Abstract
Purpose
Hyperhomocysteinemia is well recognized as an independent risk factor for the development of premature atherosclerosis. Atherosclerosis, however, may be prevented by polyphenols, potent antioxidant compounds with anti-atherogenic properties. Previously, we used cystathionine beta synthase-deficient mice [Cbs (±)] fed a high-methionine diet—a murine model of hyperhomocysteinemia—to show that daily intake of a red wine polyphenolic extract, mainly comprised of catechin and epicatechin, has a beneficial effect on aortic expression of endothelial dysfunction biomarkers and pro-inflammatory cytokines. The aim of the present study was to understand whether catechin and epicatechin, in purified forms, have anti-atherogenic effects in hyperhomocysteinemia.
Methods
Cbs (±) mice received 50 μg of catechin and/or epicatechin daily in drinking water for 1 month. Plasma homocysteine (Hcy) level and aortic expression of several endothelial dysfunction biomarkers (Vcam-1, Icam-1, E-selectin, and Lox-1) and pro-inflammatory cytokines (Tnf-α, Il-6) were assessed.
Results
We found that both catechin and epicatechin had a beneficial effect on plasma homocysteine levels and endothelial dysfunction biomarker expression; however, only catechin had a beneficial effect on pro-inflammatory cytokine expression. Further, when both polyphenols were given, a beneficial effect was observed only on pro-inflammatory cytokine expression.
Conclusions
Catechin seems to be a more potent anti-atherogenic compound than epicatechin in hyperhomocysteinemia and should be considered as a novel therapeutic approach against endothelial dysfunction induced by this condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atherosclerosis is a chronic inflammatory disease of the arteries, initiated by endothelial dysfunction and accumulation of monocyte-derived macrophage-like foam cells in the intima. At endothelial cell surfaces, expression of adhesion proteins such as vascular cell adhesion molecule 1 (Vcam-1), intercellular cell adhesion molecule 1 (Icam-1), and E-selectin facilitates the recruitment and internalization of circulating monocytes, which then differentiate into macrophages. Subsequent activity of lectin-like oxidized low-density lipoprotein receptor 1 (Lox-1) allows oxidized LDL particles (OxLDL) to penetrate macrophages and induce their transformation into foam cells [1, 2].
Several causes and risk factors for atherosclerosis, which can lead to ischemic events and death, have been identified. Among them, hyperhomocysteinemia, defined as an abnormal elevation of total plasma homocysteine (tHcy), is recognized as an important vascular risk factor associated with atherosclerosis in the coronary, cerebrovascular, and peripheral arterial circulation [3, 4]. Elevated homocysteine results when one of the enzymes involved in methionine metabolism fails to properly metabolize homocysteine, a thiol-containing amino acid byproduct of methionine metabolism. Once formed, homocysteine may be recycled to methionine via the remethylation pathway or, in the liver and kidney, via betaine-homocysteine methyltransferase. Homocysteine can also undergo condensation with serine to form cystathionine via the vitamin B6-dependent cystathionine beta synthase (Cbs), the first enzyme involved in the transsulfuration pathway [4, 5].
Experimental evidence suggests that elevated homocysteine concentration leads to endothelial dysfunction [6, 7], which plays a crucial role in the pathogenesis of atherothrombotic vascular disease. Heterozygous Cbs-deficient [Cbs (±)] mice [8, 9] fed a normal diet have, among other phenotypes induced by a twofold increase in plasma tHcy level (~9 μmol/L), endothelial dysfunction characterized by an abnormal vasodilatation due to increased oxidative stress [10, 11]. In a previous study, we showed that Cbs (±) mice fed a methionine-enriched diet have a plasma tHcy level around 40 μmol/L and exhibit increased aortic expression of endothelial dysfunction biomarkers Vcam-1, Icam-1, E-selectin, and Lox-1, concomitant with increased expression of proinflammatory cytokines such as Tnf-α (tumor necrosis factor α) and Il-6 (interleukin 6) [8, 12]. These mice also show decreased expression and activity of paraoxonase-1 (Pon-1), an enzyme with anti-atherogenic properties synthesized in the liver, anchored on circulating HDL particles. In that study, we administered a red wine polyphenolic extract, mainly composed of catechin and epicatechin (a catechin diastereomer), daily in the drinking water and found not only a decreased plasma tHcy level in Cbs (±) mice fed a high-methionine diet, but also normalized expression of endothelial dysfunction biomarkers and restored hepatic expression and hepatic and plasma activities of Pon-1 [8, 13].
Reports of the antioxidant properties of polyphenolic compounds have emerged in the recent years [14–16], making these molecules a new field of research. Compared to vitamin C or E, the antioxidant properties of polyphenols are greater [15, 17], and they result from the ability of the phenolic group to accept an electron and to form a stable phenoxyl radical, which is believed to disturb the oxidative reaction [18]. Importantly, dietary polyphenols show potential for vascular protection (for review see [19]). However, catechin alone could suppress plasma tHcy levels in Cbs (±) mice fed a high-methionine diet [10]. Thus, the aim of the present study was to determine whether purified catechin and/or epicatechin, at a dose corresponding to the amount found in red wine extract, could explain the anti-atherogenic properties of the red wine polyphenolic extract on aorta of Cbs (±) mice fed a high-methionine diet and, further, would therefore offer a novel approach in treating endothelial dysfunction induced by hyperhomocysteinemia.
Experimental methods
Mice, genotyping, and experimental protocol
Mice were maintained in a controlled environment with unlimited access to food and water on a 12-h light/dark cycle. All procedures were carried out in accordance with internal guidelines of the French agriculture ministry for animal handling. Number of mice and suffering were minimized. Mice heterozygous for targeted disruption of the Cbs gene [Cbs (±)] were generously donated by Dr. N. Maeda (Department of Pathology, University of North Carolina, Chapel Hill, NC, USA) [9]. Cbs (±) mice on a C57BL/6 genetic background were generated by mating male Cbs (±) mice with female wild-type C57BL/6 mice. DNA isolated from tail biopsies of 4 week-old mice was subjected to genotyping of the targeted Cbs allele using polymerase chain reaction [9].
Mice were fed a standard laboratory diet (A03, Safe-UAR, Augy, France) ad libitum. Forty-four 3-month-old female Cbs (±) mice from the same litters were divided into eight groups and maintained on the following diets for 3 months before experiments: (a) control diet (Control), consisting of the standard A03 rodent diet; (b) high-methionine diet (Met), consisting of a control diet supplemented with 0.5 % l-methionine (Sigma-Aldrich, France) in drinking water for 3 months; (c) high-methionine diet with 0.001 % catechin and/or 0.001 % epicatechin for the last month (Met/Cat, Met/Epi, or Met/Cat/Epi); and (d) control diet with 0.001 % catechin and/or 0.001 % epicatechin for the last month (Cat, Epi, or Cat/Epi). Catechin or epicatechin was given in the drinking water, and the daily intake was 50 μg. For mice fed the standard diet, the daily methionine intake was 21 mg, and for mice fed the high-methionine diet it was 36 mg. Animals were given a fresh portion of supplemented diet twice a week. Dietary supplementation did not affect the growth or food consumption of mice during the experimental feeding period.
Preparation of serum samples, tissue collection, and plasma assay
When mice were euthanized, blood samples were collected into tubes containing a 1/10 volume of 3.8 % sodium citrate and placed on ice immediately. Plasma was isolated by centrifugation at 2,500 × g for 15 min at 4 °C. Liver and aorta were harvested, snap-frozen, and stored at –80 °C until use. Plasma tHcy was assayed by using the fluorimetric high-performance liquid chromatography method described by Fortin and Genest [1]. Levels of plasma Lox-1 were determined using an ELISA from R&D Systems, Inc. (R&D Systems Europe, Lille, France).
RNA extraction and determination of mRNA levels
mRNA was prepared from liver or aorta with the micro fast track mRNA isolation kit (Invitrogen). Quantity and purity of RNA were assessed by measuring absorbance at 260 and 280 nm. Reverse transcription was carried out on 150 ng mRNA. mRNA was heated for 5 min at 65 °C with random nonamer primers (Biolabs) and 0.8 mM dNTP (Invitrogen), then ice-cooled. Reverse transcription was performed in the presence of 5 × prime script buffer, 20 U RNase inhibitor (Biolabs), and 200 U PrimeScript™ reverse transcriptase (Takara Bio, Inc) and incubated at 42 °C for 1 h. Reverse transcriptase was inactivated at 95 °C for 5 min. mRNA levels of individual mice were assessed by real-time quantitative reverse transcription-polymerase chain reaction (Q-PCR). cDNA (0.4 μL) was diluted with PCR mix (Light Cycler 480 SYBR Green I Master, Roche Diagnostics) containing a final concentration of 3 mM MgCl2 and 0.5 μM of primers in a final volume of 7 μL. Primers were designed by Primer 3 software. Primer pairs were selected to yield a single amplicon based on dissociation curves. Mouse peptidyl prolyl isomerase A (Ppia) and peptidyl prolyl isomerase B (Ppib) mRNA were used as endogenous controls. Primer sequences are shown in Table 1. Vcam-1, Icam-1, E-selectin, Lox-1, Tnf-α, and Il-6 were quantified in aorta, and Pon-1 was quantified in liver. Thermal cycler parameters were as follows: activation for 8 min at 95 °C; amplification of cDNA over 40 cycles with melting for 10 s at 95 °C, annealing for 10 s at 65 °C, and extension for 10 s at 72 °C. Each reaction was performed in triplicate. ΔΔCp analysis of the results was used to assess the ratio of target mRNA to control mRNA [3].
Determination of Pon-1 activity
Pon-1 activity assay was performed using 200 μg of total proteins obtained from liver samples or 5 μL of plasma. Pon-1 arylesterase activity toward phenyl acetate was quantified spectrophotometrically using 20 mM Tris HCl, pH 8.3, with 1 mM CaCl2 and 10 mM phenyl acetate (Sigma-Aldrich). The reaction was performed at room temperature for 1 min by measuring the appearance of phenol at 270 nm with the use of a continuous and automated recording spectrophotometer. All values were corrected for nonenzymatic hydrolysis.
Data analysis
Statistical analysis was done with one-way ANOVA followed by Bonferroni–Dunn post hoc test using Statview software. Correlation between plasma tHcy level and plasma Lox-1 level was determined by using Bravais–Pearson correlation test since data were normally distributed according to Shapiro–Wilk test. The results are expressed as mean ± SEM and are considered significant when p < 0.05.
Results
Effect of catechin and/or epicatechin supplementation on plasma and hepatic parameters in hyperhomocysteinemic mice
High-methionine diet induced an increase in plasma tHcy in Cbs (±) mice (Table 2). Additional supplementation with catechin or epicatechin resulted in a significant decrease in plasma tHcy in these mice, but supplementation with both polyphenols resulted in a nonsignificant decrease in plasma tHcy (Table 2). Catechin and/or epicatechin supplementation had no effect on plasma tHcy in Cbs (±) mice fed a control diet (Table 2).
Consistent with our previous report, plasma Pon-1 activity was significantly reduced in Cbs (±) mice fed a high-methionine diet (Table 2). However, catechin supplementation alone induced a significant increase in plasma Pon-1 activity in Cbs (±) mice fed a high-methionine diet; in contrast, epicatechin alone induced a significant decrease in activity (Table 2). Supplementation with both polyphenols had no effect on plasma Pon-1 activity in Cbs (±) mice fed a high-methionine diet (Table 2). Additionally, catechin and/or epicatechin supplementation had no effect on plasma Pon-1 activity in Cbs (±) mice fed a control diet (Table 2). To explain the variations in plasma Pon-1 activity, we analyzed the expression and activity of Pon-1 in the liver. We found that Cbs (±) mice fed a high-methionine diet had decreased liver expression and activity of Pon-1 (Table 3). Catechin or epicatechin supplementation induced a significant increase in expression and activity of Pon-1 in liver of Cbs (±) mice fed a high-methionine diet (Table 3). However, supplementation with both polyphenols had no effect on Pon-1 activity, despite a significant increase in Pon-1 expression in liver of Cbs (±) mice fed a high-methionine diet (Table 3). Supplementation with catechin alone in Cbs (±) mice fed a control diet resulted in significantly higher hepatic Pon-1 expression but no effect on its activity, while supplementation with epicatechin alone or in conjunctions with catechin produced a significant increase in Pon-1 hepatic expression and a significant decrease in hepatic activity (Table 3).
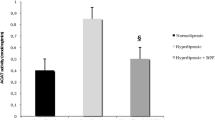
The plasma level of soluble Lox-1, determined by ELISA, was increased in Cbs (±) mice fed a high-methionine diet (Table 2). However, supplementation with catechin or epicatechin restored plasma Lox-1 to near-normal levels in Cbs (±) mice fed a high-methionine diet, though catechin exhibited a more potent effect (Table 2). Supplementation with both polyphenols had no effect on plasma Lox-1 level in Cbs (±) mice fed a high-methionine diet (Table 2). In contrast, supplementation with both polyphenols induced a significant increase in plasma Lox-1 in Cbs (±) mice fed a control diet, but catechin or epicatechin alone produced no effect (Table 2). Additionally, we found a positive correlation between plasma tHcy level and plasma Lox-1 level using a Bravais–Pearson correlation test (Fig. 1).
Effect of catechin and/or epicatechin supplementation on aortic parameters in hyperhomocysteinemic mice
Cbs (±) mice fed a high-methionine diet had a significant increase in expression of Vcam-1, Icam-1, E-selectin, Tnf-α, Il-6, and Lox-1 (Table 4). Catechin supplementation alone induced a significant decrease in expression of these biomarkers in the aorta of Cbs (±) mice fed a high-methionine diet (Table 4). Similarly, epicatechin supplementation alone in Cbs (±) mice fed a high-methionine diet also induced a significant decrease in expression of Vcam-1, Icam-1, and Lox-1 and a small, but significant, decrease in expression of Tnf-α (Table 4). Further, high-methionine diet and supplementation with epicatechin induced a strong increase in expression of E-selectin and Il-6 in aorta of Cbs (±) mice in comparison with littermates fed a control diet (Table 4). Supplementation with both polyphenols induced a significant decrease in expression of E-selectin, Tnf-α, and Il-6, but had no effect on Vcam-1, Icam-1, and Lox-1 expression (Table 4). Catechin supplementation alone had no effect on aortic expression of E-selectin, Tnf-α, and Il-6, but significantly increased expression of Vcam-1, Icam-1, and Lox-1, in Cbs (±) mice fed a control diet (Table 4). Similarly, epicatechin supplementation alone induced a strong and significant increase in expression of Vcam-1, Icam-1, E-selectin, and Lox-1, but had no effect on Tnf-α and Il-6 expression, in aorta of Cbs (±) mice fed a control diet (Table 4). Supplementation with both polyphenols induced a strong and significant increase in expression of Vcam-1, Icam-1, E-selectin, and Lox-1, but a significant decrease in expression of Tnf-α and Il-6, in aorta of Cbs (±) mice fed a control diet (Table 4).
Discussion
Hyperhomocysteinemia is well recognized as an independent risk factor for developing an atherothrombotic event [4], and people with moderate to intermediate hyperhomocysteinemia are predisposed to develop pathology in the cardiovascular system [6]. Here we found that Cbs (±) mice fed a methionine-enriched diet had, as previously described [8], a significant increase in plasma tHcy level and expression of Vcam-1, Icam-1, E-selectin, Lox-1, Tnf-α, and Il-6 in the aorta.
Interestingly, we found that daily consumption of catechin or epicatechin decreased plasma tHcy and Lox-1 levels and aortic expression of Vcam-1, Icam-1, and Lox-1, while catechin alone also decreased aortic expression of E-selectin, in Cbs (±) mice fed a high-methionine diet. The daily dose of 50 μg of catechin and/or epicatechin was established based on the beneficial effect obtained with 50 μg of catechin given daily to male Cbs (±) mice fed a high-methionine diet, as shown previously [10]. Daily consumption of catechin also induced a significant decrease in expression of Tnf-α and Il-6 in aorta of Cbs (±) mice fed a high-methionine diet, while epicatechin only produced an effect on Tnf-α expression.
Decreased expression of Vcam-1 and Icam-1 could be associated with decreased expression of Lox-1. Indeed, it has been shown that Lox-1 activation in human coronary artery endothelial cells [12] induces expression of CD40 and CD40L, two proteins involved in Vcam-1 and Icam-1 expression [13]. Increased expression of Lox-1 in hyperhomocysteinemia might be explained by the oxidative stress induced by elevated homocysteine levels. Indeed, it has been shown that plasma homocysteine influences oxidation of circulating LDL particles (OxLDL) [14], which has been shown to be involved in Lox-1 gene expression by fixation of OxLDL particles on Lox-1 protein at the surface of human aortic endothelial cells [17]. Interestingly, Pon-1 activity may prevent oxidation of LDL particles [20]. Plasma Pon-1 activity is decreased in hyperhomocysteinemic patients [21] and in Cbs (±) mice fed a high-methionine diet [8], and hepatic activity and expression is decreased in Cbs (±) mice fed normal chow [22, 23]. Thus, decreased expression of Lox-1 in Cbs (±) mice fed a high-methionine diet supplemented with catechin may result from a beneficial effect of this polyphenol on plasma Pon-1 activity and hepatic gene expression. Indeed, Gouedard et al. showed that polyphenols such as catechin can induce Pon-1 expression through a mechanism involving the arylhydrocarbon receptor signaling pathway [24]. However, Cbs (±) mice fed a methionine-enriched diet supplemented with epicatechin alone had decreased plasma Pon-1 activity despite increased hepatic expression and activity. Our hypothesis, therefore, is that epicatechin, or one of its metabolites, may directly inhibit Pon-1 in the plasma. Thus, the diastereomery between catechin and epicatechin may explain the observed difference in Pon-1 activity as well as the observed increase in Pon-1 hepatic expression and decrease in Pon-1 hepatic activity when epicatechin was given.
Nevertheless, the beneficial effect of catechin or epicatechin supplementation on Lox-1, Vcam-1, and Icam-1 expression was not evident when Cbs (±) mice fed a high-methionine diet were given both polyphenols. Thus, the beneficial effect previously observed with red wine polyphenolic extract supplementation [8] seems to be due not only to the presence of catechin but also to the concentration mice received. Daily consumption of catechin and epicatechin in Cbs (±) mice fed a high-methionine diet did, however, induce a significant decrease in expression of Tnf-α and Il-6. This decrease seems to be due to a direct effect of catechin because plasma tHcy level was not decreased in Cbs (±) mice fed a high-methionine diet. Interestingly, one study reported that epigallocatechin-3-gallate, the most abundant catechin found in green tea, could counteract the expression of Lox-1 in oxLDL-stimulated human umbilical vein cells (HUVEC) [25]. Moreover, the potential anti-inflammatory activity of catechin may result from its antioxidant properties [15, 16]. Along this line, activity and expression of oxidative stress markers such as NF-κB, a transcription factor regulating the expression of pro-inflammatory cytokines and adhesion molecules, can be enhanced by homocysteine in HUVEC [26]. On the other hand, inhibition of NF-κB translocation by procyanindin extract, another class of polyphenols, can modulate the inflammatory response in activated macrophages [27]. Thus, our results could suggest anti-inflammatory properties of catechin and epicatechin acting as antioxidants, by inhibiting the expression of genes (such as Il-6) regulated by the activation of NF-κB [28], a pro-oxidant signaling pathway.
Furthermore, we found a discrepancy in the effect of catechin and/or epicatechin supplementation between Cbs (±) mice fed a high-methionine diet and Cbs (±) mice fed a control diet. Indeed, it seems that catechin and/or epicatechin supplementation produces a strong increase in Lox-1 and, therefore, Vcam-1 and Icam-1 expression, making these molecules potentially atherogenic even in normohomocysteinemia. How polyphenols can have a contradictory effect between two conditions is not well understood, but it is a well-described phenomenon. This apparent contradictory effect on normohomocysteinemia and hyperhomocysteinemia could be due to the dose administrated but remains to be elucidated.
Our study design did not allow us to determine which cell types were the direct or indirect targets of catechin and/or epicatechin supplementation (i.e., endothelial cells vs. smooth muscle cells). The oral bioavailability of flavonoids is relatively low, and the major site of absorption is the small intestine [29]. Moreover, a study on human volunteers after consumption of red wine showed that if flavonoids are protective nutrients, the active forms are likely to be metabolites, which are more abundant in plasma [30]. Therefore, we cannot fully elucidate whether the observed effects were mediated by the ingested catechin or epicatechin or their metabolites. However, the conversion of catechin proceeds five times faster than that of epicatechin, which may affect their bioavailability and could explain the observed differences in treatment effects between these two diastereomers [31].
In conclusion, a daily consumption of 50 μg of catechin or epicatechin appears to be protective in hyperhomocysteinemic mice, while the daily consumption of both polyphenols seems to be deleterious. We found that both catechin and epicatechin, given individually, decrease plasma tHcy, but that catechin seems to be a more potent anti-atherogenic and anti-inflammatory agent than epicatechin. Furthermore, our results show that a daily consumption of catechin and/or epicatechin in normohomocysteinemic mice seems to have negative effects on expression of endothelial dysfunction biomarkers but not on proinflammatory cytokine expression. Our results highlight a novel approach to reduce plasma homocysteine level and its associated endothelial dysfunction. However, the combination of the two polyphenols does not appear to be an effective approach to treating endothelial dysfunction in hyperhomocysteinemia.
References
Fortin LJ, Genest J (1995) Measurement of homocyst(e)ine in the prediction of arteriosclerosis. Clin Biochem 28(2):155–162
Weber C, Noels H (2011) Atherosclerosis: current pathogenesis and therapeutic options. Nat Med 17(11):1410–1422
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acid Res 29(9):e45
Welch GN, Loscalzo J (1998) Homocysteine and atherothrombosis. N Engl J Med 338(15):1042–1050
Selhub J (1999) Homocysteine metabolism. Annu Rev Nutr 19:217–246
Refsum H, Nurk E, Smith AD, Ueland PM, Gjesdal CG, Bjelland I, Tverdal A, Tell GS, Nygård O, Vollset SE (2006) The Hordaland homocysteine study: a community-based study of homocysteine, its determinants, and associations with disease. J Nutr 136(6 Suppl):1731S–1740S
Lentz SR (2005) Mechanisms of homocysteine-induced atherothrombosis. J Thromb Haemost 3(8):1646–1654
Noll C, Hamelet J, Matulewicz E, Paul J-L, Delabar J-M, Janel N (2009) Effects of red wine polyphenolic compounds on paraoxonase-1 and lectin-like oxidized low-density lipoprotein receptor-1 in hyperhomocysteinemic mice. J Nutr Biochem 20(8):586–596
Watanabe M, Osada J, Aratani Y, Kluckman K, Reddick R, Malinow MR, Maeda N (1995) Mice deficient in cystathionine beta-synthase: animal models for mild and severe homocyst(e)inemia. Proc Natl Acad Sci USA 92(5):1585–1589
Hamelet J, Demuth K, Dairou J, Ledru A, Paul J-L, Dupret J-M, Delabar J-M, Rodrigues-Lima F, Janel N (2007) Effects of catechin on homocysteine metabolism in hyperhomocysteinemic mice. Biochem Biophys Res Commun 355(1):221–227
Eberhardt RT, Forgione MA, Cap A et al (2000) Endothelial dysfunction in a murine model of mild hyperhomocyst(e)inemia. J Clin Investiga 106(4):483–491
Li D, Liu L, Chen H, Sawamura T, Mehta JL (2003) LOX-1, an oxidized LDL endothelial receptor, induces CD40/CD40L signaling in human coronary artery endothelial cells. Arteriosclero Thrombo Vasc Biol 23(5):816–821
Hammwöhner M, Ittenson A, Dierkes J, Bukowska A, Klein HU, Lendeckel U, Goette A (2007) Platelet expression of CD40/CD40 ligand and its relation to inflammatory markers and adhesion molecules in patients with atrial fibrillation. Exp Biol Med (Maywood) 232(4):581–589
Kassab A, Ajmi T, Issaoui M, Chaeib L, Miled A, Hammami M (2008) Homocysteine enhances LDL fatty acid peroxidation, promoting microalbuminuria in type 2 diabetes. Ann Clin Biochem 45(Pt 5):476–480
Rice-Evans C, Miller N, Paganga G (1997) Antioxidant properties of phenolic compounds. Trends Plant Sci 2(4):152–159
Sies H (2010) Polyphenols and health: update and perspectives. Arch Biochem Biophys 501(1):2–5
Mattaliano MD, Huard C, Cao W, Hill AA, Zhong W, Martinez RV, Harnish DC, Paulsen JE, Shih HH (2009) LOX-1-dependent transcriptional regulation in response to oxidized LDL treatment of human aortic endothelial cells. Am J Physiol Cell Physiol 296(6):C1329–C1337
Scalbert A, Manach C, Morand C, Rémésy C, Jiménez L (2005) Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr 45(4):287–306
Stoclet J-C, Chataigneau T, Ndiaye M, Oak M-H, El Bedoui J, Chataigneau M, Schini-Kerth VB (2004) Vascular protection by dietary polyphenols. Eur J Pharmacol 500(1–3):299–313
Kaplan M, Aviram M (1999) Oxidized low density lipoprotein: atherogenic and proinflammatory characteristics during macrophage foam cell formation. An inhibitory role for nutritional antioxidants and serum paraoxonase. Clin Chem Lab Med 37(8):777–787
Kerkeni M, Addad F, Chauffert M, Chuniaud L, Miled A, Trivin F, Maaroufi K (2006) Hyperhomocysteinemia, paraoxonase activity and risk of coronary artery disease. Clin Biochem 39(8):821–825
Robert K, Chassé J-F, Santiard-Baron D, Vayssettes C, Chabli A, Aupetit J, Maeda N, Kamoun P, London J, Janel N (2003) Altered gene expression in liver from a murine model of hyperhomocysteinemia. J Biol Chem 278(34):31504–31511
Janel N, Robert K, Chabert C, Ledru A, Gouédard C, Barouki R, Delabar J-M, Chassé J-F (2004) Mouse liver paraoxonase-1 gene expression is downregulated in hyperhomocysteinemia. Thromb Haemost 92(1):221–222
Gouédard C, Barouki R, Morel Y (2004) Dietary polyphenols increase paraoxonase 1 gene expression by an aryl hydrocarbon receptor-dependent mechanism. Mol Cell Biol 24(12):5209–5222
Ou H-C, Song T-Y, Yeh Y-C et al (2010) EGCG protects against oxidized LDL-induced endothelial dysfunction by inhibiting LOX-1-mediated signaling. J Appl Physiol 108(6):1745–1756
Foncea R, Carvajal C, Almarza C, Leighton F (2000) Endothelial cell oxidative stress and signal transduction. Biol Res 33(2):89–96
Terra X, Valls J, Vitrac X et al (2007) Grape-seed procyanidins act as antiinflammatory agents in endotoxin-stimulated RAW 264.7 macrophages by inhibiting NFkB signaling pathway. J Agric Food Chem 55(11):4357–4365
Libermann TA, Baltimore D (1990) Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol 10(5):2327–2334
Raab T, Barron D, Vera FA, Crespy V, Oliveira M, Williamson G (2010) Catechin glucosides: occurrence, synthesis, and stability. J Agric Food Chem 58(4):2138–2149
Donovan JL, Bell JR, Kasim-Karakas S, German JB, Walzem RL, Hansen RJ, Waterhouse AL (1999) Catechin is present as metabolites in human plasma after consumption of red wine. J Nutr 129(9):1662–1668
Kutschera M, Engst W, Blaut M, Braune A (2011) Isolation of catechin-converting human intestinal bacteria. J Appl Microbiol 111(1):165–175
Acknowledgments
This work was supported in part by an EU grant AnEUploïdie and by Viniflhor (Office national interprofessionnel des fruits, des légumes, des vins et de l’horticulture), Ministère de l’Agriculture, programme Vin et Santé, Pathologie et biologie vasculaires. Christophe Noll is supported by a fellowship from the Ministère de l’Enseignement Supérieur et de la Recherche. We thank Dr N. Maeda (Department of Pathology, University of North Carolina, Chapel Hill, NC) for providing heterozygous Cbs mice. We thank C. Imbert, A. Djemat, and A. Kourdouli for technical assistance. We acknowledge the technical platform “Séparation, caractérisation et quantification de biomolécules” (Unité de Biologie Fonctionnelle et Adaptative, Université Paris Diderot-Paris 7, CNRS EAC 4413) for provision of Q-PCR facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Noll, C., Lameth, J., Paul, JL. et al. Effect of catechin/epicatechin dietary intake on endothelial dysfunction biomarkers and proinflammatory cytokines in aorta of hyperhomocysteinemic mice. Eur J Nutr 52, 1243–1250 (2013). https://doi.org/10.1007/s00394-012-0435-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-012-0435-0