Abstract
Alzheimer’s disease (AD) is the most prevalent neurodegenerative disease that results in progressive dementia, and exhibits high disability and fatality rates. Recent evidence has demonstrated that neuroinflammation is critical in the pathophysiological processes of AD, which is characterized by the activation of microglia and astrocytes. Under different stimuli, microglia are usually activated into two polarized states, termed the classical ‘M1’ phenotype and the alternative ‘M2’ phenotype. M1 microglia are considered to promote inflammatory injury in AD; in contrast, M2 microglia exert neuroprotective effects. Imbalanced microglial polarization, in the form of excessive activation of M1 microglia and dysfunction of M2 microglia, markedly promotes the development of AD. Furthermore, an increasing number of studies have shown that the transition of microglia from the M1 to M2 phenotype could potently alleviate pathological damage in AD. Hence, this article reviews the current knowledge regarding the role of microglial M1/M2 polarization in the pathophysiology of AD. In addition, we summarize several approaches that protect against AD by altering the polarization states of microglia. This review aims to contribute to a better understanding of the pathogenesis of AD and, moreover, to explore the potential of novel drugs for the treatment of AD in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a multifactorial neurodegenerative disease that is caused by genetic and non-inheritable components. Most cases are sporadic, as only 5–20% of AD cases have familial history. Intracellular neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau and extracellular deposits of amyloid β (Aβ) are considered to be the two key hallmarks of AD. Importantly, more and more evidence has demonstrated that neuroinflammation is also a crucial player in the onset and development of AD, which is characterized by astrocytic and microglial activation. Excessive neuroinflammation promotes the generation of inflammatory mediators such as cytokines and chemokines, which results in neuronal injury and neurodegeneration. A noticeable neuroinflammatory response has been detected in both sporadic and familial AD, as well as in transgenic models of the disease (Akiyama et al. 2000). In vivo studies have shown that Aβ treatment activates microglia and aggravates inflammatory responses by binding to innate immune receptors on microglia (Stewart et al. 2010; Liu et al. 2012a, b; Wirz 2013). Taken together, these observations have formed the inflammatory cascade hypothesis of Alzheimer’s disease.
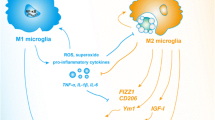
In addition to being crucial cellular mediators of neuroinflammatory processes, microglia play a vital role in overall brain maintenance, and participate in inflammatory and immune responses in the central nervous system (CNS) (Lawson et al. 1992; Gehrmann et al. 1995). Microglia display neurotoxic or neuroprotective functions in the CNS depending upon the phenotypic polarization, thereby acting as a ‘double-edged sword’. Microglia are usually activated into two polarized states, termed the classical ‘M1’ phenotype and the alternative ‘M2’ phenotype. M1 microglia are considered to enhance pro-inflammatory responses by secreting large numbers of inflammatory cytokines that lead to tissue damage (Orihuela et al. 2016). In contrast, M2 microglia exert neuroprotective effects by inhibiting neuroinflammation, thereby promoting tissue repair (Mantovani et al. 2004; Edwards et al. 2006).
In the early stage of AD, even before the formation of senile plaques, activated microglia exert protective effects by reducing Aβ deposition (Solito and Sastre 2012), alleviating tau hyperphosphorylation (Jiang et al. 2016) and secreting neurotrophic factors (Fillit et al. 1991). However, during the development of AD, the excessive activation of microglia promotes inflammatory injury and aggravates AD-related pathological damage (Mcdonald et al. 1997; Lue et al. 2010). Recent studies have revealed that microglial polarization may be a promising therapeutic target in AD. In both in vivo study and in vitro studies, experimentally inducing microglial polarization towards the neuroprotective M2 phenotype, has been shown to significantly alleviate neuroinflammatory responses and ameliorate pathological damage in AD models (Jiang et al. 2016; Oh et al. 2017; Yu et al. 2017; Zhang et al. 2017). Nevertheless, the regulatory mechanisms involved in microglial polarization are not well known. Hence, the current article aims to review the role of microglial polarization in AD, and to summarize potential microglial-based therapeutic targets for treating AD.
Microglia in the CNS
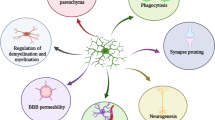
The micro-environment of the CNS is mainly composed of neurons and glial cells, the latter of which includes microglial cells, astrocytes and oligodendrocytes. Derived from embryonic mesodermal-marrow precursor cells, microglia provide potent neurotrophic and neuroprotective effects in the CNS (Hume et al. 2002). Additionally, as the major immunological effector cells in the CNS, microglia exhibit scavenger-like immune activity in terms of inflammatory and immune responses. Under physiologically healthy conditions, microglia have small cell bodies, slender branching, and do not engage in phagocytosis (Nimmerjahn et al. 2005). Under several pathological conditions, activated microglia are rapidly converted into an amoeba-like large morphology, engage in synaptic pruning and migrate to the lesion region to provide strong phagocytic activities (Nakamura et al. 1999). Consequently, abundant pro-inflammatory cytokines such as tumour necrosis factor-α (TNF-α), interferon γ (IFN-γ) and interleukin-1 (IL-1) are generated, and lead to neuroinflammatory responses.
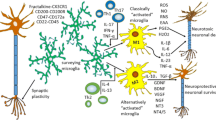
Microglia act like a ‘double-edged’ sword to provide beneficial or harmful effects in the CNS, depending on the phenotypic polarization. Microglia are usually activated into two polarized states, termed the classical ‘M1’ phenotype and the alternative ‘M2’ phenotype. The M1 phenotype is induced by immune stimulation such as from lipopolysaccharides (LPSs) and INF-γ, which induces microglia to secrete large amounts of pro-inflammatory factors including nitric oxide (NO), TNF-α, IL-6 and IL-lβ (Ponomarev et al. 2007; Orihuela et al. 2016). Hence, the classic activation state of the M1 phenotype promotes neuroinflammatory responses. In contrast, in response to inflammatory stimuli such as IL-4 and IL-13, microglia are converted into the M2 phenotype (Tang and Le, 2016). M2 microglia display beneficial effects by releasing neuroprotective cytokines such as IL-10, transforming growth factor-β (TGF-β) and insulin-like growth factor 1 (IGF-1). Additionally, the alternative activation state of the M2 phenotype inhibits excessive neuroinflammation induced by M1 microglia, which leads to tissue repair and reestablishment (Mosser 2003; Mantovani et al. 2004; Edwards et al. 2006; Mosser and Edwards 2008). During the clearance of apoptotic cells or myelin fragments by M2 microglia, M2 markers such as arginase-1 (Arg1) and mannose receptor (CD206) are generated to aid in tissue reconstruction and synaptic remodelling (Boche et al. 2006; Suh et al. 2013). M2 activation is divided into additional sub-categories. Accompanied by different markers, the expressions of mannose receptor (MRC1) and Arg1 are considered to be indicative of M2a activation. In contrast, elevated expressions of CD86 and the major histocompatibility complex II (MHCII) receptor are consistently observed during M2b activation. Finally, the M2c phenotype is characterized by amplified expressions of transforming growth factor β1 (TGFβ1) and sphingosine kinase 1 (SPHK1) (Mosser 2003; Mosser and Edwards 2008). Despite these established subclassifications, the functions of M2 microglia sub-phenotypes are not well understood and require future investigations for their further elucidation.
Microglia in AD
Depending on the polarized state, microglia manifest dual toxic and protective roles in the process of AD. Previous studies have shown that moderate microglial activation alleviates AD pathological damage and reduces Aβ levels via phagocytosis and induction of tissue repair. However, excessive neuroinflammation releases toxins such as nitric oxide (NO) and pro-inflammatory cytokines/chemokines, which exacerbates neuronal injury and, consequently, accelerates AD progression (Michaud et al. 2013).
Toxic function of microglia in AD
It has been reported that excessive activation of M1 microglia aggravates the pathological damage in AD via multiple mechanisms. First, M1 microglia promote the production of large amounts of pro-inflammatory cytokines such as TNF-α, IL-1 and macrophage inflammatory protein-1 (MIP-1) that consequently exacerbate neuronal damage, Aβ deposition (Mcdonald et al. 1997; Lue et al. 2010; Krabbe et al. 2013) and cholinergic neuronal injury (Raleigh 2006; Wyss-Coray 2006). Second, aggregation of activated microglia has been shown to surround NFTs at both early and late stages of AD (Sheng et al. 1997; Sheffield et al. 2000). The inflammatory cytokines secreted by M1 microglia such as IL-1, IL-6 and fractalkine (CX3CL1) modulate the structure and function of tau, moreover, promote tau hyperphosphorylation and formation of NFTs (Bhaskar et al. 2010). In addition, during the progression of AD, the persistent activation of M1 microglia releases many neurotoxic substances, such as pyridinedicarboxylic acid and amines, that result in neuronal excitotoxicity (Giulian et al. 1995; Leipnitz et al. 2007). Furthermore, more and more evidence has suggested that microglial phagocytosis of Aβ is significantly inhibited during AD because of diminished expression of specific proteins in microglia/macrophages, including scavenger receptor (SR-A), receptor for advanced end glycation products (RAGE) and insulin degrading enzyme (IDE) (Koenigsknechttalboo and Landreth 2005). Other M1 microglia-mediated pro-inflammatory cytokines, such as IFN-γ and TNFα, not only inhibit uptake of Aβ, but also block internalized Aβ degradation (Yamamoto et al. 2008; Michelucci 2009). Interestingly, recent studies have found that excessive M1 microglial activation facilitates the spread of Aβ and tau. Venegas et al. (2017) found that apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) specks is released by microglia. ASC specks promote the production of Aβ oligomers and Aβ aggregation, and injection of ASC specks into hippocampus aggravates the spread of Aβ in different brain regions in APPSwePSEN1dE9 mice, which is considered to be a key hallmark of AD progression. Another study used an adeno-associated virus-based model that exhibited rapid tau propagation from the entorhinal cortex to the dentate gyrus within 4 weeks. The results of this study showed that depleting microglia markedly prevented the propagation of tau and reduced tau spreading from the entorhinal cortex to the hippocampal region, indicated that microglia exacerbated tau spreading via exosome secretion (Asai et al. 2015).
Neuroprotective function of microglia in AD
The alleviated scavenging activity of Aβ has been reported to be the main reason for the progression of pathology in the majority of sporadic AD cases (Sollvander et al. 2016). In the early stage of AD, even before the formation of senile plaques, activated microglia exert protective effects in Aβ deposition by phagocytosis and releasing Aβ-degrading enzymes (Solito and Sastre 2012). Pro-inflammatory M1 microglia appear to be impaired in their ability to clear Aβ; in contrast, M2 microglia have been shown to be efficient phagocytes. Many studies have shown that Aβ activates microglia and neuroinflammation in the CNS (D’Andrea et al. 2004; Liu et al. 2018), and that misfolding and aggregated Aβ protein can be phagocytosed and cleared by activated microglia. Scavenger receptors are a group of evolutionally conserved proteins that are expressed on the surface of microglia and act as receptors for Aβ. SCARA-1 (scavenger receptor A-1), CD36 and RAGE are some examples of scavenger receptors (Wilkinson and El 2012). Simulating M2 activation with cytokine IL-4 and IL-10 effectively blocks lipopolysaccharide-induced inhibition of Aβ phagocytosis (Koenigsknechttalboo and Landreth 2005; Michelucci, 2009; Kawahara et al. 2012). Treatment with IL-4, a strong inducer of M2 polarization, facilitates the degradation of internalized Aβ by phagosomes and lysosomes (Majumdar et al. 2007; Balce et al. 2011). Different subtypes of M2 microglia have been shown to exhibit unique functions. IL-4-induced M2a microglia have significant Aβ scavenging activity, while M2c microglia—induced by IL-10, TGFβ1 and glucocorticoids—may play a crucial role in tissue repair (Mecha et al. 2015). Additionally, a previous study demonstrated that M2 microglial products prevented inter-neuronal transfer of Aβ and reduced the spread of Aβ in the AD brain (Sackmann et al. 2017). Furthermore, M2 microglia markedly alleviate neuroinflammatory responses and prevent tau hyperphosphorylation, which ameliorates pathological damage in AD (Jiang et al. 2016). Additionally, M2 microglia provide neuroprotective effects by releasing anti-inflammatory cytokines such as IL-10 and TGF-β (Colton 2009), and secreting neurotrophic factors such as nerve growth factor (NGF). M2 microglia also potently suppress the generation of neuronal toxins (e.g., glutamic acid), which promotes tissue repair and synaptic regeneration (Fillit et al. 1991; Lambeth 2004; Gandy and Heppner 2013).
Microglia-based therapy in AD
It has been reported that inhibitors of excessive neuroinflammatory responses alleviate pathological damage in AD. In vitro studies have shown that non-selective inhibitors of cyclooxygenase (COX) can preferentially decrease the levels of the highly amyloidogenic Aβ1–42 peptide. In murine models of AD, similarly, non-selective NSAIDs reduce Aβ plaque deposition in the brains of rodents (Pasinetti 2002). A prospective study demonstrated that the long-term use of NSAIDs might protect against AD, but not against vascular dementia (In et al. 2001). Other inflammatory regulators have also been shown to provide neuroprotective functions in AD, such as phosphodiesterases (PDEs) (Zhang et al. 2013; Guo et al. 2017a, b), histone deacetylase (Ke et al. 2011) and NADPH oxidase (NOX) (Laibaik et al. 2008). These controversial results were provided by the Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT). The authors found that anti-inflammatory drugs (i.e., naproxen and celecoxib) delayed cognitive decline in slow decliners while accelerating decline in fast decliners (Ji et al. 2018). Besides, more and more evidence has revealed that modulators of microglial phenotypes may be a promising therapeutic approach for the treatment of AD.
AMPK-signalling agonists
AMP-activated protein kinase (AMPK) plays a crucial role in mitochondrial biogenesis, lipid metabolism and inflammation (Giri et al. 2004). AMPK signalling is activated in response to stresses that deplete cellular ATP supplies, such as low glucose, hypoxia and ischemia. Moreover, AMPK signalling can be activated by upstream AMPK kinases, such as LKB1 and calmodulin-dependent protein kinase β (CaMKKβ). Recent evidence has demonstrated that AMPK signalling is involved in microglial polarization. For instance, peroxisome proliferator-activated receptor γ (PPARγ), a ligand‐activated transcription factor, regulates microglial polarization and inflammatory responses, as well as glucose and lipid metabolism (Zhao et al. 2016; Ji et al. 2018). Many studies have revealed that treatment with PPARγ agonists reduces CNS Aβ levels and alleviates AD pathology (Escribano et al. 2010; Mandrekarcolucci et al. 2012; Yamanaka et al. 2012). Additionally, PPARγ agonists have been considered to efficiently provide neuroprotective properties via increasing mRNA levels of the M2 marker, YM1 (Mandrekarcolucci et al. 2012), as well as the scavenger receptor, CD36 (Yamanaka et al. 2012). The modulatory effect of PPARγ in microglial polarization might be due to the activation of the LKB1–AMPK signalling pathway (Ji et al. 2018). Furthermore, the LKB1 inhibitor, radicicol, or knockdown of LKB1 prevented AMPK-signalling activation and the T0070907-induced M1-to-M2 phenotypic shift in LPS-treated BV2 microglial cells (Ji et al. 2018). Another study explored the effects of the CaMKKβ inhibitor, STO-609, and CaMKKβ siRNA. The results demonstrated that CaMKKβ promoted downstream betulinic acid (BA)-mediated AMPK activation and microglial M2 polarization. Pre-administration of the AMPK inhibitor blocked M2 microglial polarization in the cerebral cortex of LPS-injected mice brains (Li et al. 2018). In addition, telmisartan, an angiotensin II type 1 receptor blocker, promoted cerebral AMPK activation and M2 microglial gene expression in a mouse model of LPS-induced neuroinflammation (Xu et al. 2015). Taken together, AMPK-signalling agonists have potential positive effects in the regulation of microglial polarization and neuroinflammatory responses, which may represent promising therapeutic approach in AD.
mTOR-signalling inhibitors
As a self‐digestion process, cell autophagy degrades useless proteins and organelles through the autophagy–lysosome pathway. Numerous studies have revealed that moderate autophagy exerts protective effects in several neurodegenerative diseases, including AD. However, excessive and uncontrolled autophagy leads to cellular injury and promotes the development of disease (Zare-Shahabadi et al. 2015). As a vital regulatory signalling pathway in cellular autophagy, the mechanistic target of rapamycin (mTOR) pathway inhibits autophagy when activated by upstream kinases such as AKT and MAPK. Recent studies have indicated that mTOR pathway inhibitors promote M2 macrophage polarization (Saxton and Sabatini 2017). Zhu et al. (2014) found that tuberous sclerosis complex 1 (TSC1) facilitated M2 properties by mTOR-dependent CCAAT/enhancer-binding protein-β pathways, and also showed that mTOR inhibition promoted M1 to M2 macrophage polarization. In a model of spinal cord injury (SCI) and in an LPS-treated BV-2 cell model, salidroside (Sal) pre-treatment significantly induced M2 microglia activation and M1 polarization inactivation via enhanced AMPK phosphorylation and reduced mTOR phosphorylation; this effect was reversed by CQ, a specific lysosome inhibitor, that is commonly used to block autophagic flux (Wang et al. 2017). In a model of vascular dementia, paeoniflorin (PF), a cannabinoid receptor 2 (CB2R) agonist, facilitated an M1 to M2 phenotypic transition in microglia/macrophages in the hippocampus of rats; this manipulation consequently improved animal learning and memory. Moreover, PF treatment significantly inhibited the mTOR/NF-κB pro-inflammatory pathway and enhanced the PI3K/Akt anti-inflammatory pathway (Luo et al. 2018). Similarly, the mTOR inhibitor, everolimus (RAD001), inhibited mTORC1 activity and ameliorated VaD by promoting the M1 to M2 microglial shift (Huang et al. 2017). In a mouse model of traumatic brain injury (TBI), Huang et al. (2017) found that miR-124-3p promoted the generation of anti-inflammatory M2 microglia and blocked neuronal inflammation by inhibiting mTOR signalling. Additionally, by crossing Raptor loxed (Raptorflox/flox) mice with CX3CR1CreER mice, blocking the mTORC pathway significantly reduced the post-stroke lesion size by decreasing CNS neuroinflammatory responses via a shift in microglial phenotype from M1 to M2 (Li et al. 2016). In spite of the lack of AD-related studies, the data above are suggestive of a promising neuroprotective property of mTOR-signalling inhibitors in AD, via regulation of microglial polarization.
Rho/Rho kinase (ROCK)-pathway inhibitors
Belonging to the Ras superfamily of small GTP binding proteins, Rho provides an important regulatory function in cellular migration and proliferation. Rho-associated protein kinase (ROCK), a member of the AGC (PKA/PKG/PKC) family of serine–threonine kinases, is a downstream effector protein of the small GTPase Rho (Knaus 2000). Being widely distributed in immune-related cells such as T cells, B cells and NK cells, the ROCK-signalling pathway potently promotes infectious and immune inflammation (Wei and Jun-Qi 2017). Furthermore, more and more studies have shown that ROCK inhibitors exert positive regulatory effects on microglial polarization in neurodegenerative diseases. Roser et al. (2017) found that Rho/ROCK-pathway inhibitors could induce the shift from M1 to M2 microglial phenotype, which has become a promising treatment option for Parkinson’s disease (PD) and amyotrophic lateral sclerosis (ALS). Zhao et al. (2015) also reported that fasudil, a selective ROCK inhibitor, could prevent MPTP-induced degeneration of dopaminergic neurons. Additionally, fasudil has been shown to convert inflammatory M1 microglia to anti-inflammatory M2 microglia in an MPTP-mouse model of PD. Similarly, in mouse BV-2 microglia, treatment with fasudil regulated microglia polarization towards the beneficial M2 phenotype. In experimental autoimmune encephalomyelitis (EAE) mice, both CD11b(+)ins(+) and CD11b(+)TNF-α(+) M1 microglia were significantly decreased, whereas CD11b(+)IL-10(+) M2 microglia were increased by fasudil administration, which resulted in the amelioration of demyelination and neuroinflammation (Chen et al. 2014). Additionally, the novel ROCK inhibitor, WAR-5 (which is a Y-27632 derivative), protected against myelin impairment and neuroinflammatory injury in EAE C57BL/6 mice, via promoting the M1-to-M2 microglial transition (Li et al. 2015). In a model of traumatic SCI, blocking the Rho/ROCK pathway promoted a shift from M1 microglia/macrophages towards the M2 phenotype, and alleviated CNS inflammatory damage (Dyck et al. 2018). Additionally, inhibition of the Rho/Rho kinase by the prostaglandin E2 receptor EP3 reduced thrombin-induced brain injury, neurologic deficits and numbers of CD68(+) microglia, whereas it increased the number of Ym-1(+) M2 microglia (Han et al. 2015). Another study investigated the link between ROCK signalling and microglial polarization in an AD transgenic model. Yu et al. (2017) found that fasudil improved spatial cognitive impairment in APP/PS1 mice by facilitating the M1-to-M2 microglial transformation. Taken together, ROCK-pathway inhibitors may mitigate AD pathology by modulating microglial phenotypes.
NF-κB pathway inhibitors
Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) is a protein complex that is implicated in cytokine production, cellular survival and synaptic plasticity in response to external stimuli such as infection, stress and free radicals. Besides, NF-κB is a major transcription factor that modulates genes responsible for both the innate and adaptive immune responses (Smith et al. 2006). Many studies have revealed that NF-κB signalling is involved in AD-associated neuroinflammation. Moreover, NF-κB pathway blockage alleviates inflammatory responses and pathological damage in AD models (Kim et al. 2017; Spagnuolo et al. 2018; Zhao et al. 2018). The NF-κB pathway also plays a crucial role in microglial polarization in AD. In vitro, tianeptine treatment has been shown to attenuate neuroinflammation and promote the transition of M1 towards the M2 microglial phenotype in lipopolysaccharide-stimulated cultures via suppression of both the NLRP3 inflammasome and TLR4/NF-κB signalling (Ślusarczyk et al. 2018). Another study found that silencing TRAM1 effectively inhibited LPS/IFN-γ-induced neuroinflammation by M1 microglia in BV2 cells. Moreover, TRAM1 is also essential for phosphorylation of IκB and P65-NF-kB translocation to the nucleus (Wang et al. 2016). In vivo studies have also confirmed the relationship between NF-κB signalling and microglial polarization. In a rat model of neuropathy via chronic-constriction injury to the sciatic nerve (CCI), M1-mediated cytokines (IL-1β, IL-18, and iNOS) were reduced by parthenolide (PTL) treatment, and M2 (IL-10, TIMP1) factors were enhanced. In addition, PTL downregulated the phosphorylated form of NF-κB, p38MAPK, and ERK1/2 protein levels (Popiolekbarczyk et al. 2015). Zhang et al. found that acute hypoxia upregulated M1 microglial markers (e.g., differentiation 86 [CD86]) and downregulated M2 markers (e.g., Arg-1, CD206, IL-4 and IL-10) in both APPswe/PS1dE9 transgenic (Tg) and wild type (Wt) mice. The effects were associated with NF-κB induction through the toll-like receptor 4 (TLR4) (Zhang et al. 2017). Our previous study demonstrated that M1/M2 microglial phenotypes could be modulated by aging via TLR2/NF-κB signalling in MPTP-PD mice (Yao and Zhao 2018). Wang et al. (2015b) also found that tanshinone I administration markedly increased anti-inflammatory M2 gene expression and reduced pro-inflammatory M1 gene expression in LPS-induced BV-2 microglial cells and MPTP-PD mice via inhibiting NF-κB activation. In a murine model of experimental autoimmune uveitis, silencing aryl hydrocarbon receptor (AhR) led to significantly increased macrophage/microglia cells and transition from the M2 to the M1 phenotype as compared to that of AhR+/+EAU mice. Moreover, this result was associated with the activation of NF-κB and signalling transducers and activators of transcriptional (STAT) pathways. Furthermore, AhR-agonist treatment could prevent macrophage/microglia activation, and shifted their polarization from M1 to M2 (Huang et al. 2018). The precise modulation of microglial polarization by the NF-κB pathway requires further investigation in AD models.
NOTCH-signalling inhibitors
As a highly conserved signalling system in most multicellular organisms (Artavanistsakonas et al. 1999), the Notch-signalling pathway is involved in multiple cellular differentiation processes during embryonic and adult life, such as neuronal development (Bolós et al. 2007) and angiogenesis (Liu et al. 2003). Notch signalling has also been reported to participate in inflammatory and immune processes (Dimitris and Nussenzweig 2007). Recent studies have demonstrated that Notch signalling could modulate polarization of macrophages/microglia in the CNS. Wu et al. (2017) found that the simvastatin and Notch-signalling inhibitor DAPT could enhance M2 microglia polarization and reduce M1-marker expression in LPS-treated BV-2 cells. A study using morphometric and phenotypic analyses of microglial cells found that arginase-1(+) cells were markedly increased in mice induced by pituitary-adenylate cyclase-activating polypeptide (PACAP)-expressing cells. Additionally, some key transcriptional factors (e.g., Notch/RBP-J) are potential targets of PACAP (Brifault et al. 2015). Liu et al. (2012a, b) found that Notch signalling was related to microglial polarization. Also, Notch-signalling blockage resulted in suppressed M1 polarization and increased M2 polarization. Another study showed that primary microglial cells treated with ol-Aβ and f-Aβ expressed high levels of M1 markers (e.g., IL-1β, IL-6, TNF-α, NOS-II, COX-2), whereas the M2 microglial marker, arginase1, was downregulated. Hes-1, a target molecule of the Notch pathway, was also decreased (Michelucci 2009). The data above indicate that Notch signalling is importantly involved in Aβ-induced microglial polarization and neuroinflammatory responses. Hence, Notch-pathway inhibitors deserve more attention in AD-related investigations.
GSK3-signalling inhibitors
GSK-3β, a serine/threonine kinase, is considered to affect cellular proliferation and inflammation. GSK-3β also aggravates tau phosphorylation, Aβ deposition and promotes progression of AD (Maqbool and Hoda 2017). A selective GSK-3 peptide-derivative inhibitor exhibited neuroprotective properties via alleviating Aβ levels and inflammatory injury in a transgenic AD mouse model (Licht-Murava et al. 2016). Previous studies have reported that the GSK-3β signalling pathway is closely related to CNS microglial polarization. Jiang et al. (2018) found that overexpression of TREM2 could prevent pro-inflammatory cytokines, such as IL-1β, via inhibiting the activity of GSK-3β. Increased Arg1 expression and improved behaviour were also observed in the transgenic mice following TREM2 overexpression. Another study used a lentiviral-mediated strategy to selectively overexpress TREM2 in microglia in the brains of P301S tau-transgenic mice. The study showed that TREM2 overexpression significantly suppressed neuroinflammation by promoting M2 microglia generation, which consequently ameliorated neuronal/synaptic loss, tau hyperphosphorylation and cognitive deficits. The protective effect was due to the inhibition of GSK3β and cyclin-dependent kinase 5 (CDK5) (Jiang et al. 2016). In a model of traumatic brain injury (TBI), the class I/II histone deacetylase (HDAC) inhibitor, scriptaid, could shift microglia/macrophage polarization from the M1-to-M2 phenotype and mitigate inflammatory responses via GSK3β/PTEN/Akt signalling. Moreover, scriptaid significantly increased myelin-basic protein expression and improved neuronal function (Wang et al. 2015a). Zhou et al. (2016) found that regulatory T lymphocytes (Tregs) alleviated intracerebral haemorrhage (ICH)-induced inflammatory injury by modulating microglia/macrophage polarization toward the M2 phenotype through the IL-10/GSK3β/PTEN axis. The data above suggest that GSK3 blockage may inhibit neuroinflammatory damage by regulating microglial polarization in several disease models, which deserves further investigation in AD-related models (Tables 1, 2).
Other signalling pathways
Some other signalling pathways also play an important role in the regulation of microglial polarization. A recent study investigated the effects of the brain renin–angiotensin system (RAS) on microglial polarization. Angiotensin II (Ang II) exerts pro-oxidative and pro-inflammatory effects via its type-1 receptor (AT1). However, Ang II/AT2 receptor signalling and Angiotensin 1-7/Mas receptor (MasR) signalling provide anti-inflammatory functions and promote M2 polarization, which has the prospect of becoming a novel therapeutic approach in AD (Labandeiragarcia et al. 2017). Oh et al. injected sRAGE-secreting mesenchymal stem cells (sRAGE-MSCs), along with Aβ1-42, into the entorhinal cortices of male Sprague–Dawley rats. The results showed that sRAGE-MSCs significantly downregulated RAGE and RAGE- ligand expressions; simultaneously, the number of M2 microglia increased and the number of M1 microglia decreased. Consequently, sRAGE-MSC transplantation provided a neuroprotective effect in Aβ1-42-treated rat brains. These observations suggest that RAGE signalling is involved in microglial polarization in AD (Oh et al. 2017). Glatiramer acetate (GA) is commonly used in the treatment of multiple sclerosis, and has been reported to alter the inflammatory environment by upregulating IL-4 and recruiting Th2 T cells to the CNS. GA administration accelerates Aβ clearance and switches microglial phenotypes, similar to those of treatments with IL-4, by activating IGF-1 signalling (Butovsky et al. 2006; 2007). This study indicated the potential regulatory effect of IGF-1 signalling in microglial polarization in AD. Cytokines also exert crucial effects in microglial-phenotypic modulation. Interleukin-4, the prototypic M2-inducing cytokine, reduces Aβ production and improves cognitive ability in an AD model (Kiyota et al. 2010). Furthermore, as a potent anti-inflammatory cytokine, TGF-β polarizes microglia to the M2c phenotype, which consequently enhances microglial uptake of Aβ (Tichauer and von Bernhardi 2012) and alleviates AD-related pathology (Wyss-Coray et al. 2001; Tesseur et al. 2006). Interestingly, another in vivo study reported that deferoxamine enhanced alternative M2 microglial activation and inhibited Aβ deposits in 12-month-old APP/PS1 mice. This result indicated the potential interaction between iron metabolism and microglial polarization in AD (Zhang and He 2017). Among the MAPKs, JNK is one of the most important microglial inflammatory modulators (Waetzig et al. 2005). In vivo treatment of the JNK inhibitor, SP600125, markedly reduced Aβ production, neuroinflammatory responses, synaptic loss and cognitive impairment in a transgenic AD mouse model (Zhou et al. 2015). In rats with CCH, Jiang et al. (2017) found that physical exercise improved cognitive function, alleviated myelin injury, shifted microglia polarization to M2 and reduced ERK and JNK phosphorylation. In cultured primary microglial cells, treatment with exenatide, a GLP-1 receptor agonist, stimulated the expression of M2 markers (e.g., Arg 1, CD206 and IL-4). The effect was blocked by the p38 MAPK inhibitor, SB203580, and the gene silencer, siRNA/p38β, indicating that p38/MAPK signalling is strongly associated with exenatide-induced microglial polarization (Wu et al. 2018) (Fig. 1).
Conclusions
Microglia-mediated neuroinflammation plays a crucial role in the onset and development of AD. During the procession of AD, the dysfunction of M2 microglia and the excessive activation of M1 microglia promote inflammatory injury and pathological damage. Furthermore, many studies have demonstrated that modulation of microglial polarization from the M1 to the M2 phenotype ameliorates neuroinflammatory responses, Aβ deposits and tau hyperphosphorylation in AD. Hence, modulation of microglial phenotypes may represent a promising therapeutic approach for the treatment of AD. Importantly, several signalling pathways—such as AMPK, mTOR, ROCK, NF-κB, NOTCH and GSK3—may be critically involved in microglial polarization in AD. However, the underlying mechanisms of microglial polarization in AD are still not well understood, and future experiments are required for their further elucidations.
References
Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL (2000) Inflammation and Alzheimer’s disease. Neurobiol Aging 21:383–421
Artavanistsakonas S, Rand MD, Lake RJ (1999) Notch signaling: cell fate control and signal integration in development. Science 284:770–776
Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T, Wolozin B, Butovsky O, Kügler S, Ikezu T (2015) Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci 18:1584–1593
Balce DR, Li B, Allan ER, Rybicka JM, Krohn RM, Yates RM (2011) Alternative activation of macrophages by IL-4 enhances the proteolytic capacity of their phagosomes through synergistic mechanisms. Blood 118:4199–4208. https://doi.org/10.1182/blood-2011-01-328906
Bhaskar K, Konerth M, Kokikocochran ON, Cardona A, Ransohoff RM, Lamb BT (2010) Regulation of tau pathology by the microglial fractalkine receptor. Neuron 68:19–31
Boche D, Cunningham C, Docagne F, Scott H, Perry VH (2006) TGFbeta1 regulates the inflammatory response during chronic neurodegeneration. Neurobiol Dis 22:638–650. https://doi.org/10.1016/j.nbd.2006.01.004
Bolós V, Grego-Bessa J, Pompa JLDL (2007) Notch signaling in development and cancer. Endocr Rev 28:339–363
Brifault C, Gras M, Liot D, May V, Vaudry D, Wurtz O (2015) Delayed pituitary adenylate cyclase-activating polypeptide delivery after brain stroke improves functional recovery by inducing m2 microglia/macrophage polarization. Stroke J Cereb Circ 46:520–528
Butovsky O, Koronyo-Hamaoui M, Kunis G, Ophir E, Landa G, Cohen H, Schwartz M (2006) Glatiramer acetate fights against Alzheimer’s disease by inducing dendritic-like microglia expressing insulin-like growth factor 1. Proc Natl Acad Sci USA 103:11784–11789
Butovsky O, Bukshpan S, Kunis G, Jung S, Schwartz M (2007) Microglia can be induced by IFN-gamma or IL-4 to express neural or dendritic-like markers. Mol Cell Neurosci 35:490–500. https://doi.org/10.1016/j.mcn.2007.04.009
Chen C, Li YH, Zhang Q, Yu JZ, Zhao YF, Ma CG, Xiao BG (2014) Fasudil regulates T cell responses through polarization of BV-2 cells in mice experimental autoimmune encephalomyelitis. Acta Pharmacol Sin 35:1428–1438
Colton CA (2009) Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol 4:399–418
D’Andrea MR, Cole GM, Ard MD (2004) The microglial phagocytic role with specific plaque types in the Alzheimer disease brain. Neurobiol Aging 25:675–683
Dimitris S, Nussenzweig MC (2007) CD8–DCs induce IL-12–independent Th1 differentiation through Delta 4 Notch-like ligand in response to bacterial LPS. J Exp Med 204:1525–1531
Dyck S, Kataria H, Alizadeh A, Santhosh KT, Lang B, Silver J, Karimiabdolrezaee S (2018) Perturbing chondroitin sulfate proteoglycan signaling through LAR and PTPσ receptors promotes a beneficial inflammatory response following spinal cord injury. J. Neuroinflamm 15:90
Edwards JP, Zhang X, Frauwirth KA, Mosser DM (2006) Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol 80:1298–1307
Escribano L, Simón AM, Gimeno E, Cuadradotejedor M, Maturana RLD, Garcíaosta A, Ricobaraza A, Pérezmediavilla A, Río JD, Frechilla D (2010) Rosiglitazone rescues memory impairment in Alzheimer’s transgenic mice: mechanisms involving a reduced amyloid and tau pathology. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 35:1593
Fillit H, Ding W, Buee L, Kalman J, Altstiel L, Lawlor B, Wolf-Klein G (1991) Elevated circulating tumor necrosis factor levels in Alzheimer’s disease. Neurosci Lett 129:318–320
Gandy S, Heppner FL (2013) Microglia as dynamic and essential components of the amyloid hypothesis. Neuron 78:575–577
Gehrmann J, Matsumoto Y, Kreutzberg GW (1995) Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev 20:269–287
Giri S, Nath N, Smith B, Viollet B, Singh AK, Singh I (2004) 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside inhibits proinflammatory response in glial cells: a possible role of AMP-activated protein kinase. J Neurosci Off J Soc Neurosci 24:479–487
Giulian D, Haverkamp LJ, Li J, Karshin WL, Yu J, Tom D, Li X, Kirkpatrick JB (1995) Senile plaques stimulate microglia to release a neurotoxin found in Alzheimer brain. Neurochem Int 27:119–137
Guo H, Cheng Y, Wang C, Wu J, Zou Z, Niu B, Yu H, Wang H, Xu J (2017a) FFPM, a PDE4 inhibitor, reverses learning and memory deficits in APP/PS1 transgenic mice via cAMP/PKA/CREB signaling and anti-inflammatory effects. Neuropharmacology 116:260–269
Guo M, Li C, Lei Y, Xu S, Zhao D, Lu XY (2017b) Role of the adipose PPARγ-adiponectin axis in susceptibility to stress and depression/anxiety-related behaviors. Mol Psychiatr 22:1056
Han X, Lan X, Li Q, Gao Y, Zhu W, Cheng T, Maruyama T, Wang J (2015) Inhibition of prostaglandin E2 receptor EP3 mitigates thrombin-induced brain injury. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 36:1059
Huang S, Ge X, Yu J, Han Z, Yin Z, Li Y, Chen F, Wang H, Zhang J, Lei P (2017) Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. Faseb J Off Publ Fed Am Soc Exp Biol 32:512–528
Huang Y, He J, Liang H, Hu K, Jiang S, Yang L, Mei S, Zhu X, Yu J, Kijlstra A, Yang P, Hou S (2018) Aryl hydrocarbon receptor regulates apoptosis and inflammation in a murine model of experimental autoimmune uveitis. Front Immunol 9:1713. https://doi.org/10.3389/fimmu.2018.01713
Hume DA, Ross IL, Himes SR, Sasmono RT, Wells CA, Ravasi T (2002) The mononuclear phagocyte system revisited. J Leukoc Biol 72:621–627
In TVB, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T, Breteler MM, Stricker BH (2001) Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N Engl J Med 345:1515–1521. https://doi.org/10.1056/NEJMoa010178
Ji J, Xue TF, Guo XD, Yang J, Guo RB, Wang J, Huang JY, Zhao XJ, Sun XL (2018) Antagonizing peroxisome proliferator-activated receptor γ facilitates M1-to-M2 shift of microglia by enhancing autophagy via the LKB1-AMPK signaling pathway. Aging Cell 17:e12774
Jiang T, Zhang YD, Chen Q, Gao Q, Zhu XC, Zhou JS, Shi JQ, Lu H, Tan L, Yu JT (2016) TREM2 modifies microglial phenotype and provides neuroprotection in P301S tau transgenic mice. Neuropharmacology 105:196–206
Jiang T, Zhang L, Pan X, Zheng H, Chen X, Li L, Luo J, Hu X (2017) Physical exercise improves cognitive function together with microglia phenotype modulation and remyelination in chronic cerebral hypoperfusion. Front Cell Neurosci 11:404
Jiang Y, Li Z, Ma H, Cao X, Liu F, Tian A, Sun X, Li X, Wang J (2018) Upregulation of TREM2 ameliorates neuroinflammatory responses and improves cognitive deficits triggered by surgical trauma in appswe/PS1dE9 mice. Cell Physiol Biochem 46:1398–1411
Kawahara K, Suenobu M, Yoshida A, Koga K, Hyodo A, Ohtsuka H, Kuniyasu A, Tamamaki N, Sugimoto Y, Nakayama H (2012) Intracerebral microinjection of interleukin-4/interleukin-13 reduces β-amyloid accumulation in the ipsilateral side and improves cognitive deficits in young amyloid precursor protein 23 mice. Neuroscience 207:243–260
Ke X, Xue-Ling D, Han-Chang H, Zhao-Feng J (2011) Targeting HDACs: a promising therapy for Alzheimer’s disease. Oxid Med Cell Longev 2011:143269
Kim YE, Hwang CJ, Lee HP, Kim CS, Son DJ, Ham YW, Hellström M, Han SB, Kim HS, Park EK (2017) Inhibitory effect of punicalagin on lipopolysaccharide-induced neuroinflammation, oxidative stress and memory impairment via inhibition of nuclear factor-kappaB. Neuropharmacology 117:21–32
Kiyota T, Okuyama S, Swan RJ, Jacobsen MT, Gendelman HE, Ikezu T (2010) CNS expression of anti-inflammatory cytokine interleukin-4 attenuates Alzheimer’s disease-like pathogenesis in APP + PS1 bigenic mice. Faseb J Off Publ Fed Am Soc Exp Biol 24:3093
Knaus UG (2000) Rho GTPase signaling in inflammation and transformation. Immunol Res 21:103–109
Koenigsknechttalboo J, Landreth GE (2005) Microglial phagocytosis induced by fibrillar beta-amyloid and IgGs are differentially regulated by proinflammatory cytokines. J Neurosci 25:8240–8249
Krabbe G, Halle A, Matyash V, Rinnenthal JL, Eom GD, Bernhardt U, Miller KR, Prokop S, Kettenmann H, Heppner FL (2013) Functional impairment of microglia coincides with beta-amyloid deposition in mice with alzheimer-like pathology. PLoS One 8:e60921
Labandeiragarcia JL, Rodríguezperez AI, Garridogil P, Rodriguezpallares J, Lanciego JL, Guerra MJ (2017) Brain renin-angiotensin system and microglial polarization: implications for aging and neurodegeneration. Front Aging Neurosci 9:129
Laibaik P, Ping Z, Rose P, Carmen C, Josef A, Norris EH, Linda Y, Steven Y, George C, Mcewen BS (2008) Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci USA 105:1347–1352
Lambeth JD (2004) NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4:181–189
Lawson LJ, Perry VH, Gordon S (1992) Turnover of resident microglia in the normal adult mouse brain. Neuroscience 48:405
Leipnitz G, Schumacher C, Dalcin KB, Scussiato K, Solano A, Funchal C, Dutrafilho CS, Wyse AT, Wannmacher CM, Latini A (2007) In vitro evidence for an antioxidant role of 3-hydroxykynurenine and 3-hydroxyanthranilic acid in the brain. Neurochem Int 50:83–94
Li YH, Yu JZ, Xin YL, Feng L, Chai Z, Liu JC, Zhang HZ, Zhang GX, Xiao BG, Ma CG (2015) Protective effect of a novel Rho kinase inhibitor WAR–5 in experimental autoimmune encephalomyelitis by modulating inflammatory response and neurotrophic factors. Exp Mol Pathol 99:220–228
Li D, Wang C, Yao Y, Chen L, Liu G, Zhang R, Liu Q, Shi FD, Hao J (2016) mTORC1 pathway disruption ameliorates brain inflammation following stroke via a shift in microglia phenotype from M1 type to M2 type. Faseb J Off Publ Fed Am Soc Exp Biol 30:3388
Li C, Zhang C, Zhou H, Feng Y, Tang F, Hoi MPM, He C, Ma D, Zhao C, Lee SMY (2018) Inhibitory effects of betulinic acid on LPS-induced neuroinflammation involve M2 microglial polarization via CaMKKβ-dependent AMPK activation. Front Mol Neurosci 11:98
Licht-Murava A, Paz R, Vaks L, Avrahami L, Plotkin B, Eisenstein M, Eldar-Finkelman H (2016) A unique type of GSK-3 inhibitor brings new opportunities to the clinic. Sci Signal 9:ra110
Liu ZJ, Shirakawa T, Li Y, Soma A, Oka M, Dotto GP, Fairman RM, Velazquez OC, Herlyn M (2003) Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol 23:14–25
Liu HC, Zheng MH, Du YL, Wang L, Kuang F, Qin HY, Zhang BF, Han H (2012a) N9 microglial cells polarized by LPS and IL4 show differential responses to secondary environmental stimuli. Cell Immunol 278:84–90
Liu S, Liu Y, Hao W, Wolf L, Kiliaan AJ, Penke B, Rube CE, Walter J, Heneka MT, Hartmann T, Menger MD, Fassbender K (2012b) TLR2 is a primary receptor for Alzheimer’s amyloid beta peptide to trigger neuroinflammatory activation. J Immunol 188:1098–1107. https://doi.org/10.4049/jimmunol.1101121
Liu M, Jevtic S, Markham-Coultes K, Ellens N, O’Reilly MA, Hynynen K, Aubert I, McLaurin J (2018) Investigating the efficacy of a combination Abeta-targeted treatment in a mouse model of Alzheimer’s disease. Brain Res 1678:138–145. https://doi.org/10.1016/j.brainres.2017.10.015
Lue LF, Kuo YM, Beach T, Walker DG (2010) Microglia activation and anti-inflammatory regulation in Alzheimer’s disease. Mol Neurobiol 41:115–128. https://doi.org/10.1007/s12035-010-8106-8
Luo XQ, Li A, Yang X, Xiao X, Hu R, Wang TW, Dou XY, Yang DJ, Dong Z (2018) Paeoniflorin exerts neuroprotective effects by modulating the M1/M2 subset polarization of microglia/macrophages in the hippocampal CA1 region of vascular dementia rats via cannabinoid receptor 2. Chin Med 13:14
Majumdar A, Cruz D, Asamoah N, Buxbaum A, Sohar I, Lobel P, Maxfield FR (2007) Activation of microglia acidifies lysosomes and leads to degradation of Alzheimer amyloid fibrils. Mol Biol Cell 18:1490
Mandrekarcolucci S, Karlo JC, Landreth GE (2012) Mechanisms underlying the rapid peroxisome proliferator-activated receptor-γ-mediated amyloid clearance and reversal of cognitive deficits in a murine model of alzheimer’s disease. J Neurosci Off J Soc Neurosci 32:10117–10128
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25:677–686
Maqbool M, Hoda N (2017) GSK3 inhibitors in the therapeutic development of diabetes, cancer and neurodegeneration: past, present and future. Curr Pharm Des 23:4332–4350. https://doi.org/10.2174/1381612823666170714141450
Mcdonald DR, Brunden KR, Landreth GE (1997) Amyloid fibrils activate tyrosine kinase-dependent signaling and superoxide production in microglia. J Neurosci Off J Soc Neurosci 17:2284
Mecha M, Feliú A, Carrillosalinas FJ, Ruedazubiaurre A, Ortegagutiérrez S, de Sola RG, Guaza C (2015) Endocannabinoids drive the acquisition of an alternative phenotype in microglia. Brain Behav Immun 49:233–245
Michaud JP, Hallé M, Lampron A, Thériault P, Préfontaine P, Filali M, Triboutjover P, Lanteigne AM, Jodoin R, Cluff C (2013) Toll-like receptor 4 stimulation with the detoxified ligand monophosphoryl lipid A improves Alzheimer’s disease-related pathology. Proc Natl Acad Sci USA 110:1941–1946
Michelucci A (2009) Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: effects of oligomeric and fibrillar amyloid-beta. J Neuroimmunol 210:3–12
Mosser DM (2003) The many faces of macrophage activation. J Leukoc Biol 73:209–212
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8:958–969
Nakamura Y, Si QS, Kataoka K (1999) Lipopolysaccharide-induced microglial activation in culture: temporal profiles of morphological change and release of cytokines and nitric oxide. Neurosci Res 35:95–100
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308:1314–1318
Oh S, Son M, Choi J, Lee S, Byun K (2017) sRAGE prolonged stem cell survival and suppressed RAGE-related inflammatory cell and T lymphocyte accumulations in an Alzheimer’s disease model. Biochem Biophys Res Commun 495:807–813
Orihuela R, Mcpherson CA, Harry GJ (2016) Microglial M1/M2 polarization and metabolic states. Br J Pharmacol 173:649–665
Pasinetti GM (2002) From epidemiology to therapeutic trials with anti-inflammatory drugs in Alzheimer’s disease: the role of NSAIDs and cyclooxygenase in β-amyloidosis and clinical dementia. J Alzheimers Dis 4:435–445
Ponomarev ED, Maresz K, Tan Y, Dittel BN (2007) CNS-derived interleukin-4 is essential for the regulation of autoimmune inflammation and induces a state of alternative activation in microglial cells. J Neurosci 27:10714–10721. https://doi.org/10.1523/JNEUROSCI.1922-07.2007
Popiolekbarczyk K, Kolosowska N, Piotrowska A, Makuch W, Rojewska E, Jurga AM, Pilat D, Mika J (2015) Parthenolide relieves pain and promotes M2 microglia/macrophage polarization in rat model of neuropathy. Neural Plast 2015:1–15
Raleigh DJ (2006) Mechanisms of Disease: astrocytes in neurodegenerative disease. Nat Rev Neurol 2:679–689
Roser AE, Tönges L, Lingor P (2017) Modulation of microglial activity by rho-kinase (ROCK) inhibition as therapeutic strategy in Parkinson’s disease and amyotrophic lateral sclerosis. Fronti Aging Neurosci. 9:94
Sackmann V, Ansell A, Sackmann C, Lund H, Harris RA, Hallbeck M, Nilsberth C (2017) Anti-inflammatory (M2) macrophage media reduce transmission of oligomeric amyloid beta in differentiated SH-SY5Y cells. Neurobiol Aging 60:173
Saxton RA, Sabatini DM (2017) mTOR signaling in growth, metabolism, and disease. Cell 168:960–976
Sheffield LG, Marquis JG, Berman NE (2000) Regional distribution of cortical microglia parallels that of neurofibrillary tangles in Alzheimer’s disease. Neurosci Lett 285:165–168
Sheng JG, Mrak RE, Griffin WST (1997) Glial-neuronal interactions in Alzheimer disease: progressive association of IL-1α + microglia and S100β + astrocytes with neurofibrillary tangle stages. J Neuropath Exp Neur 56:285–290
Ślusarczyk J, Trojan E, Głombik K, Piotrowska A, Budziszewska B, Kubera M, Popiołekbarczyk K, Lasoń W, Mika J, Bastakaim A (2018) Targeting the NLRP3 inflammasome-related pathways via tianeptine treatment-suppressed microglia polarization to the M1 phenotype in lipopolysaccharide-stimulated cultures. Int J Mol, Sci, p 19
Smith EM, Gregg M, Hashemi F, Schott L, Hughes TK (2006) Corticotropin releasing factor (CRF) activation of NF-kappaB-directed transcription in leukocytes. Cell Mol Neurobiol 26:1019–1034
Solito E, Sastre M (2012) Microglia function in Alzheimer’s disease. Front Pharmacol 3:14. https://doi.org/10.3389/fphar.2012.00014
Sollvander S, Nikitidou E, Brolin R, Soderberg L, Sehlin D, Lannfelt L, Erlandsson A (2016) Accumulation of amyloid-beta by astrocytes result in enlarged endosomes and microvesicle-induced apoptosis of neurons. Mol Neurodegener 11:38. https://doi.org/10.1186/s13024-016-0098-z
Spagnuolo C, Moccia S, Russo GL (2018) Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur J Med Chem 153:105
Stewart CR, Stuart LM, Wilkinson K, Gils JMV, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA (2010) CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol 11:155–161
Suh HS, Zhao ML, Derico L, Choi N, Lee SC (2013) Insulin-like growth factor 1 and 2 (IGF1, IGF2) expression in human microglia: differential regulation by inflammatory mediators. J Neuroinflamm 10:37. https://doi.org/10.1186/1742-2094-10-37
Tang Y, Le W (2016) Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol Neurobiol 53:1181–1194
Tesseur I, Zou K, Esposito L, Bard F, Berber E, Can JV, Lin AH, Crews L, Tremblay P, Mathews P (2006) Deficiency in neuronal TGF-β signaling promotes neurodegeneration and Alzheimer’s pathology. J. Clin. Invest. 116:3060–3069
Tichauer JE, von Bernhardi R (2012) Transforming growth factor-beta stimulates beta amyloid uptake by microglia through Smad3-dependent mechanisms. J Neurosci Res 90:1970–1980. https://doi.org/10.1002/jnr.23082
Venegas C, Kumar S, Franklin BS, Dierkes T, Brinkschulte R, Tejera D, VieiraSaecker A, Schwartz S, Santarelli F, Kummer MP (2017) Microglia-derived ASC specks cross-seed amyloid-β in Alzheimer’s disease. Nature 552:355
Waetzig V, Czeloth K, Hidding U, Mielke K, Kanzow M, Brecht S, Goetz M, Lucius R, Herdegen T, Hanisch UK (2005) c-Jun N-terminal kinases (JNKs) mediate pro-inflammatory actions of microglia. Glia 50:235–246. https://doi.org/10.1002/glia.20173
Wang G, Shi Y, Jiang X, Leak RK, Hu X, Wu Y, Pu H, Li WW, Tang B, Wang Y, Gao Y (2015a) HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3β/PTEN/Akt axis. Proc Natl Acad Sci USA 112:2853–2858
Wang S, Jing H, Yang H, Liu Z, Guo H, Chai L, Hu L (2015b) Tanshinone I selectively suppresses pro-inflammatory genes expression in activated microglia and prevents nigrostriatal dopaminergic neurodegeneration in a mouse model of Parkinson’s disease. J Ethnopharmacol 164:247–255
Wang H, Liu C, Han M, Cheng C, Zhang D (2016) TRAM1 promotes microglia M1 polarization. J Mol Neurosci 58:287–296
Wang C, Wang Q, Lou Y, Xu J, Feng Z, Chen Y, Tang Q, Zheng G, Zhang Z, Wu Y (2017) Salidroside attenuates neuroinflammation and improves functional recovery after spinal cord injury through microglia polarization regulation. J Cell Mol Med 22:1148–1166
Wei Z, Jun-Qi Y (2017) Role of Rho GTPases in the immune regulation of infection and inflammation. Zhongguo xue xi chong bing fang zhi za zhi = Chinese journal of schistosomiasis control 29:807
Wilkinson K, El JK (2012) Microglial scavenger receptors and their roles in the pathogenesis of alzheimer’s disease. Int J Alzheimers Dis 2012:489456
Wirz KT (2013) Cortical beta amyloid protein triggers an immune response, but no synaptic changes in the APPswe/PS1dE9 Alzheimer’s disease mouse model. Neurobiol Aging 34:1328–1342
Wu F, Luo T, Mei Y, Liu H, Dong J, Fang Y, Peng J, Guo Y (2017) Simvastatin alters M1/M2 polarization of murine BV2 microglia via Notch signaling. J Neuroimmunol 316:56–64
Wu HY, Tang XQ, Liu H, Mao XF, Wang YX (2018) Both classic Gs-cAMP/PKA/CREB and alternative Gs-cAMP/PKA/p38β/CREB signal pathways mediate exenatide-stimulated expression of M2 microglial markers. J Neuroimmunol 316:17–22
Wyss-Coray T (2006) Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med 12:1005–1015
Wyss-Coray T, Lin CF, Yu G, Rohde M, Mcconlogue L, Masliah E, Mucke L (2001) TGF-beta1 promotes microglial amyloid-beta clearance and reduces plaque burden in transgenic mice. Nat Med 7:612–618
Xu Y, Xu Y, Wang Y, Wang Y, He L, Jiang Z, Huang Z, Liao H, Li J, Saavedra JM (2015) Telmisartan prevention of LPS-induced microglia activation involves M2 microglia polarization via CaMKKβ-dependent AMPK activation. Brain Behav Immun 50:298–313
Yamamoto M, Kiyota T, Walsh SM, Liu J, Kipnis J, Ikezu T (2008) Cytokine-mediated inhibition of fibrillar amyloid-β peptide degradation by human mononuclear phagocytes. J Immunol 181:3877–3886
Yamanaka M, Ishikawa T, Griep A, Axt D, Kummer MP, Heneka MT (2012) PPARγ/RXRα-induced and CD36-mediated microglial amyloid-β phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. J Neurosci 32:17321–17331
Yao K, Zhao YF (2018) Aging modulates microglia phenotypes in neuroinflammation of MPTP-PD mice. Exp Gerontol 111:86–93. https://doi.org/10.1016/j.exger.2018.07.010
Yu J, Gu Q, Yan Y, Yu H, Guo M, Liu C, Song G, Chai Z, Wang Q, Xiao B (2017) Fasudil improves cognition of APP/PS1 transgenic mice via inhibiting the activation of microglia and shifting microglia phenotypes from M1 to M2. XI Bao Yu Fen Zi Mian Yi Xue Za Zhi 33:1585–1593
Zare-Shahabadi A, Masliah E, Johnson GV, Rezaei N (2015) Autophagy in Alzheimer’s disease. Rev Neurosci 26:385–395. https://doi.org/10.1515/revneuro-2014-0076
Zhang Y, He ML (2017) Deferoxamine enhances alternative activation of microglia and inhibits amyloid beta deposits in APP/PS1 mice. Brain Res 1677:86–92
Zhang J, Guo J, Zhao X, Chen Z, Wang G, Liu A, Wang Q, Zhou W, Xu Y, Wang C (2013) Phosphodiesterase-5 inhibitor sildenafil prevents neuroinflammation, lowers beta-amyloid levels and improves cognitive performance in APP/PS1 transgenic mice. Behav Brain Res 250:230–237
Zhang F, Zhong R, Li S, Fu Z, Cheng C, Cai H, Le W (2017) Acute hypoxia induced an imbalanced M1/M2 activation of microglia through NF-κB signaling in Alzheimer’s disease mice and wild-type littermates. Front Aging Neurosci 9:282
Zhao YF, Zhang Q, Xi JY, Li YH, Ma CG, Xiao BG (2015) Multitarget intervention of Fasudil in the neuroprotection of dopaminergic neurons in MPTP-mouse model of Parkinson’s disease. J Neurol Sci 353:28–37
Zhao Q, Wu X, Yan S, Xie X, Fan Y, Zhang J, Peng C, You Z (2016) The antidepressant-like effects of pioglitazone in a chronic mild stress mouse model are associated with PPARγ-mediated alteration of microglial activation phenotypes. J Neuroinflamm 13:259
Zhao H, Wang Q, Cheng X, Li X, Li N, Liu T, Li J, Yang Q, Dong R, Zhang Y (2018) Inhibitive effect of resveratrol on the inflammation in cultured astrocytes and microglia induced by Aβ1–42. Neuroscience 379:390–404
Zhou Q, Wang M, Du Y, Zhang W, Bai M, Zhang Z, Li Z, Miao J (2015) Inhibition of JNK activation reverses AD-phenotypes in APPswe/PS1dE9 mice. Ann Neurol 77:637–654
Zhou K, Zhong Q, Wang YC, Xiong XY, Meng ZY, Zhao T, Zhu WY, Liao MF, Wu LR, Yang YR (2016) Regulatory T cells ameliorate intracerebral hemorrhage-induced inflammatory injury by modulating microglia/macrophage polarization through the IL-10/GSK3β/PTEN axis. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 37:967
Zhu L, Yang T, Li L, Sun L, Hou Y, Hu X, Zhang L, Tian H, Zhao Q, Peng J (2014) TSC1 controls macrophage polarization to prevent inflammatory disease. Nat Commun 5:4696
Acknowledgements
The study was supported by research grants from Research Project of Jinshan District Health and Family Planning Commission (No. JSKJ-KTMS-2018-19), Qi Hang project of Jinshan Hospital (No. 2018-JSYYQH-06). We thank LetPub (https://www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
KY was responsible for the design and manuscript drafting. HZ contributed to figure generation and manuscript writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yao, K., Zu, Hb. Microglial polarization: novel therapeutic mechanism against Alzheimer’s disease. Inflammopharmacol 28, 95–110 (2020). https://doi.org/10.1007/s10787-019-00613-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-019-00613-5