Abstract
Neuropathic pain is a complication of cancer and diabetes mellitus and the most commonly used drugs in the treatment of the diabetic neuropathic pain have only limited efficacy. The aim of this study was to evaluate the role of the biomarker interleukin-1beta (IL-1ß) in the pharmacological interaction of gabapentin with tramadol in a model of diabetic neuropathic pain. CF-1 male mice, pretreated with 200 mg/kg i.p. of streptozocin (STZ), were used and at day 3 and 7 were evaluated by the hot plate test and the spinal cord level of IL-1ß was determined. Antinociceptive interaction of the coadministration i.p. of gabapentin with tramadol, in basic of the fixed the ratio 1:1 of their ED50 values alone, was ascertained by isobolographic analysis. Tramadol was 1.13 times more potent than gabapentin in saline control mice, 1.40 times in STZ mice at 3 days and 1.28 times in STZ at 7 days. The interaction between gabapentin and tramadol was synergic, with an interaction index of 0.30 and 0.22 for mice pretreated with STZ at 3 and 7 days. The combination of gabapentin with tramadol reversed the increased concentration of IL-1β induced by STZ in diabetic neuropathic mice. These findings could help clarify the mechanism of diabetic neuropathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pain is an unpleasant sensation with physical and emotional components, and among the different types neuropathic pain is defined as “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system” (Treede et al. 2008).
Neuropathic pain is a common complication of cancer, diabetes mellitus, acquired immunodeficiency syndrome and various other infectious diseases. The pathogenesis of diabetic neuropathy (DN) is complicated, and the mechanism of this disease remains poorly understood. It has been suggested that hyperglycemia is responsible for changes in the nerve tissue (Zychowska et al. 2013). Also, the participation of glial cells has been involved in the genesis of DN (Pabreja et al. 2011; Old et al. 2015). Therefore, to better understand the mechanisms underlying DN, animal models of diabetes have been used. Between these models, the destruction of the pancreatic beta cells, inducing a deficiency in insulin production, is achieved by streptozocin (STZ) (King 2012; Kitada et al. 2016).
The pharmacotherapy of neuropathic pain includes of gabapentin, tramadol, morphine, carbamazepine, topiramate, duloxetine, venlafaxine, amitriptyline, desipramine, and lidocaine (Finnerup et al. 2015). Gabapentin is an anticonvulsant used in DN, associated with Ca and Na channels, modulation of monoamines neurotransmitter and NMDA current (Cheng and Chiou 2006; Finnerup et al. 2015). In addition, tramadol induces antinociception as an MOP receptor agonist and blocks norepinephrine and serotonin reuptake (Vazzana et al. 2015; Miotto et al. 2017).
It is recognized that DN is a pain in which the mechanism of the disease is slightly understood and monotherapy are inefficient to available antinociceptive drugs. The purpose of this study was to evaluate the role of the biomarker IL-1ß in the multimodal interaction of gabapentin with tramadol in a murine model of diabetic neuropathy.
Methods
Animals
CF-1 male mice, 28–30 g, housed in a 12-h light–dark cycle at 22 ± 1 °C, with free access to food and water, were used and the animals were acclimatized to the laboratory environment for at least 1 h before use. Experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals issued by the National Institute of Health, and experimental protocols accepted by the Institutional Animal Care and Use Committee at the Universidad de Chile, Santiago, Chile (protocol CBA No. 0852, 04/2016). Each animal was used only once and received only one dose of the drugs tested. All drugs were freshly prepared in normal saline and administered intraperitoneally (i.p.) in a saline solution at a constant volume of 10 ml/kg. The behavior test was performed by investigators blinded to the treatment groups. Control saline animals were run interspersed concurrently with the drug-treated animals (at least two mice per group), which prevented all the controls being run on a single group of mice at one time.
Algesiometric assay
The hot plate test was performed at 50 ± 0.5 °C with an automatic device (Ugo Basile, Italy) according to Miranda et al. (2017). The animals were free to move, and the conduct considered as signs of pain was the licking of the forelegs which was expressed in seconds (latency time) with a cutoff time of 30 s to elude skin damage The following measurements were set: before and after administration of the test drug. Hot plate latencies were converted to % of maximum possible effect (% MPE). The latency period, in seconds, for saline sham control group animals was 24.39 ± 1.58 (n = 12).
Isobolographic analysis
Isobolographic analysis was used to characterize the interaction between gabapentin and tramadol in the hot plate test, according to Miranda et al. (2016).
Determinations of IL-1ß
Interleukin-1beta concentrations were determined using commercially available ELISA kits (Miranda et al. 2017). Small spinal samples from mice were lysed in Sigma buffer (20 mM Tris, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 1 mM EDTA and 0.1% SDS (pH 7.5) with protease inhibitors cocktail). Then, samples were homogenized and centrifuged for 20 min at 13,000 rpm at 4 °C. Supernatants were collected and protein was determined by microassay (Bio-Rad, Hercules, CA, USA). The protein concentrations in all samples were diluted to 5 mg/mL. IL-1β was determined by ELISA kits (eBioscience, San Diego, CA, USA) according to the manufacturer’s instructions (500 μg proteins of sample). Results were expressed as IL-β concentration (pg/mg protein).
Protocol
Dose–response curves for the antinociceptive effect of gabapentin or tramadol and their combinations and determinations of spinal IL-1ß were obtained using at least six animals for each dose administered i.p. Testing procedures were conducted on days 3 and 7 after STZ administration. The concentration that produced 50% antinociception (ED50) for each drug was expressed as % MPE. A dose–response curve was also obtained by the i.p. co-administration of fractions of their respective ED50 values: 1/2, 1/4, 1/8 and 1/16. Synergism is defined as the effect of a drug combination that is higher and statistically different than the theoretically calculated effects of the drug combination with the same proportions in addition. The interaction index (I.I) was calculated according to experimental ED50/theoretical ED50.
Drugs
Experimental DN was induced by i.p. administration of 200 mg/kg of STZ (Miranda et al. 2017) and animals were fasted for 3 h before drug administration. With Hemoglucotest (Roche Diagnostic labs), blood glucose levels were determined and animals were considered diabetic if levels were 200 ≥ mg/dL. Fasting blood glucose was taken at 3 and 7 days following STZ injection and controls mice were injected with saline.
The antinociception of the drugs, individually and in combination, was evaluated after 30 min of administration of the drugs. Streptozocin, gabapentin and tramadol hydrochloride were purchased from Sigma Chemical Co., St. Louis, MO, USA.
Statistical analysis
Results are presented as mean values ± SEM, or ED50 values with 95% CI. Statistical analysis of the isobolograms was calculated by t test for independent means with the program Pharm Tools Pro (version 1.27; The McCary Group Inc., Allentown, PA, USA). p values < 0.05 (p < 0.05) were considered significant.
Results
Streptozocin (STZ) induction of diabetes
Control mice had an average fasting blood glucose level of 108.80 ± 17.71 mg/dL after STZ (200 mg/kg, i.p.), glycemia increase on day 3 to 241.54 ± 32.20 mg/dL, and day 7 to 298.46 ± 21.61 mg/dL, as can be seen in Fig. 1.
Antinociception by gabapentin
Gabapentin (3–100 mg/kg, i.p.) produced antinociception dose-dependent on the hot plate assay, with an ED50 of 18.02 ± 1.20 mg/kg (n = 24), and pretreatment of the mice with STZ decreased the value of latency on day 3 to 7.51 ± 0.57 mg/kg and on day 7 to 6.10 ± 0.48 mg/kg (see Fig. 2).
Dose response (mg/kg) of the antinociceptive activity of gabapentin (GBP) and tramadol (TRAM) i.p. in the mice hot plate test. Each point is the mean ± SEM of six animals. % MPE = percentage of maximum possible effect. a Control GBP, b 3 days and c 7 days GBP post-STZ pretreatment. d Control TRAM, e 3 days and f 7 days TRAM post-STZ pretreatment
Antinociception by tramadol
The i.p. administration of tramadol (3–100 mg/kg) induced a dose-dependent antinociception, with an ED50 ED50 of 15.87 ± 2.10 mg/kg (n = 24), and the pretreatment of STZ reduced the latency on day 3 to 5.34 ± 0.23 mg/kg (n = 24) and on day 7 to 4.76 ± 0.68 mg/kg (n = 24) (see Fig. 2).
Tramadol was 1.13 times more potent than gabapentin in control mice, 1.40 times in mice pretreated with STZ at 3 days and 1.28 times in mice pretreated with STZ at 7 days.
Antinociception by the combination of gabapentin with tramadol
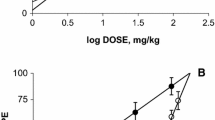
The coadministration i.p. of gabapentin with tramadol was synergistic with the following ED50: for theoretical control 8.90 ± 0.52 mg/kg; in STZ DN mice at 3 days 5.01 ± 0.78 mg/kg and at 7 days 3.78 ± 0.65 mg/kg (see Fig. 3). Besides, the I.I values were 0.30 and 0.22 for animals pretreated with STZ at 3 days and 7 days, respectively.
Isobolograms for the i.p. administration of the combination of gabapentin (GBP) and tramadol (TRAM), in the hot plate assay of mice after 3 days and 7 days of STZ pretreatment. Theoretical ED50 value with 95% CI (closed circle). Experimental ED50 value with 95% CI at 3 days (open circle) and 7 days (closed square)
Evaluation of IL-1ß
The control value of mice spinal cord levels of IL-1ß were significantly elevated at 3 and 7 days after induction of DN by STZ (see Fig. 4). Administration of gabapentin or tramadol reversed significantly the increase in concentration of IL-1ß induced by STZ either at 3 or 7 days. Besides, the combination of gabapentin with tramadol reversed significantly the elevated concentration of IL-1ß induced by DN-STZ, demonstrating a synergistic effect of the combination, as shown in Fig. 4.
Effect of gabapentin (GBP) and tramadol (TRAM) as ED50, via i.p. on the mice spinal cord levels of IL-1β, expressed as pg/ml protein, in control saline mice and mice pretreated with STZ after 3 days and 7 days in the hot plate assay. Each point is the mean ± SEM of 12 animals. *Significance vs. saline control: p < 0.05; **significance vs. STZ control: p < 0.05
Discussion
In this study, using a murine neuropathic model induced by the i.p. administration of STZ, gabapentin, tramadol and their combination were able to induce a dose-dependent antinociception, in which tramadol was 1.13 times more potent than gabapentin in control mice; 1.40 times in DN mice of 3 days and 1.28 times in DN mice of 7 days. Besides, the combination of gabapentin with tramadol displayed potency, expressed as ED50 in mg/kg, of 1.77 times more than that of control at 3 days and 2.35 times more than that of control at 7 days in DN mice. These results are concordant with previous reports of the antinociceptive effect of gabapentin and tramadol in other algesiometer tests, i.e. tail flick, acetone, von Frey assays (Dai et al. 2008; Miranda et al. (2016); Corona-Ramos et al. (2016).
The pharmacological interaction between gabapentin and tramadol was synergistic, since the results showed a significantly greater reduction in pain intensity with the combination compared to gabapentin–tramadol control in ND 3 days and 7 days. This increased pain relief could be explained by the different mechanisms of action of each component of the combination, according to multimodal analgesia.
Gabapentin is an anticonvulsant with high-affinity binding to the α2δ subunit of voltage-activated calcium channels, inhibition of voltage-activated sodium channels, alteration of monoamine neurotransmitter release and blood serotonin levels, and selective enhancing of the NMDA current (Cheng and Chiou 2006; Finnerup et al. 2015).
Tramadol is a synthetic analgesic drug with antinociceptive properties induced by activation of opioid receptors and by acting on monoamine receptor systems, blocking norepinephrine and serotonin reuptake (Vazzana et al. 2015; Miotto et al. 2017).
There is considerable evidence that the pro-inflammatory cytokine IL-1β is involved in the pathogenesis of DN. Increased levels of IL-1ß are correlated with the progression of nerve degeneration in DN and this proinflammatory cytokine affects glial cells and neurons to set the pathological process of DN (Zhou and Zhou 2014; Muhammad et al.2016).
The significantly increased levels of the pro-nociceptive and pro-inflammatory cytokine IL-1ß in the spinal cord of the mice, induced through STZ administration, suggests that the pain process during DN neuropathy is mediated through enhanced proinflammatory cytokines that are released from activated microglia cells (Bishnoi et al. 2011; Old et al. 2015). Since anti-inflammatory drugs are used as symptomatic pain therapies in DN, advances in the role of inflammatory cytokines, such as IL-ß, induced by gabapentin and tramadol, informed in the present study, could be a new therapeutic pathway for inflammatory and neuropathic pain.
It has been reported that gabapentin and tramadol reverse microglial activation in the spinal cord of streptozocin-induced diabetic rats (Wodarski et al. 2009; Zychowska et al. 2013). Consequently, the present study suggested that pharmacological interaction of the combination of tramadol and gabapentin could exert synergistic effects on DN pain by suppressing neuronal and glial activation.
Pharmacotherapy, the main treatment option for DN pain, remains a major clinical challenge. The most commonly studied drug classes in the context of neuropathic pain—antidepressants, anticonvulsants, and opioids—have only limited efficacy and frequent dose-limiting adverse effects. Furthermore, due to the narrow usefulness of available treatments in DN pain, drug combinations are often used (Eisenberg and Suzan 2014; Finnerup et al. 2015).
The present results suggest that treatment of DN pain with gabapentin and tramadol combination therapy results in pain relief than treatment with either gabapentin or tramadol as a single agent, as indicated by latency time of the hot plate assay.
Conclusion
Since the treatment of neuropathic pain is usually limited and prolonged, the findings of this work are important, because the interaction described involves a potent relation between IL-1ß and DN.
References
Bishnoi M, Bosgraaf CA, Abooj M et al (2011) Streptozotocin-induced early thermal hyperalgesia is independent of glycemic state of rats: role of transient receptor potential vanilloid 1(TRPV1) and inflammatory mediators. Mol Pain 7:52–63
Cheng JK, Chiou LCh (2006) Mechanism of the antinociceptive action of gabapentin. J Pharmacol Sci 100:471–486
Corona-Ramos JN, De la O-Arciniega M et al (2016) The antinociceptive effects of tramadol and/or gabapentin on rat neuropathic pain induced by a chronic constriction injury. Drug Dev Res 77:217–226
Dai X, Brunson CD, Rockhold RW et al (2008) Gender differences in the antinociceptive effect of tramadol, alone or in combination with gabapentin, in mice. J Biomed Sci 15:645–651
Eisenberg E, Suzan E (2014) Drug combinations in the treatment of neuropathic pain. Curr Pain Headache Rep 18:463–470
Finnerup NB et al (2015) Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 14:162–173
King AJ (2012) The use of animal models in diabetes research. Br J Pharmacol 166:877–894
Kitada M, Ogura Y, Koya D (2016) Rodent models of diabetic nephropathy: their utility and limitations. Int J Nephrol Renovascular Dis 9:279–290
Miotto K, Cho AK, Khalil MA et al (2017) Trends on tramadol: pharmacology, metabolism and misuse. Anesth Analg 124:44–51
Miranda HF, Noriega V, Prieto JC et al (2016) Antinociceptive interaction of tramadol with gabapentin in experimental mononeuropathic pain. Basic Clin Pharmacol Toxicol 119:210–214
Miranda HF, Sierralta F et al (2017) Antinociceptive interaction of gabapentin with minocycline in murine diabetic neuropathy. Inflammopharmacology 25:91–97
Muhammad AA, Arulselvan P, Cheah PS et al (2016) Evaluation of wound healing properties of bioactive aqueous fraction from Moringa oleifera Lam on experimentally induced diabetic animal model. Drug Des Dev Ther 10:1715–1730
Old EA, Clark AK, Malcangio M (2015) The role of glia in the spinal cord in neuropathic and inflammatory pain. Handb Exp Pharmacol 227:145–170
Pabreja K, Dua K, Sharma S, Padi SS et al (2011) Minocycline attenuates the development of diabetic neuropathic pain: possible anti-inflammatory and antioxidant mechanisms. Eur J Pharmacol 661:15–21
Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW et al (2008) Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 70:1630–1635
Vazzana M, Andreani T, Fangueiro J et al (2015) Tramadol hydrochloride: pharmacokinetics, pharmacodynamics, adverse side effects, co-administration of drugs and new drug delivery systems. Biomed Pharmacother 70:234–238
Wodarski R, Clark AK, Grist J et al (2009) Gabapentin reverses microglial activation in the spinal cord of streptozotocin-induced diabetic rats. Eur J Pain 13:807–811
Zhou J, Zhou S (2014) Inflammation: therapeutic targets for diabetic neuropathy. Mol Neurobiol 49:536–546
Zychowska M, Rojewska E, Kreiner G et al (2013) Minocycline influences the antiinflammatory interleukins and enhances the effectiveness of morphine under mice diabetic neuropathy. J Neuroimmunol 262:35–45
Acknowledgements
This work was partially supported by the project Fondecyt 11140757, Chile.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Miranda, H.F., Poblete, P., Sierralta, F. et al. Interleukin-1beta in synergism gabapentin with tramadol in murine model of diabetic neuropathy. Inflammopharmacol 27, 151–155 (2019). https://doi.org/10.1007/s10787-018-0532-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-018-0532-7