Abstract
Purpose
To investigate the influence of preoperative biometric parameters on the accuracy of Haigis and SRKT formulae in predicting postoperative target refraction.
Methods
Retrospective analysis of 108 eyes (70 patients) underwent uneventful phacoemulsification surgery with implant of Alcon-SN60WF intraocular lens (IOL). Forty-five eyes were intentionally targeted to myopia (−0.75 to −1.25 dpt), while the others targeted between 0 and −0.75 dpt. Preoperative axial length and keratometry (K) were measured with optical biometry (LENSTAR—Haag-Streit). Postoperative spherical equivalent was assessed 3 ± 2 months after surgery.
Results
There is a significant correlation between the mean keratometry (K) and the Haigis–SRKT prediction differences (P < 0.001; r = 0.749). Linear regression indicates that a decrease of 1 diopter (D) on K implies an increase of 0.23 D on the difference between formulae prediction. K alone does not influence the prediction error for both formulas. The difference between the two formulae is dependent on K (r = −0.75; P < 0.01). Moreover, eyes with K <43.75 targeted at myopia (n = 23) showed a significant myopic shift of −0.26 ± 0.09 dpt (P < 0.05) with Haigis, but a hyperopic shift of 0.24 ± 0.09 dpt (P < 0.05) with SRKT.
Conclusion
Divergences between Haigis and SRKT formulae cause uncertainty in choosing the IOL. Our results indicate that, in eyes with lower preoperative K, an IOL targeted at myopia might result in a small, but significant myopic shift with the Haigis formula, while a hyperopic shift with the SRKT formula.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Precise prediction of postoperative refraction has become pivotal in the actual scenario of cataract surgery. In our days, patients demand independency of glasses after surgery.
Multifocal intraocular lenses (IOL) have been developed on early 1990s and can be used to improve far and near vision [1], but there are still several drawbacks, such as risk of macular diseases and consequent eccentric fixation [2], dry eye [3], and astigmatism [4], that might compromise the visual outcome with these lenses [5]. Besides, multifocal IOL implant is related to glare, visualization of halos, and lower contrast sensitivity that restricts its use [5].
In this context, one broadly used approach is the pseudophakic monovision, in which an IOL targeted for emmetropia is implanted in one eye, but intentional myopic overcorrection is targeted for the contralateral eye, providing near, intermediate, and distance vision [6,7,8]. There are reports of 92% patient satisfaction with monovision [8], and clinical trials comparing this strategy with multifocal IOLs show similar results for near and far visual acuity and patients spectacle dependency [6].
Achieving success with monovision requires careful preoperative planning. The ideal amount of intentional anisometropia is controversial; however, there are evidences that the difference between eyes should be around 1.5 D [9]. In any case, achieving intentional myopia should be rationally and precisely predicted, since the worst consequences for higher postoperative anisometropia are related to the loss of stereopsis [9].
Recently, high-accuracy technologies for ocular axial measurements using partial coherence interferometry, coupled with precise keratometry, and the new prediction formulae, particularly Haigis, SRKT and Holladay have provided improvements on IOL calculation [10, 11], but their reports usually show precision when the target is emmetropia [11]. In this study, we investigate the accuracy of IOL calculation based on Haigis and SRKT formulae in predicting postoperative myopic intentional overcorrection using Lenstar.
Materials and methods
Patients
We performed a retrospective study including 108 eyes of 70 patients who underwent uneventful cataract surgeries with implantation of SN60WF IOL (Alcon). All patients signed informed consent, agreeing with the procedure. The approval of local ethics committee for data analysis was obtained. Age ranged from 45 to 87 years old (mean 67.6); 43 patients were female.
We selected consecutive patients referred for surgery who had cataract with no other ocular pathology, such as diabetic retinopathy, glaucoma, history of ocular trauma or uveitis. Exclusion criteria were missing follow-up, lacking of medical chart data, presence of endophthalmitis, posterior capsule rupture or any other intra- or peri-operative complication, and postoperative macular edema affecting visual acuity.
Surgery and intraocular lens
Experienced surgeons performed all operations using the same standard phacoemulsification protocol (Infinity—Ozil 0.9 mm—Alcon) with the phaco-chop technique, through a 2.75 mm temporal clear corneal tunnel incision and a 5.0–5.5 mm capsulorhexis. Phacoemulsification was followed by in-the-bag implantation of the IOL.
All surgeries were performed in the Clinics Hospital of Ribeirão Preto (University of São Paulo) from June 2013 to October 2015.
In 45 eyes the postoperative refraction was intentionally target to myopia (−0.75 to −1.25 dpt) in order to obtain pseudophakic monovision. The other 63 eyes were targeted to emmetropia or slight myopia (0 and −0.75 dpt).
Postoperative refraction and visual acuity were assessed by the same examiner at the medical appointment 3 ± 2 months; after procedure this data was obtained through assessment of medical records.
Measurements
All biometry measurements were taken using the same optical biometer (Lenstar LS900—Haag-Streit) including axial length, keratometry, anterior chamber depth, lens thickness, central cornea thickness, and white to white. Also by Lenstar were obtained IOL power, refractive target using the Haigis and SRKT formulas. Only eyes with complete biometry measurements achieved by Lenstar were included in the study.
SN60WF biometric optimized constants used for IOL power calculations at the study were a0 = −0.576, a1 = 0.311, and a2 = 0.197 (Haigis formula); and A = 119.2 (SRK/T formula). (http://www.doctor-hill.com/physicians/lenstar-constants.htm).
Statistics
A paired t test was used to investigate whether the difference found between predicted and postoperatively observed spherical equivalent is significant.
Multivariate linear regressions (fitted as an ensemble) were explored between formulas prediction differences and preoperative biometric variables, including: axial length, corneal central thickness, aqueous depth, anterior chamber depth, lens thickness, astigmatism, white-to-white distance, and mean keratometry.
Regression parameters are reported along with bivariate correlation coefficient (r − Pearson) and P values. All calculations were performed using JMP-IN 10.0 (SAS).
Results
To verify the biometric variables could be involved in the discrepancy between the Haigis and SRKT predictions, multifactorial linear regressions along with Pearson correlations coefficients were calculated for the interrelations between all Lenstar measured variables and the intra-individual difference between Haigis and SRKT formulas predictions. Pearson coefficients are listed in Table 1.
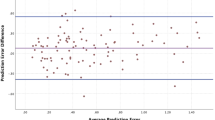
Noticeably, the correlation between the mean keratometry (K) and the Haigis–SRKT prediction differences is highly significant (P < 0.001; with r = 0.749) (Fig. 1a; Table 1). The linear regression parameter for variable K = −0.23 ± 0.01 indicates that a decrease of 1 diopter (D) on K implies an increase of 0.23 D on the difference between formulas prediction.
Scatter plots showing the interrelation between mean keratometry (K) and A Haigis–SRKT prediction difference; B SRKT prediction deviation to postoperative spherical equivalent; and C SRKT prediction deviation to postoperative spherical equivalent. Gray circles: eyes with difference Haigis–SRKT > 0.25 D, black circles: between −0.25 and +0.25 D, and white circles Haigis-SRKT < −0.25 D
Other variables showed significant bivariate correlations, but no significant multivariate linear regression parameters (Table 1).
However, only weak correlation is observed between overall prediction errors for the two formulas and K (Fig. 1b, c), showing that K alone does not influence the prediction error for both formulas. Nonsignificant correlations and estimates for multivariate linear regression were found for all other biometric variable.
Interestingly, if an intentional myopia is aimed (postoperative spherical equivalent aiming ≤ −0.75 D), the influence of K on Haigis formula prediction error is positive (myopic shift; r = −0.300; P = 0.049), but is negative for SRKT (hyperopic shift; r = 0.420; P = 0.004).
In eyes aimed myopia with K < 43.5, the mean prediction error for Haigis formula was 0.24 ± 0.09 D, but -0.27 ± 0.09 D for SRKT, but this difference was not observed for eyes with K ≥ 43.5 (Fig. 2).
Discussion
Pseudophakic monovision is broadly used in clinical practice with high rates of patient satisfaction [8], but this strategy is not free of patient disappointment with postoperative near and/or far visual acuity.
This retrospective study was designed to help surgeons planning a postoperative myopia with choosing the best IOL, particularly if Haigis and SRTK formula disagree. Our data indicate that in those cases, namely in eyes with lower mean keratometry, an IOL targeted at myopia might result in a small (around 0.25 D), but significant myopic shift using the Haigis formula, while with the SRKT formula, a hyperopic shift can be expected.
There are only a few reports, so far, discussing accuracy of both formulae. Recently, Sharma et al. [11] reported that Haigis formula, with optimized constants, is slightly better than SRKT, and Miraftab et al. [12] showed that Haigis formula is the preferred choice for eyes with anterior chamber depth >3.5 mm. Both studies compared formulas predictability for emmetropia, which is also very similar in our patients’ eyes aimed to emmetropia. Nevertheless, a difference between formulas predictability was only found in our data, when myopia is target for eyes with K < 43.5, and no influence of anterior chamber depth was found for prediction precision in our cases.
A recent study comparing Haigis and SRKT formulas for corneal astigmatism correction with toric intraocular lenses stated that Haigis is more accurate predicting the refractive outcome and astigmatism correction with these IOLs [13]. They included eyes with mean preoperative K of 44.82 ± 1.66 and apparently aimed emmetropia, but since the prediction error pointed in our study is corneal dependent, it could be interesting to investigate whether similar findings would be observed with toric IOLs aimed to myopia.
Interestingly, Lundqvist et al. [14] associated prediction errors of SRKT and Haigis formulas to patient gender, but they also indicate that flatter corneas, longer axial lengths, and deeper anterior chamber depth also worse prediction errors. Gender is not a significant factor determining prediction error in our cohort for neither formulas, but since authors also indicate that corneal steepness differed significantly between the sexes, we hypothesize that, in agreement with our data, the myopic shift found for Haigis and the hyperopic shift for SRKT could be, at least partially, due to the flat cornea in their cohort.
Moreover, Behndig et al. showed that Haigis performs better than SRKT in predicting postoperative refraction in women. Here again women had steeper corneas and shorter axial lengths than men, and keratometry seems to play an important role on the differences between the two formulas [15].
Despite the relatively small cohort included in these analyses, we were able to determine a strong correlation between preoperative keratometry and the difference among Haigs and SRKT predictions (Fig. 1) and to determine that, if an intentional myopia is aimed, the influence of keratometry on Haigis formula prediction error is positive, but is negative for SRKT (Fig. 2).
In summary, if surgeons aim to implant an IOL targeted at myopia in eyes with flatter corneas, they should consider a small, but significant myopic shift for Haigis formula and hyperopic shift with SRKT.
References
Petermeier K, Messias A, Gekeler F, Spitzer MS, Szurman P (2009) Outcomes of the Acrysof ReSTOR IOL in myopes, emmetropes, and hyperopes. J Refract Surg 25:1103–1109
Klyce SD, McDonald MB, Morales MU (2015) Screening patients with cataract for premium IOL candidacy using microperimetry. J Refract Surg 31:690–696
Woodward MA, Randleman JB, Stulting RD (2009) Dissatisfaction after multifocal intraocular lens implantation. J Cataract Refract Surg 35:992–997
Hayashi K, Manabe S, Yoshida M, Hayashi H (2010) Effect of astigmatism on visual acuity in eyes with a diffractive multifocal intraocular lens. J Cataract Refract Surg 36:1323–1329
Pepose JS (2008) Maximizing satisfaction with presbyopia-correcting intraocular lenses: the missing links. Am J Ophthalmol 146:641–648
Ito M, Shimizu K, Iida Y, Amano R (2012) Five-year clinical study of patients with pseudophakic monovision. J Cataract Refract Surg 38:1440–1445
Ito M, Shimizu K, Amano R, Handa T (2009) Assessment of visual performance in pseudophakic monovision. J Cataract Refract Surg 35:710–714
Finkelman YM, Ng JQ, Barrett GD (2009) Patient satisfaction and visual function after pseudophakic monovision. J Cataract Refract Surg 35:998–1002
Hayashi K, Yoshida M, Manabe S, Hayashi H (2011) Optimal amount of anisometropia for pseudophakic monovision. J Refract Surg 27:332–338
Holzer MP, Mamusa M, Auffarth GU (2009) Accuracy of a new partial coherence interferometry analyser for biometric measurements. Br J Ophthalmol 93:807–810
Sharma R, Maharajan P, Kotta S, Maharajan S (2014) Prediction of refractive outcome after cataract surgery using partial coherence interferometry: comparison of SRK/T and Haigis formulae. Int Ophthalmol 34:451–455
Miraftab M, Hashemi H, Fotouhi A, Khabazkhoob M, Rezvan F, Asgari S (2014) Effect of anterior chamber depth on the choice of intraocular lens calculation formula in patients with normal axial length. Middle East Afr J Ophthalmol 21:307–311
Eom Y, Song JS, Kim YY, Kim HM (2015) Comparison of SRK/T and Haigis formulas for predicting corneal astigmatism correction with toric intraocular lenses. J Cataract Refract Surg 41:1650–1657
Lundqvist O, Westin O, Koskela T, Behndig A (2015) Gender differences in refractive prediction in refractive lens exchange surgery. Eur J Ophthalmol 25:108–111
Behndig A, Montan P, Lundstrom M, Zetterstrom C, Kugelberg M (2014) Gender differences in biometry prediction error and intra-ocular lens power calculation formula. Acta Ophthalmol 92:759–763
Acknowledgments
This work was supported by FAPESP, Grant: 2012/19496‐3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict interests
The authors declare that they have no conflict interests.
Rights and permissions
About this article
Cite this article
Dalto, R.F., Ferreira, M.A., Queiroz, W. et al. Haigis and SRKT formulae accuracy for intentional myopic overcorrection. Int Ophthalmol 38, 1459–1463 (2018). https://doi.org/10.1007/s10792-017-0607-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-017-0607-2