superior and inferior quadrants (p = 0.026, p = 0.046, and p = 0.024, respectively), (Figs. 1, 2, 3). No significant difference was observed among the groups in terms of nasal and temporal quadrant RNFL thickness or CMT values (p > 0.05). PCA PI and RI indices were statistically significantly higher in the severe OSAS group compared to the other groups (p < 0.05), while no significant difference was observed among the groups in terms of OA and CRA PI and RI indices (p > 0.05), (Figs. 4, 5). No significant difference was observed among the groups in terms of temporal, subfoveal or mean CT values (p > 0.05) (Table 4), (Fig. 6).
Negative correlation was observed between AHI and mean, superior and inferior RNFL thickness values, while there was a positive correlation with IOP values (r = − 0.313, p = 0.015; r = − 0.3, p = 0.02; r = − 0.278, p = 0.032 and r = 0.472, p < 0.01, respectively), (Fig. 7). In addition, AHI was positively correlated with PCA PI and RI indices (r = 0.521, p < 0.01 and r = 0.428, p < 0.01, respectively), (Figs. 8, 9). IOP values were significantly positively correlated with PCA PI and RI indices (r = 0.336, p = 0.009 and r = 0.395, p = 0.002, respectively). However, when the RNFL thickness decrease was determined independent of IOP, the decrease was not statistically significant (p = 0.13) (Fig. 10). A negative correlation was determined between AHI and CT values, and this was not also statistically significant (p > 0.05). In addition, negative correlation was observed between PCA RI indice and mean CT value (r = 0.264, p = 0.042).
Discussion
The purpose of this study was to determine the changes in orbital flow in patients newly diagnosed as OSAS without any other systemic disease, and the effect of OSAS on RNFL, CMT and CT values measured by SD-OCT with the enhanced depth imaging modality.
OSAS causes various levels of neural, humoral and endothelial abnormality in vascular regulation and therefore represents an important risk factor in terms of disposition to cardiovascular and neurovascular diseases [21]. Repetitive airway obstructions occurring during sleep result in a series of hemodynamic, humoral and neuroendocrine responses by leading to hypoxia and hypercapnia [22]. Compared with the waking state, a decrease occurs in partial O2 and an increase in partial CO2 [23]. Since ocular blood pressure is a component of the cerebral circulation, the above cycle is repeated in the same way there [17]. However, this autoregulation mechanism causes an increase in vascular resistance indicating vascular damage by being insufficient in the event of persisting pathological processes. As a result, an increase will occur in PI and RI in order to preserve perfusion pressure, particularly in the PCA. Our study findings revealed an increase in RI and PI indices of PCA in the severe OSAS group compared to the control group.
This study considered OCT findings together with retrobulbar blood flow as a cause of changes in posterior ocular tissues. Previous studies have considered retrobulbar flows and OCT findings separately at various levels [24, 25]. To the best of our knowledge, this is the first study that retrobulbar blood flow is investigated as a major cause of changes in macular, choroid and RNFL thicknesses. RNFL thickness changes identified with optic nerve head analysis represent the most important part of these in terms of vascular activation. Hayreh et al. used angiofluoresceinography to show that the short PCA supplies the optic nerve head [26]. The optic nerve head is the area most affected when ocular perfusion pressure (OPP) decreases. OPP is defined as the difference between median arterial pressure (MAP) and IOP (OPP = MAP − IOP). Decreased MAP or increased IOP can reduce ocular perfusion pressure. Perfusion in ocular tissues gradually starts to decrease together with the severity of OSAS. If autoregulation continues, a continuous decrease will also continue in OPP [27]. Some have suggested that physiological nocturnal hypotension, in the presence of other vascular risk factors, can reduce optic nerve head circulation to below critical levels and thus be involved in the pathogenesis of anterior ischemic neuropathy and glaucoma [28,29,30,31]. Karakucuk et al. revealed a relation between OA and CRA RI values and the visual field in patients with OSAS, and determined a positive correlation between visual field MD values and OA RI and CRA RI values [25]. However, they determined no significant difference among the control and OSAS groups in terms of OA or CRA RI values. They explained this as indicating that pathological changes in the optic nerve occur during periods of apnea and the RI values measured sonographically in patients with OSAS being similar to those of the healthy group show that these values return to normal during the day. However, one significant limitation of that study was that it did not include RI values for the PCA, which has a smaller diameter compared to that OA and CRA and that is frequently affected in OSAS. In our study, although OA and CRA RI and PI values were similar in the patients with OSAS and the control group, we determined significantly elevated PCA RI and PI values. In addition, despite a negative correlation between mean, superior and inferior RNFL thickness values and PCA PI and RI values, we observed no significant relation with OA and CRA RI values.
In one of the few studies on this subject, Erdem et al. reported significantly higher flow rates in the PCA in patients with OSAS compared to a control group, but observed no significance between the groups in terms of flow rates in either OA or SRA [32]. These findings were similar to our own study. However, in contrast to our study, RI values measured in all three arteries in that study were similar to those of the control group.
Karakucuk et al. [25] reported a 12.9% (4 of 31 patients) level of glaucoma in patients with OSAS. They also determined a positive correlation between OA RI values and MD values. However, PCA flow values were not given in that study. We determined that PCA PI and RI values were negatively correlated with RNFL thickness values. Moreover, we determined positive correlation between IOP values and PCA PI and RI values. Similarly to the above study, 6.8% of the patients (3 of 34 patients) in this study had glaucoma and were in the severe OSAS group.
Previous studies on the effect of OSAS on CT among posterior ocular tissues have reported that choroidal tissue can be affected to various degrees depending on the duration of OSAS and associated hemodynamic complications. In a study of OSAS patients with a mean age of 41, Özge et al. reported greater submacular CT in an OSAS group compared to the control group [33]. In contrast, Zengin et al. determined no difference in CT between OSAS and healthy groups [34]. Another study reported choroidal thinning in patients with OSAS [35, 36]. The studies all show that choroidal vascular changes in patients with OSAS can develop over time. The difference between our study and the studies cited above is that they investigated changes in CT independent of retrobulbar blood flow. The mean ages of the OSAS and control groups in our study were 53 and 51, respectively. We observed similar mean, nasal, temporal, subfoveal and mean CTs in the OSAS group and the control group. However, there was a negative correlation between PCA RI values and mean choroid thickness. At the same time, we observed similar CMT values in the OSAS and control groups. Blood CO2 concentrations increase as a result of recurring apnea attacks in patients with OSAS. CO2 concentrations play an important role in ocular blood flow modulation, a component of the cerebral circulation. The dilator effect of CO2 is mainly limited to small vessels and the peripheral vascular bed [34]. Since the PCA has a smaller vascular diameter than the OA and CRA, it will probably be more affected by CO2 levels.
There are a number of limitations to this study. The most important of these is that retrobulbar flows and OCT findings could not be evaluated using an appropriate method during polysomnography. We performed Doppler and OCT measurements the day after polysomnography. We also think that larger patient numbers will increase the sensitivity of OCT data concerning choroid tissue in particular.
In conclusion, PI and RI values in the PCA were higher in the patients with OSAS. With its smaller diameter, PCA is more prone to stenosis and this situation may explain the optic nerve ischemia and the decrease in RNFL thickness. However, the absence of significant variation at CT and CMT values among the OSAS patients and the control group may be explained by normal PI and RI values in the OA and CRA. As a result, we observed significantly lower mean, superior and inferior RNFL values in the OSAS patients compared to the control group. We think that neurodegeneration emerging due to vascular dysregulation in OSAS leads to ischemic optic neuropathy. We therefore think that vascular regulation should not be overlooked in the diagnosis, follow-up and treatment of glaucomatous optic neuropathy.
References
Punjabi NM (2008) The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 5:136–143. https://doi.org/10.1513/pats.200709-155MG
Waller EA, Bendel RE, Kaplan J (2008) Sleep disorders and the eye. Mayo Clin Proc 83:1251–1261. https://doi.org/10.4065/83.11.1251
Caples SM, Garcia-Touchard A, Somers VK (2007) Sleep-disordered breathing and cardiovascular risk. Sleep 30:291–303
Arnaud C, Dematteis M, Pepin J-L et al (2009) Obstructive sleep apnea, immuno-inflammation, and atherosclerosis. Semin Immunopathol 31:113–125. https://doi.org/10.1007/s00281-009-0148-5
Lévy P, Pépin J-L, Arnaud C et al (2009) Obstructive sleep apnea and atherosclerosis. Prog Cardiovasc Dis 51:400–410. https://doi.org/10.1016/j.pcad.2008.03.001
Mojon DS, Hess CW, Goldblum D et al (1999) High prevalence of glaucoma in patients with sleep apnea syndrome. Ophthalmology 106:1009–1012. https://doi.org/10.1016/S0161-6420(99)00525-4
Li J, McGwin G, Vaphiades MS, Owsley C (2007) Non-arteritic anterior ischaemic optic neuropathy and presumed sleep apnoea syndrome screened by the sleep apnea scale of the sleep disorders questionnaire (SA-SDQ). Br J Ophthalmol 91:1524–1527. https://doi.org/10.1136/bjo.2006.113803
Bloomfield RL, Felts JH, Burkart JM, Cashwell FL (1987) Optic disc edema in a pickwickian man mimicking hypertensive crisis. J Clin Hypertens 3:27–30. https://doi.org/10.1016/S0161-6420(99)00525-4
Kloos P, Laube I, Thoelen A (2008) Obstructive sleep apnea in patients with central serous chorioretinopathy. Graefe’s Arch Clin Exp Ophthalmol 246:1225–1228. https://doi.org/10.1007/s00417-008-0837-0
Stein JD, Kim DS, Mundy KM et al (2011) The association between glaucomatous and other causes of optic neuropathy and sleep apnea. Am J Ophthalmol 152(989–998):e3. https://doi.org/10.1016/j.ajo.2011.04.030
Muniesa M, Sánchez-de-la-Torre M, Huerva V et al (2014) Floppy eyelid syndrome as an indicator of the presence of glaucoma in patients with obstructive sleep apnea. J Glaucoma 23:e81–e85. https://doi.org/10.1097/IJG.0b013e31829da19f
Mojon DS, Hedges TR, Ehrenberg B et al (2002) Association between sleep apnea syndrome and nonarteritic anterior ischemic optic neuropathy. Arch Ophthalmol 120:601–605
Sergi M, Salerno DE, Rizzi M et al (2007) Prevalence of normal tension glaucoma in obstructive sleep apnea syndrome patients. J Glaucoma 16:42–46. https://doi.org/10.1097/01.ijg.0000243472.51461.24
Purvin VA, Kawasaki A, Yee RD (2000) Papilledema and obstructive sleep apnea syndrome. Arch Ophthalmol (Chicago, Ill. 1960) 118:1626–1630
Glacet-Bernard A, les Jardins GL, Lasry S et al (2010) Obstructive sleep apnea among patients with retinal vein occlusion. Arch Ophthalmol 128:1533. https://doi.org/10.1001/archophthalmol.2010.272
Nickla DL, Wallman J (2010) The multifunctional choroid. Prog Retin Eye Res 29:144–168. https://doi.org/10.1016/j.preteyeres.2009.12.002
Mojon DS, Mathis J, Zulauf M et al (1998) Optic neuropathy associated with sleep apnea syndrome. Ophthalmology 105:874–877. https://doi.org/10.1016/S0161-6420(98)95030-8
Fuchsjäger-Mayrl G, Luksch A, Malec M et al (2003) Role of endothelin-1 in choroidal blood flow regulation during isometric exercise in healthy humans. Investig Opthalmol Vis Sci 44:728. https://doi.org/10.1167/iovs.02-0372
Epstein L, Kristo D, Strollo P Jr et al (2009) Adult obstructive sleep apnea task force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 5:263–276
Spaide RF, Koizumi H, Pozonni MC (2008) Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol 146:496–500. https://doi.org/10.1016/j.ajo.2008.05.032
Davies RJ, Crosby J, Prothero A et al (1994) Ambulatory blood pressure and left ventricular hypertrophy in subjects with untreated obstructive sleep apnoea and snoring, compared with matched control subjects, and their response to treatment. Clin Sci (Lond) 86:417–424. https://doi.org/10.7326/0003-4819-120-5-199403010-00005
Young T, Palta M, Dempsey J et al (1993) The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328:1230–1235
Deegan PC, McNicholas WT (1995) Pathophysiology of obstructive sleep apnoea. Eur Respir J 8:1161–1178. https://doi.org/10.1183/09031936.95.08071161
Yuvacı İ, Pangal E, Bayram N et al (2016) Evaluation of posterior ocular changes using enhanced depth imaging-optical coherence tomography in patients with obstructive sleep apnea syndrome. Arq Bras Oftalmol 79:247–252. https://doi.org/10.5935/0004-2749.20160070
Karakucuk S, Goktas S, Aksu M et al (2007) Ocular blood flow in patients with obstructive sleep apnea syndrome (OSAS). Graefe’s Arch Clin Exp Ophthalmol 246:129–134. https://doi.org/10.1007/s00417-007-0656-8
Hayreh SS (2015) Ocular Vascular Occlusive Disorders. Springer, Cham. https://doi.org/10.1007/978-3-319-12781-1
Singleton CD, Robertson D, Byrne DW, Joos KM (2003) Effect of posture on blood and intraocular pressures in multiple system atrophy, pure autonomic failure, and baroreflex failure. Circulation 108:2349–2354. https://doi.org/10.1161/01.CIR.0000097114.11038.26
Arimoto T, Toratani A, Ito K et al (1995) Relationship between sleep stage and blood pressure variability during apnea in patients with sleep apnea syndrome. Japanese J Thorac Dis 33:917–923
Graham SL, Drance SM, Wijsman K et al (1995) Ambulatory blood pressure monitoring in glaucoma. Ophthalmology 102:61–69. https://doi.org/10.1016/S0161-6420(95)31053-6
Leroy M, Van Surell C, Pilliere R et al (1996) Short-term variability of blood pressure during sleep in snorers with or without apnea. Hypertension 28:937
Shepard JW (1985) Gas exchange and hemodynamics during sleep. Med Clin North Am 69:1243–1264
Erdem CZ, Altin R, Erdem LO et al (2003) Doppler measurement of blood flow velocities in extraocular orbital vessels in patients with obstructive sleep apnea syndrome. J Clin Ultrasound 31:250–257. https://doi.org/10.1002/jcu.10171
Ozge G, Dogan D, Koylu MT et al (2016) Retina nerve fiber layer and choroidal thickness changes in obstructive sleep apnea syndrome. Postgrad Med 128:317–322. https://doi.org/10.1080/00325481.2016.1159118
Zengin MÖ, Öz T, Baysak A et al (2014) Changes in choroidal thickness in patients with obstructive sleep apnea syndrome. Ophthalmic Surg Lasers Imaging Retin 45:298–304. https://doi.org/10.3928/23258160-20140624-02
Bayhan HA, Aslan Bayhan S, İntepe YS et al (2015) Evaluation of the macular choroidal thickness using spectral optical coherence tomography in patients with obstructive sleep apnoea syndrome. Clin Exp Ophthalmol 43:139–144. https://doi.org/10.1111/ceo.12384
Xin C, Wang J, Zhang W et al (2014) Retinal and choroidal thickness evaluation by SD-OCT in adults with obstructive sleep apnea–hypopnea syndrome (OSAS). Eye 28:415–421. https://doi.org/10.1038/eye.2013.307
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was performed between December, 2016, and March, 2017, in line with the principles of the Declaration of Helsinki. Approval was granted by the Recep Tayyip Erdoğan University Ethical Committee (16.12.2016/83).
Informed consent
Written and verbal consent was obtained from the participants.
Rights and permissions
About this article
Cite this article
Fındık, H., Çeliker, M., Aslan, M.G. et al. The relation between retrobulbar blood flow and posterior ocular changes measured using spectral-domain optical coherence tomography in patients with obstructive sleep apnea syndrome. Int Ophthalmol 39, 1013–1025 (2019). https://doi.org/10.1007/s10792-018-0892-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-018-0892-4