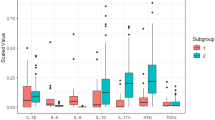
To determine the concentration of inflammatory mediators in the tear film of patients with keratoconus. Basal tears from patients with keratoconus and from normal controls were collected using a capillary tube. Patients with keratoconus were examined in a routine fashion, and keratometric readings were also taken from corneal topographic maps .The concentration of cytokines including Interleukin 6,10,1b and Interferon-γ was measured by enzyme-linked immunoadsorbent assay. Seventy-two subjects were enrolled in the study including 42 patients with keratoconus and 30 normals. Patients with keratoconus had significantly higher levels of Interlukin 6,1b and Interferon-γ (17.49 ± 1.92 pg/ml), (8.58 ± 1.15 pg/ml), and (33.33 ± 7.57 pg/ml) compared with control subjects (13.81 ± 1.71 pg/ml), (4.98 ± 0.52 pg/ml), and (22.99 ± 4.68 pg/ml), (P = 0.0001, P = 0.0001, and P = 0.0001). But the level of Interlukin-10 in keratoconus patients was significantly lower (6.07 ± 1.35 pg/ml) than controls (8.99 ± 0.70 pg/ml) (P = 0.0001). We realized that the proinflammatory markers (Interlukin-6,1-b and Interferon-γ) are over expressed, whereas the anti-inflammatory marker (Interlukin-10) is under expressed, indicating that the pathogenesis of keratoconus may involve complex chronic inflammatory events. Additional future studies will reveal the exact molecular and biochemical mechanisms that are required to better manage the disease and halt its progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Keratoconus (KC) is an asymmetric, progressive ectatic condition that may lead to significant visual impairment. Prevalence rate for KC varies widely across geographic areas and studies, ranging from 0.0002 to 2.34 %. It generally affects young adults with a mean age of onset at 15.4 years. The disorder typically progresses until the third or the fourth decade of life, and the factors that determine the progression or stabilization of the disease are not well characterized [1–3].
Although the exact etiology of KC is not well understood, the disease is currently thought to be triggered by various genetics, as well as environmental factors. KC is historically defined as a non inflammatory condition. The literature uncovers some compelling evidence of inflammatory molecules being presented in these patients [4]. Allergic history, atopy, corneal injury, eye rubbing, and rigid contact lens usage have been associated with the development of KC [4–7].
Recent studies have suggested pro-inflammatory factors as key to keratoconus pathogenesis based on their findings of elevated interleukin (IL)-6, Tumor Necrosis Factor (TNF)-α and matrix metalloproteinase (MMP)-9 in the tear fluid of KC patients. Moreover, increased binding of IL-1 by KC corneal fibroblasts has led another group to suggest a role for inflammation in the onset or progression of KC as well [8, 9].
Despite initial findings about the role of tear film inflammatory cytokines in the pathogenesis of KC, there are some controversial or even conflicting results. Furthermore, to the best of our knowledge, there are no detailed studies to have examined a range of cytokines to determine whether KC is associated with an imbalance in the repertoire of cytokines that regulate inflammatory and immune responses driven by subsets of T-helper cells, TH1, TH2, and TH17 in the corneal environment. To address this issue, we quantified one of the TH1 and TH2 cytokines (IFN-γ, IL-10 respectively) and two other inflammatory cytokines (IL-1b, IL-6) in the tear film of KC patients and compared the results with normal subjects.
Materials and methods
Subjects and examinations
We have designed a prospective case-controlled study in which 42 eyes of 42 KC patients (24 males and 18 female) aged between 14 and 42 years (mean, 24.09 ± 6.50 years), as well as 30 eyes of 30 normal subjects aged from 16 to 32 years (mean 24.43 ± 4.55 years; 13 males and 17 females) were enrolled. The criterion for the inclusion in the study was the presence of KC as determined by clinical examination and corneal topography.
Exclusion criteria included the existence of active inflammatory or infective systemic or ocular disease, current treatment with systemic or topical anti-inflammatory drugs, history of ocular surgery, and history of wearing any type of contact lens.
Patients with KC and normal subjects were recruited from Nikookary Eye Hospital,Tabriz, Iran, from December 2012 to September 2013. This study was approved by the Institutional Ethics Committee of Tabriz University of Medical Sciences and followed the tenets of the Declaration of Helsinki. Thus, written informed consent was obtained from all participants prior to their enrollment.
All examinations were performed by the same researcher (N.T). All eyes underwent ophthalmic examinations consisting of best-corrected visual acuity, slit-lamp examination, and corneal topography. The instrument employed for corneal topography was Allegro Oculyzer (Wavelight GmbH, Erlangen, Germany). The Belin / Ambrosio Enhanced Ectasia Display was used for KC screening.
Severity of KC was graded by the steepest keratometry measurement (K max) with <45 dioters(D) being mild, 45D ≤ Kmax ≤ 52D being moderate, and severe >52D).
Tear analysis
Tear samples were obtained atraumatically by glass capillary tube from the inferior tear meniscus. Care was taken to avoid touching the corneal and conjunctival surface. A new capillary tube was used for each tear sampling and for adequate tear collection. The procedure was repeated 2 to 3 times in each subject. All tear samples were obtained approximately at the same time of day and by the same researcher (N.T). Within 1 h of obtaining the samples, they were frozen and stored at −70 °C.
IL-10 was measured by immunoassay (Orgenium Laboratories. AviBion IL-10 ELISA kit). The assay employs an antibody specific for human IL-10 coated on a well plate. Standards, samples, and biotinylated anti-human IL-10 were pipetted into the wells and IL-10 present in the sample was captured by the antibody immobilized to the wells and by the biotinylated IL-10 specific detection antibody. After adding HRP-conjugated streptavidin into the wells, TMB substrate was used to color the development. The stop solution changed color from blue to yellow, and the intensity of the color was measured at 450 nm. The sensitivity of the assay was <2 pg/ml, with Inter-Assay Precision of <4 % and Intra-Assay Precision of <6 %.
IL-1b was quantitatively measured by ELISA method (Bender MedSytems GmbH). In this method, an anti–human IL-1b coating antibody was adsorbed on to microwells. IL-1b present in the samples binds with antibodies and biotin-conjugated anti-human IL-1b was added to the antibody coated wells before washing to remove unbound sample. Streptavidin- HRP was added and could bind wih biotin-conjugated anti-human IL-1b antibody. Following this step,substrate solution reactive with HRP was added to the wells. The reaction was terminated by adding acid absorbance was measured at 450 nm. The intra-assay and inter-assay coefficients of variation were 5.1 and 8.6 %, respectively, while the sensitivity was 0.3 pg/ml.
Il-6 was determined by ELISA (Orgenium Laboratories. AviBion) method with sensitivity of <7 pg/ml. In this method, human Il-6 specific antibodies were coated onto 96-well plates. Standards, samples, and biotinylated anti-human IL-6 were pipetted into the wells and IL-6 present in a sample was captured the antibody immobilized to the wells and by the biotinylated IL-6 specific detection antibody. After adding HRP—conjugated streptavidin into the wells, TMB (3, 32, 5, 52-tetramethylbenzidine) was used to visualize HRP enzymatic reaction. TMB was catalyzed by HRP to produce a blue color product that turned into yellow after adding acidic stop solution. The density of produced yellow color is proportional to the human IL-6 amount of the sample. The sensitivity of the assay was <7 pg/ml, with Inter-Assay Precision of <8.6 % and Intr-Assay Precision of <9.4 %.
IFN-γ concentration was measured using a sandwich Elisa kit (Boster Biological Technology.,LTD), which has sensitivity of 2.8–5.7 % and measurement ranging between 15.6–1,000 pg/ml.
Statistical analysis
Statistical analyses were carried out through SPSS version 18 for windows software. All data were described as mean ± standard deviation (SD). The Mann–Whitney U test and t test were performed in order to compare groups. The Spearman Correlation Coefficient was used to analyze the statistical significance between topographic indices and the concentration of cytokines. A value of P < 0.05 was considered to be statistically significant.
Results
Age- or gender-related differences were not statistically significant between KC and control groups (P = 0.8, P = 0.7, respectively). Fifteen (33.3 %) of the KC eyes were graded as mild, 15 (33.3 %) as moderate, and 15 (33.3 %) as severe. Some topographic characteristics of KC group are demonstrated in Table 1.
This study revealed that tear levels of IL-6, IL-1b, and IFN-γ among KC patients significantly increased compared with those of the control group subjects. In contrast, IL-10 level was significantly lower among KC patients. (Table 2)
Discussion
There have been extensive studies on biochemical and pathologic changes in structural and cellular levels of cornea in the literature, but specific mechanisms which lie behind the development of KC are not yet clearly understood. Recent studies support the idea that an inflammatory component plays a role in the pathogenesis of KC. Tissue degradation in KC involves expression of inflammatory mediators, pro-inflammatory cytokines, and matrix degrading enzymes [6–11]. A role for reactive oxygen species was also demonstrated among these patients [12, 13].
The presence of cytokines and chemokines in the healthy tear film has been demonstrated. However, the available literature on actual baseline cytokine concentration in tear fluid is scarce [14].
In this study, ocular surface healthiness in control subjects was specially checked based on history and biomicroscopic examinations. It may be hypothesized that following various inflammatory stimuli such as chronic eye rubbing, allergic eye diseases, ocular surface inflammation, and contact lens use; corneal epithelium may secrete inflammatory cytokines and degrading enzymes. These enzymes in turn cause tissue damage, corneal thinning, and increased risk for KC [15, 16]. To avoid the confounding effect of ocular surface inflammation on the level of tear film cytokines, we recruited patients without any inflammatory process and excluded any subject with a history of ocular allergy or atopy.
Since only a limited amount of tear fluid is obtainable without stimulation and measuring multiple cytokines is challenging, in the present study, tear samples were pooled from different cessions of the same patients.
To compare the findings of this study with similar ones, we reviewed the literature and found only a few instances where tear fluids were assessed for multiple cytokines. Jun, Carreno et al. used an immune-bead-based multiplex system, whereas Lema et al. have examined cytokines using ELISA [7, 8, 16]. In general, the conventional, standard ELISA (high sensitivity) is less sensitive than multiplex immuno-bead assays. Therefore, it is necessary to pool the tear samples from different KC and control subjects for these ELISA experiments.
We examined IL 1b, 6, 10, and IFN-γ in tear film of KC patient and compared the results with those of control subjects and found a significant increase in levels of IL 1b, 6 and IFN-γ in KC group.
Concerning increased levels of IL-6, the findings of this study are in line with those of of Lema, Balasubramanian and Jun [6–9]. Various cell types, including keratocytes, produce IL-6 in response to stimulation by IL-1 or TNF-α. It is, thus, conceivable that the tear may act as a reservoir of cytokines produced by the stroma, corneal, or conjunctival epithelium; alternatively, it may act as a vehicle of cytokines produced by the lacrimal gland or other epithelia of the ocular surface. Increased levels of these molecules may be sufficient to provoke slowly progressive ectasia [6, 8].
It is noteworthy that an increased level of IL-6 has also been reported in dry eye syndrome, allergic, and vernal keratoconjunctivitis [17, 18].
In this study, IL-1b level significantly increased in KC samples compared to the one among control subjects. This was in contrast with Jun et al. in which IL-1b was essentially unchanged but in accordance with Balasubramanian et al. [8, 9]. With regard to the KC corneas, Saghizadeh et al. demonstrated that the IL 1a, b mRNA was over three-folds less than those in the normal corneas. On the other hand, Zhou et al. reported that the IL-1 protein increased in the epithelium and endothelium of the KC corneas [19, 20].
In KC, keratocyte apoptosis has been suggested to contribute to the corneal thinning process. This indicates that enhanced IL-1b expression caused by the promoter polymorphism can induce the over expression of IL1-b protein, resulting in the increased corneal apoptotic activity observed in patients with KC [21, 22].
The increase in TH1-related cytokine; INF-γ in KC patients in this study was found to be significant in this study. This is in contrast with the findings of Jun et al. who reported that INF-γ in their control group was larger than that of KC patients despite no statistical significance. It is worth mentioning that there was not any correlation between concentration of IFN-γ and Mean K, TP, and Kmax (P = 0.75, P = 0.72, and P = 0.88 respectively). This finding indicates that as INF-γ is a non specific cytokine and in KC patients may be affected by many factors other than disease progression indices.
Of the TH2-related cytokines, the decrease in IL-10 was statistically significant in KC versus control subjects. This finding corresponds with that of Jun et al., but Lema et al. found similar mean values of IL-10 between KC and control groups. IL-10 plays crucial roles in the amplification of TH2 response, and the decreased levels suggest that TH2 responses may be dampened in KC patients [6, 8].
In addition to the variation observed among different studies, the range for each cytokine within a given study, including ours, is very large. Some of this variability could be due to individual-to-individual variation in cytokine levels and the extent of tearing even though samples are collected without direct stimulation. Furthermore, KC is a multifactorial disease, so geographical and ethnic discrepancies can affect the results.
One of the limitations of this study was that we have examined a few numbers of cytokines. Therefore, limited pathways were being investigated. Despite being the largest sample size among the related studies in the literature, the scope of our study was still small, and still there is a need for the expansion of the sample size in future studies to achieve more precise and accountable correlative results. Moreover, we have excluded gross inflammatory disease in this study, while some unknown and subclinical immunologic processes may exist and could confound the results.
Given the above-mentioned findings and limitations, this study revealed that the pro-inflammatory markers IL-6, IL-1b, and INF-γ are over expressed, whereas the anti-inflammatory marker IL-10 is under expressed. This finding is similar to the previous studies that analyzed the inflammatory response to contact lens wear and eye rubbing in KC patients [7, 14, 23]. Interestingly, in our patients, in spite of excluding contact lens wear or any ocular surface inflammation, similar results were obtained. To gain insight into the immunological processes that may contribute to these cytokine changes, it is important to track down the cellular sources of these changes. While historically many studies have focused on the cornea itself, the tear fluid changes imply that the conjunctiva and lacrimal gland may have some paracrine effects in this disease. This study also raises questions and promising horizons about the feasibility of prescribing medical treatments that modulate the inflammatory response in halting the progression of disease.
Conclusion
In summary, KC is a complex heterogeneous disease in which altered corneal structures and functions may be related to multiple factors. Our data confirm that there is a complex imbalance between pro-inflammatory and anti-inflammatory cytokines in KC patients. Hence, further prospective studies are required to elucidate the clinical role of inflammatory cytokines in the pathophysiology of KC and to establish an exact correlation between KC and ocular surface subclinical inflammation.
References
Rabinowitz YS (1998) Keratoconus. Surv Ophthalmol 42:297–319
Gordon-Shag A, Millodot M, Shneor E (2012) The epidemiology and etiology of KC. Int J Keratoco 1:7–15
Rabinowitz YS (2003) The genetics of KC. Ophthal Clin N Am 16:607–620
Lema I, Duran JA (2005) Inflammatory molecules in the tears of patients with KC. Ophthalmology 112:654–659
Cristina Kenney M, Brown DJ (2003) The cascade hypothesis of KC. Contact Lens Anterior Eye 26:139–146
Zadik K, Barr JT, Edrington TB, Everett DF, Jameson M, McMahon TT, Shin JA, Sterling JL, Wagner H, Gordon MO (1998) Baseline findings in the Collaborative Longitudinal Evaluation of KC (CLEK) study. Invest Ophthalmol Vis Sci 39:2537–2546
Lema I, Sobrino T, Duran JA, Brea D, Diez Feijoo E (2009) Subclinical KC and inflammatory molecules from tears. Br J Ophthalmol 93:820–824
Jun AS, Cope L, Speck C, Feng X, Lee S, Meng H, Hamad A, Chakravarti S (2011) Subnormal cytokine profile in the tear fluid of KC patients. PloS One 6:e16437
Balasubramanian SA, Mohan S, Pye DC, Willcox Perry (2012) Proteases, proteolysis and inflammatory molecules in the tears of people with KC. Acta Ophthalmol 90:e303–e309
Nemet AY, Vinker S, Bahar I, Kaiserman I (2010) The association of KC with immune disorders. Cornea 29:1261–1264
Ghosh A, Zhou L, Ghosh A, Shetty R, Beuerman R (2013) Proteomic and gene expression patterns of KC. Indian J Ophthalmol 61(8):389–391
Dudakova L, Liskova P, Trojek T, Palos M, Kalasova S, Jirsova K (2012) Changes in lysyl oxidase (LOX) distribution and its decreased activity in KC corneas. Exp Eye Res 104:74–81
Chwa M, Atilano SR, Reddy V, Jordan N, Kim DW (2006) Increased stress-induced generation of reactive oxygen species and apoptosis in human KC fibroblasts. Invest Ophthalmol Vis Sci 47:1902–1910
Balasubramanian SA, Pye DC, Willcox DP (2013) Effects of eye rubbing on the levels of protease, protease activity and cytokines in tears: relevance in KC. Clin Exp Optom 96:214–218
Fodor M, Kolozsvari BL, Petrovski G, Kettesy B, Gogolak P, Rajnavolgyi E, Ujhelyi B, Laszlo M, Petrovski B, Georgina S, Berta A, Facsko A (2013) Effect of contact lens wear on the release of tear mediators in KC. Eye Contact Lens 39:147–152
Carreno E, Enriquez-de-Salamanca A, Teson M, Garcia-Vazquez C, Stern ME (2010) Cytokine and chemokine levels in tears from healthy subjects. Acta Ophthalmol 88:e250–e258
Yoon KC, Jeong IY, Park YG, Yang SY (2007) Interleukin-6 and tumor necrosis factor-alpha levels in tears of patients with dry eye syndrome. Cornea 26:431–437
Leonardi A, Curnow SJ, Zhan H, Calder VL (2006) Multiple cytokines in human tear specimens in seasonal and chronic allergic eye disease and in conjunctival fibroblast culture. Clin Exp Allergy 36:777–784
Saghizadeh M, Chwa M, Aoki A, Lin B, Pirouzmanesh A, Brown DJ, Ljubimov AV, Kenny MC (2001) Altered expression of growth factors and cytokines in KC, bullous keratopathy and diabetic human corneas. Exp Eye Res 73:179–189
Zhou L, Yue BY, Twining SS, Sugar J, Feder RS (1996) Expression of wound healing and stress-related proteins in KC corneas. Curr Eye Res 15:1124–1131
Kaldawy RM, Wagner J, Ching S, Seigel GM (2002) Evidence of apoptotic cell death in KC. Cornea 21:206–209
Mikami T, Meguro A, Teshigawara T, Takeuchi M, Uemoto R, Kawagoe T, Nomura E, Asukata Y, Ishioka M, Iawasaki M, Fukagawa K, Konomi K, Shimazaki J, Nishida T, Mizuki N (2013) Interleukin 1 beta promoter polymorphism is associated with KC in a Japanese population. Mol Vis 19:845–851
Schultz CL, Kunert KS (2000) Interleukin-6 levels in tears of contact lens wearers. J Interferon Cytokine Res 20:309–310
Acknowledgments
This study was funded and supported by the vice-chancellor of the Research Center of Tabriz University of Medical Sciences, Tabriz, Iran. The authors also extend their gratitude to patients for their participation in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sorkhabi, R., Ghorbanihaghjo, A., Taheri, N. et al. Tear film inflammatory mediators in patients with keratoconus. Int Ophthalmol 35, 467–472 (2015). https://doi.org/10.1007/s10792-014-9971-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-014-9971-3