Abstract
Aim
Stable gastric pentadecapeptide BPC 157, administered before a high-dose magnesium injection in rats, might be a useful peptide therapy against magnesium toxicity and the magnesium-induced effect on cell depolarization. Moreover, this might be an NO-system-related effect. Previously, BPC 157 counteracts paralysis, arrhythmias and hyperkalaemia, extreme muscle weakness; parasympathetic and neuromuscular blockade; injured muscle healing and interacts with the NOS-blocker and NOS-substrate effects.
Main methods
Assessment included magnesium sulfate (560 mg/kg intraperitoneally)-induced muscle weakness, muscle and brain lesions, hypermagnesemia, hyperkalaemia, increased serum enzyme values assessed in rats during and at the end of a 30-min period and medication (given intraperitoneally/kg at 15 min before magnesium) [BPC 157 (10 µg, 10 ng), l-NAME (5 mg), l-arginine (100 mg), alone and/or together]. In HEK293 cells, the increasing magnesium concentration from 1 to 5 mM could depolarize the cells at 1.75 ± 0.44 mV.
Key findings
l-NAME + magnesium-rats and l-arginine + magnesium-rats exhibited worsened severe muscle weakness and lesions, brain lesions, hypermagnesemia and serum enzymes values, with emerging hyperkalaemia. However, l-NAME + l-arginine + magnesium-rats exhibited all control values and normokalaemia. BPC 157 abrogated hypermagnesemia and counteracted all of the magnesium-induced disturbances (including those aggravated by l-NAME or l-arginine). Thus, cell depolarization due to increasing magnesium concentration was inhibited in the presence of BPC 157 (1 µM) in vitro.
Significance
BPC 157 likely counteracts the initial event leading to hypermagnesemia and the life-threatening actions after a magnesium overdose. In contrast, a worsened clinical course, higher hypermagnesemia, and emerging hyperkalaemia might cause both l-NAME and l-arginine to affect the same events adversely. These events were also opposed by BPC 157.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
We hypothesize that the stable gastric pentadecapeptide BPC 157 (GEPPPGKPADDAGLV, M.W. 1419, PL-10, PLD-116, PL 14736) (Sikiric et al. 2014) administered before a high-dose magnesium injection in rats may serve as a useful peptide therapy against magnesium toxicity and magnesium-induced effects on cell depolarization, and that this might be an NO-system-related effect. BPC 157 was originally an anti-ulcer peptide (used in trials for ulcerative colitis and now is in trials for the treatment of multiple sclerosis) that largely interacts with NO-system (Sikiric et al. 2014); BPC 157 is known to counteract various induced potassium disturbances (Barisic et al. 2013; Stambolija et al. 2016). Moreover, BPC157 is known to affect several molecular pathways (Cesarec et al. 2013; Chang et al. 2011, 2014; Huang et al. 2015; Tkalcevic et al. 2007). This might be important considering magnesium sulfate safety in treating and preventing eclampsia and concerns regarding the possibility of hypermagnesemia toxicity in eclampsia treatment (Euser and Cipolla 2009). Also, hypermagnesemia has been reported in patients who ingest large amounts of Epsom salts and magnesium-containing cathartics (Nordt et al. 1996). Signs and symptoms of this condition include extreme muscle weakness, loss of deep tendon reflexes, a mental status of depression, and cardiac dysrhythmias (Gerard et al. 1988; Nordt et al. 1996).
Arguments for the use of BPC 157 to counteract magnesium toxicity, start with virtually no known toxicity of its own (an LD1 value has not yet been achieved, and there have been no side effects in clinical trials) (Sikiric et al. 2014). Also, many arguments have been presented to support the role of BPC 157 in counteracting magnesium toxicity. BPC 157 counteracts magnesium sulfate-induced writhing, non-inflammatory and non-prostaglandin-dependent pain (Sikiric et al. 1993). There is the association of hypermagnesemia with hyperkalaemia and succinylcholine administration (Guay et al. 1998; Sato et al. 2000), parasympathetic and neuromuscular blockade (Krendel 1990; Rizzo et al. 1993). Consequently, the effectiveness of BPC 157 in disturbances associated with hyperkalaemia and/or succinylcholine administration, neuromuscular and further parasympathetic blockade (Barisic et al. 2013; Kokot et al. 2016; Stambolija et al. 2016) is likely indicative of its effectiveness against hypermagnesemia disturbances: BPC 157 has a lifesaving effect through the treatment of arrhythmias induced by KCl overdose (>12 mmol/L hyperkalaemia)(Barisic et al. 2013); it reduces potassium-induced depolarization in vitro under severe hyperkalaemic conditions (Barisic et al. 2013); it mitigates succinylcholine-induced muscle disturbances leading to paralysis, arrhythmias and hyperkalaemia (Stambolija et al. 2016). BPC 157 also counteracts digitalis-induced arrhythmias and reduces digitalis-associated fatalities (Balenovic et al. 2009), extreme muscle weakness induced by KCl overdose (Barisic et al. 2013), the effects of cuprizone (neurotoxin producing multiple sclerosis-like patterns in rats) administration (Klicek et al. 2013), serotonin syndrome (Boban Blagaic et al. 2005), and parasympathetic blockade (Kokot et al. 2016).
Furthermore, BPC 157 counteracts pupil paralysis induced by atropine (Kokot et al. 2016). In addition, BPC 157 strongly accelerates muscle healing after various injuries, transection (Staresinic et al. 2006), or crushing (Novinscak et al. 2008). Many of these disturbances that were all consistently counteracted by BPC 157 application were also shown to be affected by NO-agent application, either a NOS-blocker, N(G)-nitro-l-arginine methyl ester (l-NAME) or a NOS-substrate, l-arginine (Balenovic et al. 2009; Barisic et al. 2013; Kokot et al. 2016); these effects were also counteracted by stable gastric pentadecapeptide BPC 157 (Balenovic et al. 2009; Barisic et al. 2013; Kokot et al. 2016).
It is noteworthy that despite the role of magnesium in counteracting l-NAME effects (Cengiz et al. 2016) in hypermagnesemia after massive doses of magnesium, the application of l-NAME and/or l-arginine was not investigated. Previously, arginine was known to induce hypermagnesemia and hyperkalaemia in nephrectomized rats (Whang et al. 1988), and finally, the mitochondrial free Mg2+concentration is commonly increased in the presence of l-arginine, an NO-donor (S-nitroso-N-acetylpenicillamine), and the NOS-inhibitors l-NAME or N(G)-monomethyl-l-arginine (L-NMMA) (Manzo-Avalos et al. 2002; Sato et al. 2000).
Thus, in the present study, pentadecapeptide BPC 157, l-NAME, and l-arginine were given alone and/or in combination to rats with a particular focus on muscle disability and encephalopathy that might appear soon after a high dose of magnesium sulfate. Particular choice of magnesium dose attempts to correlate with patients’ conditions (Gerard et al. 1988; Nordt et al. 1996). The effect of BPC 157 on the depolarization of HEK293 cells when Mg2+ concentration changed was also investigated.
Materials and methods
The experimental procedure, approved by the local Ethics Committee (UP/I 322-01/07-01/210), included male Wistar albino rats with 180–250 g body weights, randomly assigned, 10 rats, at least, per group per each experimental interval. Intraperitoneal injection of magnesium sulfate (560 mg/kg, 1 mL/rat) was given immediately before the assessment procedure. Medication (pentadecapeptide BPC 157 (manufactured by Diagen, Ljubljana, Slovenia) (Barisic et al. 2013; Kokot et al. 2016; Stambolija et al. 2016), GEPPPGKPADDAGLV, M.W. 1419, partial sequence of human gastric juice protein BPC, peptide with 99% (HPLC), freely soluble in water at pH 7.0 and in saline dissolved in saline (10 μg, 10 ng/kg b.w.); and l-NAME (5 mg/kg) and l-arginine (100 mg/kg) alone and/or together were intraperitoneally injected 15 min before the magnesium sulfate, whereas the controls simultaneously received an equivolume of saline (5.0 ml/kg). All rats were killed 30 min after magnesium sulfate administration.
Briefly, 24 h before the experiments, the rats were exercised by 20 consecutive front and hind paw grips on a vertical grid, gently held at the base of their tail as previously described (Meinen et al. 2012). Immediately after magnesium sulfate administration, the rats were placed on an upside-down grid; if the rats fell down, they were continuously placed again within 1 min until the end of the 30-min period. The amount of time they could hold on to the grid reflected the grade of fatigue and muscle weakness. The scoring assessment (0–5, healthy rats presented with scores of 4 and 5) was carried out in 1-min intervals until the end of the experiment as follows: 0/5: immediately falling, no contraction, hunched posture with flaccid paralysis; 1/5: falling (<5 s), muscle flicker, but no movement, hunched posture upon falling; 2/5: falling (<10 s), movement possible, but not against gravity, hunched posture upon falling; 3/5: falling (<20 s), movement possible against gravity, but not against resistance by the examiner, hunched posture upon falling; 4/5: no obvious fatigue, movement possible against some resistance by the examiner, normal posture and activity upon falling (>150 s); 5/5: no fatigue, movement possible against significant resistance by the examiner, normal posture and activity upon falling (>150 s).
Immediately after the animals were killed (30 min after magnesium sulfate administration), the muscle was fixed (immediately after skinning the leg) in buffered formalin (pH 7.4) for 24 h, dehydrated, and embedded in paraffin wax. Transverse sections were used for pathohistological evaluation, stained with haematoxylin and eosin, and examined in a blinded fashion. A special program called ISSA (VAMSTEC, Zagreb, Croatia) was used for morphometric analysis. Five high-power fields from the quadriceps muscle, which were examined as semi-serial muscle sections, were randomly selected for analysis. In selected areas, the smallest diameters of the smallest muscle fibres were measured as previously described, and the healthy values of the quadriceps muscle (31 ± 3 µm) were considered as normal (Novinscak et al. 2008; Pevec et al. 2010; Stambolija et al. 2016; Staresinic et al. 2006). For pathohistological evaluation, transverse sections of diaphragm muscles were used. They were measured with the same procedure used for the upper leg muscles, and the healthy values of 32 ± 4 µm were considered normal (Novinscak et al. 2008; Pevec et al. 2010; Stambolija et al. 2016; Staresinic et al. 2006).
Immediately after killing, the brain (whole 5 μm brain slices) was fixed in 10% neutral buffered formalin for 2 days. After fixation, the brain was grossly inspected and cut by consecutive coronal sections. Brain slabs were dehydrated in a graded ethanol series and embedded in paraffin. Paraffin blocks were cut into 5 μm thin sections, deparaffinated in xylene, rehydrated in graded ethanol and stained with haematoxylin and eosin. As described previously (Ilic et al. 2009, 2010, 2011a, b; Klicek et al. 2013), the intensity and distribution of brain lesions (swollen or damaged, hypoxic neurons), and brain oedema were described and evaluated semi-quantitatively as follows. While 0 generally indicated no changes, the lesions were subsequently scored as follows: 0–3, oedema (1: weak diffuse and/or perifocal; 2: moderate; 3: strong and generalized); 0–4, balonized or red neurons (1: <5% red neurons, 2: 5–30% red neurons, 3: 30–50% red neurons, 4: >50% red neurons) (note, neurons are especially vulnerable to damage from hypoxia, which causes distinctive histological changes); hypoxic (ischaemic) neurons show pronounced cytoplasmic eosinophilia (red neurons), collapse of cytoplasm with accentuated pericellular spaces, and pyknotic nuclei with indistinct nucleoli. Ultrastructurally, ischaemia produced changes in cellular necrosis, with breaks in nuclear and cell membranes and flocculent densities in mitochondria.
ECG recording
In deeply anaesthetized rats, the ECG was recorded continuously for all three main leads by positioning stainless steel electrodes on all four limbs, using an ECG monitor and 2090 Medtronic programmer (Minneapolis, MN, USA) connected to a LeCroy WaveRunner LT342 digital oscilloscope (Chestnut Ridge, NY, USA), which enabled precise recordings, measurements and analysis of ECG parameters as previously described (Barisic et al. 2013) (note, since all findings were normal, specific data are not shown).
Plasma electrolyte concentration
As previously described (Barisic et al. 2013), the blood samples of separate groups of animals were collected from the rat’s eye with heparinized tubes, and plasma electrolyte concentrations were measured by an autoanalyser (DRI-CHEM800 V, Fuji-Film, Tokyo, Japan). K+, Na+, Cl− serum values were assessed at 30 min after the administration of magnesium sulfate.
Enzyme activity
To determine serum values (IU/l) of aspartate transaminase (AST) and alanine transaminase (ALT) (Novinscak et al. 2008), blood samples were centrifuged for 15 min at 3000 rpm, immediately after death. All tests were measured using an Olympus AU2700 analyser with original test reagents (Olympus Diagnostica, Lismeehan, Ireland) as previously described (Novinscak et al. 2008).
Cell culture
HEK293 cells were grown on glass coverslips in Dulbecco’s modified Eagle’s medium (DMEM) which contained 3.7 g/L NaHCO3 in addition to 2 mM l-glutamine, 10 mL/L penicillin/streptomycin (10,000 E/10,000 mg/mL) and 10% fetal calf serum (FCS). Cells were kept at 5% CO2 at 37 °C. Cells were used from passages 123–128, 3–6 days after trypsinization (0.25% trypsin–EDTA solution, Sigma-Aldrich, in Mg2+- and Ca2+-free HANKS solution).
Patch clamp studies
Coverslips with HEK293 cells were mounted at the bottom of a perfusion chamber on an inverted microscope (Axiovert 10 Zeiss, Gottingen, Germany). Membrane voltages (V m) of HEK293 cells were measured using the slow-whole-cell patch clamp technique (Barisic et al. 2013) and a Ringer-type solution containing 145 mM NaCl, 1.6 mM K2HPO4, 0.4 mM KH2PO4, 5 mM d-glucose, 1 mM MgCl2, and 1.3 mM calcium gluconate (pH 7.4). For the high Mg2+ solution, 5 mM NaCl was replaced with 5 mM MgCl2 (140 mM NaCl, 1.6 mM K2HPO4, 0.4 mM KH2PO4, 5 mM d-glucose, 6 mM MgCl2, 1.3 mM calcium gluconate). BPC 157 was dissolved in both the solutions. All experiments were performed at 37 °C with a bath perfusion rate of 10 mL/min. Patch clamp pipettes were filled with 95 mM potassium gluconate, 30 mM KCl, 4.8 mM Na2HPO4, 1.2 mM NaH2PO4, 5 mM d-glucose, 1.3 mM calcium gluconate, 1.03 mM MgCl2, and 1 mM ATP (pH 7.2). To this solution, 160 μM of nystatin was added to permeabilize the membrane under the pipette. The pipette resistance was 5–10 MΩ. The Vm was measured with a patch-clamp amplifier (U. FröbePhysiologische Institut, Freiburg, Germany) and recorded continuously on a pen recorder (WeKa graph, Kaltbrunn, Switzerland).
Statistical analysis was performed using the parametric two-way mixed-model ANOVA (one factor is repeated-measures) and Student–Newman–Keuls test to compare the difference between groups. In addition, non-parametric Kruskal–Wallis and post hoc Mann–Whitney U tests were used. Fisher’s exact probability test was used to assess the frequency differences between groups. P values of 0.05 or less were considered statistically significant.
Results
In general, a syndrome that was counteracted by stable gastric pentadecapeptide BPC 157 was abrupt administration of magnesium overdose (Table 1; Figs. 1, 2, 3, 4, 5, 6). In rats with induced hypermagnesemia [and frequently, hyperkalaemia (with both l-NAME and l-arginine], severe muscle weakness and prostration, decreased muscle fibres in both quadriceps muscle and diaphragm, increased serum enzyme values, nerve damage and oedema in various brain areas were observed. The most prominent damage was observed in the cerebral cortex (also extended to cerebellar nuclei with both l-NAME and l-arginine) (Table 1; Figs. 1, 2, 3, 4, 5). In vitro, BPC 157 counteracted depolarization induced by an increase in magnesium concentration (Fig. 6).
Scored were oedema (0–3), cerebral cortex (red neurons), cerebellar nn (red neurons), Purkinje cells (red neurons) (0–4), Min/Med/Max *P < 0.05, at least vs. control. Medication (BPC 157 10 µg, 10 ng, l-NAME 5 mg, l-arginine 100 mg, alone and/or together) (ip/kg) given 15 min before magnesium sulfate (560 mg/kg ip)
Magnesium sulfate-brain lesions in rats at 30 min after application. Controls presented moderate oedema with numerous hypoxic neurons in the cortex, HE, ×400 (c), and numerous hypoxic neurons in the cerebellar nucleus, HE, ×200 (c). BPC 157-rats presented weak diffused oedema with sporadic hypoxic neurons in the cortex, HE, ×400 (b) and sporadic hypoxic neurons in cerebellar nucleus HE, ×200) (B)
Depolarization of HEK293 cells caused by increasing Mg2+ concentrations from 1 to 5 mM was inhibited by BPC 157 (1 µM). a Depolarization caused by increased Mg2+ concentration was negatively correlated with the starting membrane potential; b in the presence of BPC 157 (1 µM), increased Mg2+ concentration did not depolarize HEK293 cells. Data are the mean value ± SE, the number of experiments is given in the bracket. *P < 0.05, vs. control
BPC 157 vs. magnesium intoxication
Specifically, rats treated with BPC 157 continuously maintained a normal appearance, undisturbed muscle function (Table 1; Fig. 1) and normokalaemia and they exhibited less hypermagnesemia (Fig. 3). In contrast, since 5 min after magnesium sulfate administration, all saline + magnesium-rats were severely muscularly disabled and prostrated (Table 1), along with hypermagnesemia but still normokalaemia (Fig. 3). Consistent with this, their muscle fibres showed a decrease in both the quadriceps muscle and diaphragm (Fig. 1). In the brain, they clearly exhibited an exaggerated and accelerated damage process; nerve damage and oedema appeared in various brain areas, with the most prominent damage in the cerebral cortex (Figs. 2, 5). BPC 157 + magnesium-rats had consistently less nerve damage in all damaged areas, especially in those areas that, otherwise, were the most affected (Figs. 2, 5).
l-NAME-, l-arginine-, and l-NAME + l-arginine-rats
Rats that received either l-NAME or l-arginine as individual agents and then magnesium became feeble immediately after magnesium treatment and exhibited further worsening throughout the experiment. They showed more muscle weakness and prostration, even worse brain lesions (i.e., cerebellar nuclei), additionally increased hypermagnesemia, and newly emerged hyperkalaemia. Interestingly, when magnesium-rats received l-NAME and l-arginine together (Table 1; Figs. 1, 2, 3), these were all counteracted to the control values with normokalemia; thus, when given together (l-NAME + l-arginine), regularly attenuating or antagonizing each other’s response.
BPC 157 + l-arginine-, BPC 157 + l-NAME-, and BPC 157 + l-NAME + l-arginine-rats
Notably, while l-NAME-rats and l-arginine-rats presented the most severe course—muscle weakness and brain lesions, hypermagnesemia, and hyperkalaemia—a counteraction consistently appeared (Table 1; Figs. 1, 2, 3) when combined with BPC 157 (BPC 157 + l-arginine-, BPC 157 + l-NAME-, and BPC 157 + l-NAME + l-arginine-rats). BPC 157 interfered with one or the other or both the NOS-blockade and NOS-substrate administration in all of these rats (i.e., BPC 157 + l-arginine-, BPC 157 + l-NAME-, and BPC 157 + l-NAME + l-arginine-rats) and turned down muscle weakness and brain lesions as well as elevated magnesium. This occurred for the control values (BPC 157 + l-NAME-rats) or more (BPC 157 + l-arginine- and BPC 157 + l-NAME + l-arginine-rats); also, accordingly, they generally recover after muscle disability while hyperkalaemia disappeared (Table 1; Figs. 1, 2, 3).
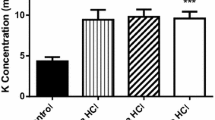
Additional biochemical analysis (Fig. 4) revealed that increased serum values in controls and a further increase in l-NAME-rats and l-arginine-rats correspondingly decreased until the control values (BPC 157 + l-NAME-rats, l-NAME + l-arginine-rats) or more (BPC 157 + l-arginine-rats, BPC 157 + l-NAME + l-arginine-rats and BPC 157-rats) (Fig. 4) resulting in normal calcium and sodium serum values (Fig. 3).
All ECG findings were normal, and data are not specifically shown.
In vitro, HEK 293 cells
The effects of increased Mg2+ concentration (5 mM) were calculated by comparing the membrane potential during Mg2+ (5 mM) effects on the cell and membrane potential with that observed while overperfusing the cells with Ringer-type solution alone (Mg2+ 1 mM). The increasing Mg2+ concentration from 1 to 5 mM depolarized the cells at 1.75 ± 0.44 mV (n = 4). The depolarization effects correlated with the starting membrane potential (r = −0.6, Fig. 6a). When we repeated the experiments in the presence of BPC 157 (1 µM), the depolarization effect disappeared (0.38 ± 0.24 mV, n = 4) (Fig. 6b).
Discussion
The clinical correlation (Euser and Cipolla 2009; Gerard et al. 1988; Nordt et al. 1996) includes producing a severe syndrome in rats immediately after an alike abrupt administration of magnesium overdose. Magnesium overdose caused hypermagnesemia (and frequently, hyperkalaemia), severe muscle weakness and prostration, decreased muscle fibres in both the quadriceps muscle and the diaphragm [as we demonstrated in rats treated with succinylcholine (Stambolija et al. 2016)], increased serum enzyme values, nerve damage and oedema in various brain areas, with the most prominent damage in the cerebral cortex. In this study, we demonstrated that the stable gastric pentadecapeptide BPC 157, which counteracted various encephalopathies, even those changes occurring rapidly (Ilic et al. 2009, 2010, 2011a, b; Lojo et al. 2016; Sikiric et al. 2016), may serve as a useful peptide therapy against magnesium toxicity and magnesium-induced effects on cell depolarization. That might be a particular NO-system-related effect that would also be particularly affected by application of the stable pentadecapeptide BPC 157 (Sikiric et al. 2014).
An especial BPC 157/NO-relationship was established in various experimental models and species, providing that it might interfere with the effects of either NOS-blockade or NOS-substrate agent application (Sikiric et al. 2014). Furthermore, as individual agents, both l-NAME and l-arginine rapidly aggravated all syndrome parameters, such as more severe and protracted muscle weakness and prostration and more hypermagnesemia and hyperkalaemia providing insight about the NO system → hypermagnesemia → hyperkalaemia relationships. Moreover, l-NAME and l-arginine caused more decreased muscle fibres in both the quadriceps muscle and the diaphragm; more increased serum enzyme values; and increased nerve damage and oedema in various brain areas, particularly cerebellar nuclei, within 30 min (at the end of the experiment). Thus, like previously with normal pupil and atropine-induced mydriasis (Kokot et al. 2016), in rats that underwent magnesium overdose, we revealed parallel l-NAME/l-arginine activity. This might also be a specific aspect of the dual role of the NO-system (l-NAME vs. l-arginine) or (vs. combination) (Kokot et al. 2016; Moncada et al. 1991; Whittle et al. 1992), where each of these effects is specific (i.e., NO-related) since given together (l-NAME + l-arginine) regularly attenuated or antagonized each other’s response. Likewise, the remaining hypermagnesemia in the l-NAME + l-arginine animals means that the other system(s) such as the BPC 157 system may function along with the NO system (previously supposed to be immobilized by the mutual actions of combined l-NAME and l-arginine). Previously, a particular l-arginine/l-arginine parallel connection with the cholinergic system was also suggested (Kokot et al. 2016). Notably, magnesium belongs to a class of substances that reduces the output of acetylcholine evoked by motor nerve impulses, and the ability of magnesium salts to interrupt neuromuscular transmission was demonstrated a long time ago (for a review see, e.g., Bowman 1983).
In support, fluctuations in the mitochondrial free Mg2+ concentration exerted by NO-related compounds (i.e., NOS-substrate, NOS-inhibitor, NO-donor) modulated NO synthesis in the heart mitochondria (Manzo-Avalos et al. 2002). However, the addition of the inhibitor l-NAME produced an even greater increase in the mitochondrial free Mg2+ concentration than l-arginine (Manzo-Avalos et al. 2002). In addition, as before (Kokot et al. 2016), this parallel activity maintains the mentioned agent’s specificity because each of these seemingly similar effects would be affected differently by BPC 157 administration. For example, in magnesium-rats, once BPC 157 counteracted l-NAME-worsening or l-arginine-worsening, the rescued muscle weakness and prostration exhibited particular and distinctive outcomes. The subsequent presentation in magnesium rats treated with l-NAME, which received BPC 157, followed the control course. On the other hand, magnesium rats treated with l-arginine, which received BPC 157, exhibited a markedly improved course. In practice, this means that two pharmacologically distinct mechanisms with opposite effects on the same signalling pathway produced the same physiological response (Kokot et al. 2016), turned out with hypermagnesemia to the alike worsened pathology, and that BPC 157 can also participate (possibly via NO-and/or cholinergic-mediated mechanisms) and beneficially maintained balance due to its effect on parasympathetic and neuromuscular blockade [atropine (Kokot et al. 2016); succinylcholine (Stambolija et al. 2016)]; and l-NAME (Sikiric et al. 2014), l-arginine (Sikiric et al. 2014) and magnesium overdose, now administered alone and/or together. Eventually, the remaining hypermagnesemia in the l-NAME + l-arginine animals was further decreased with BPC 157 addition (l-NAME + l-arginine + BPC 157 animals). This more complex approach (l-NAME vs. l-arginine vs. combination), providing a complete NO-system presentation, had not been used regularly (for review, see, e.g., Moncada et al. 1991; Whittle et al. 1992) because NO studies are usually limited to the blunted generation of NO only and are less precise (Sikiric et al. 2014). Moreover, this approach was not applied in beneficial NO–magnesium studies (Cengiz et al. 2016).
The beneficial effect of pentadecapeptide BPC 157 (no muscle weakness, markedly counteracted brain lesions, lessened hypermagnesemia, maintained normokalaemia) is common for a µg–ng/kg dose range and is significant, because the same doses used and beneficial effects were previously noted (Balenovic et al. 2009; Barisic et al. 2013; Kokot et al. 2016; Novinscak et al. 2008; Sikiric et al. 2014; Stambolija et al. 2016; Staresinic et al. 2006). Furthermore, combined hyperkalaemia/hypermagnesemia consistently appeared along with worsening and then disappeared with amelioration, indicating that clearly attenuated hypermagnesemia/maintained normokalaemia is suggestive that BPC 157 might, here, also counteract the initial event leading to hypermagnesemia. A supportive analogy is the counteraction of magnesium sulfate-induced writhing (Sikiric et al. 1993) that also appears rapidly and is quite specific as it is unaffected by anti-inflammatory drugs (including serotonin and histamine receptor antagonists) and is unaccompanied by prostaglandin release (Collier et al. 1968; Gyires and Torma 1984). This effect, along with the illustrative evidence, implies all of the counteracting effects mentioned before (i.e., on paralysis, arrhythmias, and hyperkalaemia, and extreme muscle weakness; parasympathetic and neuromuscular blockade; injured muscle) (Balenovic et al. 2009; Barisic et al. 2013; Kokot et al. 2016; Novinscak et al. 2008; Sikiric et al. 2014; Stambolija et al. 2016; Staresinic et al. 2006). In contrast, with a worsened course, higher hypermagnesemia, and emerging hyperkalaemia, it is likely that both l-NAME and l-arginine might adversely affect the same events (and that these could be again opposed by BPC 157).
Also, the BPC 157 beneficial effects in rats overdosed with magnesium should be considered with respect to its possible effect on the evidenced Mg2+ homeostasis tightly controlled by maintaining the equilibrium between intestinal Mg2+ absorption and renal Mg2+ excretion/re-absorption (Xie et al. 2011) and several Mg2+ transporters and channels implicated in Mg2+ absorption and/or re-absorption (for review see, e.g., Quamme 2010).
Therefore, it is important that BPC 157 can inhibit the effects of Mg2+ on the cell membrane potential directly by inhibiting Mg2+ entry into the cell. For example, depolarization of HEK293 cells when the Mg2+ concentration changed from 1 to 5 mM is negatively correlated with the starting membrane potential. Therefore, we suggest that depolarizations caused by increased magnesium concentration are due to the influx of Mg2+ into the cell, which could increase with an increase in electrochemical gradient by more negative starting membrane potentials. When the starting potential is more negative, more potassium channels are open, and their inhibition by increased magnesium concentration leads to higher depolarization of the cells. Likewise, we ruled out the alternative possibility that the inhibition of potassium channels is the cause. The presented results could not be explained by changes in the sodium concentration (from 145 to 140 mM), which lead to hyperpolarization and are positively correlated with the starting membrane potentials of the cells. As shown previously (Barisic et al. 2013; Zivanovic Posilovic et al. 2016) in the same cells, BPC 157 inhibited potassium channels and could, therefore, inhibit the effects of increased magnesium concentration on the membrane potential.
Thus, cell depolarization due to increased magnesium concentration was inhibited in the presence of BPC 157, and the life-threatening actions after a magnesium sulfate overdose were accordingly counteracted. This study raised several issues (i.e., no heart disturbances appeared), which need to be further elucidated because, for instance, BPC 157 interacts with several molecular pathways (Cesarec et al. 2013; Chang et al. 2011, 2014; Huang et al. 2015; Tkalcevic et al. 2007). However, considering the previous indicative evidence (Barisic et al. 2013; Kokot et al. 2016; Sikiric et al. 1993, 2014; Zivanovic Posilovic et al. 2016) and our findings, now reported, as well as the very safe profile of the stable gastric pentadecapeptide BPC 157 in clinical trials [for review see, i.e. (Sikiric et al. 2014)] (LD1 could be not achieved) (Sikiric et al. 2014), we suggest the further use of stable pentadecapeptide BPC 157 as an antidote against magnesium toxicity.
References
Balenovic D, Bencic ML, Udovicic M et al (2009) Inhibition of methyldigoxin-induced arrhythmias by pentadecapeptide BPC 157: a relation with NO-system. Regul Pept 156(1–3):83–89
Barisic I, Balenovic D, Klicek R et al (2013) Mortal hyperkalemia disturbances in rats are NO-system related. The life saving effect of pentadecapeptide BPC 157. Regul Pept 181:50–66
Boban Blagaic A, Blagaic V, Mirt M et al (2005) Gastric pentadecapeptide BPC 157 effective against serotonin syndrome in rats. Eur J Pharmacol 512(2–3):173–179
Bowman WC (1983) Peripherally acting muscle relaxants. In: Parnham MJ, Bruinvels J (eds) Discoveries in pharmacology, volume I: psycho- and neuro-pharmacology. Elsevier Science Publishers BV, Amsterdam, pp 106–159
Cengiz M, Ülker P, Üyüklü M et al (2016) Effect of magnesium supplementation on blood rheology in NOS inhibition-induced hypertension model. Clin Hemorheol Microcirc 63(1):57–67
Cesarec V, Becejac T, Misic M et al (2013) Pentadecapeptide BPC 157 and the esophagocutaneous fistula healing therapy. Eur J Pharmacol 701(1–3):203–212
Chang CH, Tsai WC, Lin MS, Hsu YH, Pang JH (2011) The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. J Appl Physiol (1985) 110(3):774–780
Chang CH, Tsai WC, Hsu YH, Pang JH (2014) Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts. Molecules 19(11):19066–19077
Collier HOJ, Dinnen LC, Johnson CA, Schneide C (1968) The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol Chemother 32:295–310
Euser AG, Cipolla MJ (2009) Magnesium sulfate treatment for the prevention of eclampsia: a brief review. Stroke 40(4):1169–1175
Gerard SK, Hernandez C, Khayam-Bashi H (1988) Extreme hypermagnesemia caused by an overdose of magnesium-containing cathartics. Ann Emerg Med 17(7):728–731
Guay J, Grenier Y, Varin F (1998) Clinical pharmacokinetics of neuromuscular relaxants in pregnancy. Clin Pharmacokinet 34(6):483
Gyires K, Torma Z (1984) The use of the writhing in mice for screening different types of analgesics. Arch Int Pharmacodyn Ther 267:131–140
Huang T, Zhang K, Sun L et al (2015) Body protective compound-157 enhances alkali-burn wound healing in vivo and promotes proliferation, migration, and angiogenesis in vitro. Drug Des Dev Ther 9:2485–2499
Ilic S, Brcic I, Mester M et al (2009) Over-dose insulin and stable gastric pentadecapeptide BPC 157. Attenuated gastric ulcers, seizures, brain lesions, hepatomegaly, fatty liver, breakdown of liver glycogen, profound hypoglycemia and calcification in rats. J Physiol Pharmacol 60(Suppl 7):107–114
Ilic S, Drmic D, Zarkovic K et al (2010) High hepatotoxic dose of paracetamol produces generalized convulsions and brain damage in rats. A counteraction with the stable gastric pentadecapeptide BPC 157 (PL 14736). J Physiol Pharmacol 61(2):241–250
Ilic S, Drmic D, Franjic S et al (2011a) Pentadecapeptide BPC 157 and its effects on a NSAID toxicity model: diclofenac-induced gastrointestinal, liver, and encephalopathy lesions. Life Sci 88(11-12):535–542 A
Ilic S, Drmic D, Zarkovic K et al (2011b) Ibuprofen hepatic encephalopathy, hepatomegaly, gastric lesion and gastric pentadecapeptide BPC 157 in rats. Eur J Pharmacol 667(1-3):322–329 B
Klicek R, Kolenc D, Suran J et al (2013) Stable gastric pentadecapeptide BPC 157 heals cysteamine-colitis and colon-colon-anastomosis and counteracts cuprizone brain injuries and motor disability. J Physiol Pharmacol 64(5):597–612
Kokot A, Zlatar M, Stupnisek M et al (2016) NO system dependence of atropine-induced mydriasis and l-NAME- and l-arginine-induced miosis: reversal by the pentadecapeptide BPC 157 in rats and guinea pigs. Eur J Pharmacol 771:211–219
Krendel DA (1990) Hypermagnesemia and neuromuscular transmission. Semin Neurol 10:42–45
Lojo N, Rasic Z, Zenko Sever A et al (2016) Effects of diclofenac, l-NAME, l-Arginine, and pentadecapeptide BPC 157 on gastrointestinal, liver, and brain lesions, failed anastomosis, and intestinal adaptation deterioration in 24 hour-short-bowel rats. PLoS One 11(9):e0162590
Manzo-Avalos S, Pérez-Vázquez V, Ramírez J et al (2002) Regulation of the rate of synthesis of nitric oxide by Mg(2 +) and hypoxia. Studies in rat heart mitochondria. Amino Acids 22(4):381–389
Meinen S, Lin S, Rüegg MA, Punga AR (2012) Fatigue and muscle atrophy in a mouse model of myasthenia gravis is paralleled by loss of sarcolemmal nNOS. PLoS One 7:441–448
Moncada S, Palmer RM, Higgs EA (1991) Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 43:109–142
Nordt SP, Williams SR, Turchen S, Manoguerra A, Smith D, Clark RF (1996) Hypermagnesemia following an acute ingestion of Epsom salt in a patient with normal renal function. J Toxicol Clin Toxicol 34(6):735–739
Novinscak T, Brcic L, Staresinic M et al (2008) Gastric pentadecapeptide BPC 157 as an effective therapy for muscle crush injury in the rat. Surg Today 38(8):716–725
Pevec D, Novinscak T, Brcic L et al (2010) Impact of pentadecapeptide BPC 157 on muscle healing impaired by systemic corticosteroid application. Med Sci Monit 16(3):BR81–88
Quamme GA (2010) Molecular identification of ancient and modern mammalian magnesium transporters. Am J Physiol Cell Physiol 298:C407–429
Rizzo MA, Fisher M, Lock JP (1993) Hypermagnesemic pseudocoma. Arch Intern Med 153:1130–1132
Sato K, Nishiwaki K, Kuno N et al (2000) Unexpected hyperkalemia following succinylcholine administration in prolonged immobilized parturients treated with magnesium and ritodrine. Anesthesiology 93(6):1539–1541
Sikiric P, Gyires K, Seiwerth S et al (1993) The effect of pentadecapeptide BPC 157 on inflmammatory, non-inflammatory, direct and indirect pain and capsaicin neortoxicity. Inflammopharmacology 2:121–127
Sikiric P, Seiwerth S, Rucman R et al (2014) Stable gastric pentadecapeptide BPC 157-NO-system relation. Curr Pharm Des 20(7):1126–1135
Sikiric P, Seiwerth S, Rucman R et al (2016) Brain-gut axis and pentadecapeptide BPC 157. Theoretical and practical implications. Curr Neuropharmacol [Epub ahead of print]
Stambolija V, Stambolija TP, Holjevac JK et al (2016) BPC 157: the counteraction of succinylcholine, hyperkalemia, and arrhythmias. Eur J Pharmacol 781:83–91
Staresinic M, Petrovic I, Novinscak T et al (2006) Effective therapy of transected quadriceps muscle in rat: gastric pentadecapeptide BPC 157. J Orthop Res 24(5):1109–1117
Tkalcević VI, Cuzić S, Brajsa K et al (2007) Enhancement by PL 14736 of granulation and collagen organization in healing wounds and the potential role of egr-1 expression. Eur J Pharmacol 570(1–3):212–221
Whang R, Papper S, Llach F (1988) Arginine-induced hypermagnesemia and hyperkalemia in nephrectomized rats. Magnesium 7(1):23–26
Whittle BJ, Lopez-Belmonte J, Moncada S (1992) Nitric oxide mediates rat mucosal vasodilatation induced by intragastric capsaicin. Eur J Pharmacol 218:339–341
Xie J, Sun B, Du J et al (2011) Phosphatidylinositol 4,5-bisphosphate [PIP(2)] controls magnesium gatekeeper TRPM6 activity. Sci Rep 1:146
Zivanovic Posilovic G, Balenovic D, Barisic I et al (2016) Stable gastric pentadecapeptide BPC 157 and bupivacaine. Eur J Pharmacol 793:56–65
Acknowledgements
This research was supported by the Ministry of Science, Education and Sports, Republic of Croatia (Grant Number 108-1083570-3635).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors indicate no potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Medvidovic-Grubisic, M., Stambolija, V., Kolenc, D. et al. Hypermagnesemia disturbances in rats, NO-related: pentadecapeptide BPC 157 abrogates, l-NAME and l-arginine worsen. Inflammopharmacol 25, 439–449 (2017). https://doi.org/10.1007/s10787-017-0323-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-017-0323-6