Abstract
Hypomagnesemia is the most concerned side effect of proton pump inhibitors (PPIs) in chronic users. However, the mechanism of PPIs-induced systemic Mg2+ deficit is currently unclear. The present study aimed to elucidate the direct effect of short-term and long-term PPIs administrations on whole body Mg2+ homeostasis and duodenal Mg2+ absorption in rats. Mg2+ homeostasis was studied by determining the serum Mg2+ level, urine and fecal Mg2+ excretions, and bone and muscle Mg2+ contents. Duodenal Mg2+ absorption as well as paracellular charge selectivity were studied. Our result showed that gastric and duodenal pH markedly increased in omeprazole-treated rats. Omeprazole significantly suppressed plasma Mg2+ level, urinary Mg2+ excretion, and bone and muscle Mg2+ content. Thus, omeprazole induced systemic Mg2+ deficiency. By using Ussing chamber techniques, it was shown that omeprazole markedly suppressed duodenal Mg2+ channel-driven and Mg2+ channel-independent Mg2+ absorptions and cation selectivity. Inhibitors of mucosal HCO3 − secretion significantly increased duodenal Mg2+ absorption in omeprazole-treated rats. We therefore hypothesized that secreted HCO3 − in duodenum decreased luminal proton, this impeded duodenal Mg2+ absorption. Higher plasma total 25-OH vitamin D, diuresis, and urine PO4 3− were also demonstrated in hypomagnesemic rats. As a compensatory mechanism for systemic Mg2+ deficiency, the expressions of duodenal transient receptor potential melastatin 6 (TRPM6), cyclin M4 (CNNM4), claudin (Cldn)-2, Cldn-7, Cldn-12, and Cldn-15 proteins were enhanced in omeprazole-treated rats. Our findings support the potential role of duodenum on the regulation of Mg2+ homeostasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Magnesium (Mg2+) is an essential cofactor or activator of at least 800 enzymes which are involved in numerous cellular functions, i.e., energy metabolism, cell cycle, and membrane transport. Mg2+ deficiency has been implicated in several diseases, e.g., Pakinson’s disease, asthma, hypertension, and osteoperosis [9, 40]. Therefore, plasma Mg2+ level is tightly regulated within a narrow range by collaborative actions of the intestinal absorption, renal excretion, and bone and muscle storage. Parathyroid hormone (PTH) and vitamin D had been reported to regulate plasma Mg2+ level [24, 45]. The bulk of intestinal Mg2+ absorption, approximately 90%, occurs through paracellular passive mechanism, whereas transcellular active Mg2+ absorption plays an important role during low dietary Mg2+ intake [31]. It has been previously proposed that small intestine absorbs Mg2+ exclusively through paracellular route, but transcellular Mg2+ uptake exists exclusively in the colon [9, 23]. While renal tubular Mg2+ handling is well documented [9, 45], cellular mechanism and regulatory factor of intestinal Mg2+absorption are largely unknown.
Acid peptic disorders are the result from either excessive gastric acid secretion or diminished mucosal defense that affects millions of people worldwide [27]. The most effective therapeutic agents for these disorders are proton pump inhibitors (PPIs), which are the fifth best-selling drug that has been taken by millions of chronic users worldwide [27, 30]. However, since 2006, there is a growing body of evidence indicating that PPIs-induced hypomagnesemia (PPIH) is a serious side effect of PPIs in chronic users [6, 7, 11, 25, 38]. The mechanism of PPIs induced systemic Mg2+ deficit is currently unclear. Previous reports suggested that PPIH might be due to chronic suppression of intestinal Mg2+ absorption and severe depletion of body Mg2+ storage pool [6, 7, 11, 38]. While oral Mg2+ failed to normalized plasma Mg2+ level, intravenous Mg2+ supplement rapidly cured hypomagnesemia [6, 11, 38]. In addition, hypomagnesemia was rapidly resolved when PPIs were discontinued and then recurred again within 1–2 weeks if PPIs was re-prescribed [6, 11]. These data suggested that PPIs rapidly suppressed intestinal Mg2+ absorption. However, short-term omeprazole administration did not affect intestinal Mg2+ absorption in human [35]. There was no evidence of urinary Mg2+ wasting in those PPIH patients [6, 7, 11, 25, 38]. On the other hand, a large-scale clinical investigation reported that PPIH was restricted to patients taking diuretics, i.e., loop and thiazide diuretics [8]. Since these diuretics could suppress Mg2+ reabsorption [3], renal Mg2+ wasting probably involved in the development of PPIH.
Recent in vivo studies proposed that PPIs mainly affected colonic Mg2+ handling by inducing magnesiotropic gene expressions in mice colon [17, 23]. However, the effect of PPIs on Mg2+ homeostasis was still controversial. Hess and colleagues [17] demonstrated that 20 mg/kg omeprazole treatment for 14 days suppressed serum Mg2+ level with normal urinary and fecal Mg2+ excretions in C57BL/J6 mice. On the other hand, Lameris et al. [23] reported that dietary Mg2+ restriction, but not 20 mg/kg omeprazole administration for 28 days, suppressed serum Mg2+ level in C57BL/J6 mice. Dietary inulin, which stimulated colonic Mg2+ absorption [33], could not normalize plasma Mg2+ level in PPIH mice [17]. Therefore, the large intestine may not be a suitable intestinal segment that should be modulated to counteract PPIH. On the other hand, previous in vitro studies proposed that PPIs impeded Mg2+ absorption in the small intestine [42–44]. Mertz-Nielsen et al. [28] reported that omeprazole significantly enhanced duodenal HCO3 − secretion in healthy subjects. Since omeprazole suppressed pancreatic secretion [48], thus, it specifically induced duodenal HCO3 − secretion. Previous study reported that omeprazole markedly enhanced apical HCO3 − secretion, decreased apical proton, and subsequently suppressed passive Mg2+ absorption [43, 44]. However, the effect of PPIs on duodenal Mg2+ absorption remains unknown.
In the present study, we aimed to elucidate the direct effect of short-term (4 weeks) and long-term (24 weeks) omeprazole-treatments on whole-body Mg2+ homeostasis in male Sprague-Dawley rats by determining serum Mg2+ level, urine and fecal Mg2+ excretions, and bone and muscle Mg2+ contents. Plasma Ca2+, PO4 3−, PTH, and total 25-OH vitamin D, as well as urine Ca2+ and PO4 3− were also determined. Duodenal total, Mg2+ channel-driven transcellular, and Mg2+ channel-independent paracellular Mg2+ absorptions, as well as paracellular charge selectivity, were studied. The involvement of mucosal HCO3 − secretion on omeprazole-affected duodenal Mg2+ absorption was also examined. The expressions of duodenal TRPM6, cyclin M4 (CNNM4), Cldn-2, -7, -12, and -15 of omeprazole-treated rats were also elucidated. The ultrastructure of duodenum and head of femurs were observed.
Materials and methods
Cell culture
Caco-2 cells were grown in Dulbecco’s modified Eagle medium (DMEM) (Gibco, Grand Island, NY, USA) supplemented with 12% fetal bovine serum (FBS-Gold) (PAA Laboratories GmbH, Pasching, Austria), 1% l-glutamine (Gibco, Grand Island, NY, USA), 1% nonessential amino acid (Sigma, St. Louis, MO, USA), and 1% antibiotic-antimycotic solution (Gibco) and maintained at a humidified atmosphere containing 5% CO2 at 37 °C. The Caco-2 monolayers were developed by seeding cells (5.0 × 105 cells/cm2) onto the Polyester Transwell Inserts (24-mm diameter and 0.4-μm pore size filter: Corning, Corning, NY, USA) and maintained for 14 days. Culture medium was changed daily after 48 h of seeding. After 14 days of seeding, Caco-2 monolayers were gently rinsed three times with Gibco™ PBS buffers (Gibco) and then exposed to normal-, low-, or high-Mg2+ conditions for 10 days. In normal Mg2+-treated group, Caco-2 monolayers were grown in HyClone™ DMEM (SH30081.02: GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 1 mmol/l sodium pyruvate, 12% fetal bovine serum (FBS-Gold) (PAA Laboratories GmbH), 1% l-glutamine (Gibco), 1% nonessential amino acid (Sigma), and 1% antibiotic-antimycotic solution (Gibco). In low-Mg2+-treated group, Caco-2 monolayers were grown in Mg2+-free HyClone™ DMEM (SH30262.02: GE Healthcare Life Sciences) supplemented with 1.25 mmol/l CaCl2, 1 mmol/l sodium pyruvate, 12% fetal bovine serum (FBS-Gold) (PAA Laboratories GmbH), 1% l-glutamine (Gibco), 1% nonessential amino acid (Sigma), and 1% antibiotic-antimycotic solution (Gibco). In high-Mg2+-treated group, Caco-2 monolayers were grown in Mg2+-free HyClone™ DMEM (SH30262.02: GE Healthcare Life Sciences) supplemented with 40 mmol/l MgSO4, 1.25 mmol/l CaCl2, 1 mmol/l sodium pyruvate, 12% fetal bovine serum (FBS-Gold) (PAA Laboratories GmbH), 1% l-glutamine (Gibco), 1% nonessential amino acid (Sigma), and 1% antibiotic-antimycotic solution (Gibco). In the omeprazole-treated group, Caco-2 monolayers were grown in 400 ng/ml omeprazole (Calbiochem, San Diego, CA, USA) containing culture media from day 7 to day 24. Culture medium of all experimental groups was changed daily.
Animals
This study was performed in strict compliance with the Animal for Scientific Purposes Act of Thailand and in accordance with Ethical Principles and Guidelines for the Use of Animals for Scientific Purposes, National Research Council of Thailand. All experimental procedures were approved by the Ethics Committee on Animal Experiment of Burapha University, Thailand. Male Sprague-Dawley rats (9 weeks old, weighting 250–350 g) were purchased from the National Laboratory Animal Centre, Mahidol University, Thailand. The animals were randomly allocated into three experimental groups, i.e., control, 4-week omeprazole treatment, and 24-week omeprazole treatment. They were acclimatized for 7 days before starting of the experiments. They were housed in a temperature-, humidity-, and light-controlled room with standard pellet chow containing 0.23% w/w magnesium, 1.0% w/w calcium, 0.9% phosphorus, and 4000 IU/kg vitamin D (CP, Bangkok, Thailand) and reverse osmosis water given ad libitum. The health, body weight, and food intake were monitored and recorded daily.
Experimental design
In the present study, we decided to use subcutaneous omeprazole injection that safely and effectively inhibited gastric acid secretion in rat [21] and human [2]. The first series of experiment aimed to elucidate the efficacy of subcutaneous omeprazole (20 mg/kg: Ocid® IV; Zydus Cadila, India) and oral gavage omeprazole (20 mg/kg: Losec®; AstraZeneca, Thailand) administrations on gastric acid suppression. The pellet chow was removed 4 h before and then retrieved 30 min after oral gavage or subcutaneous omeprazole administration. At 2 and 24 h after administration, stomach and duodenum were removed under thiopental anesthesia (70 mg/kg; Anesthal, Jagsonpal Pharmaceuticals ltd, India). Stomach and duodenum pH were determined using diagnostic test strips (MColorpHast™ pH-Indicator Strips, Merck-Millipore, German).
The second series of experiment aimed to study the effect of short-term and long-term omeprazole treatments on Mg2+ homeostasis in the rats. Control and 24-week omeprazole-treated rats were respectively received daily subcutaneous sham or subcutaneous omeprazole (20 mg/kg) injection for 24 weeks. In the 4-week omeprazole-treatment group, rats received subcutaneous sham injection daily for 20 weeks and subsequently followed by subcutaneous omeprazole injection for 4 weeks. For urine and feces collections, rats were housed in metabolic cages for 24 h. The health of all rats was checked daily throughout 24 weeks of injection. At the experiment end point, the rats were anesthetized with thiopental, blood was collected from left ventricle, and the rats were subsequently sacrificed. Duodenum, left and right femurs, and left soleus muscle were collected.
Analytical procedures
Plasma and urine Mg2+, Ca2+, and PO4 3− concentrations were respectively determined by xylidyl blue II, asenazo III, and phosphomolybdate method, and analyzed by an automate clinical chemistry analyzer (ILab Taurus; Instrumentation Laboratory, Bedford, MA, USA). Total serum 25-OH vitamin D level was determined by Tosoh™ Bioscience ST AIA-PACK 25-OH vitamin D and an automate Tosoh AIA-900 analyzer (Tosoh Bioscience, Inc., South San Francisco, CA, USA). Plasma PTH level was determined by ARCHITECT Intact PTH and ARCHITECT i2000sr automatic immunoassay analyzer (Abbott Diagnostics, Abbott Park, IL, USA). Soleus muscles were chopped and digested with nitric acid (Sigma). Left femurs and feces were dried, ashed, and subsequently extracted with nitric acid (Sigma). Muscle, bone, and fecal Mg2+ content were determined by an atomic absorption spectrophotometer (Shimadzu, Tokyo, Japan).
Epithelial electrical parameter measurement and dilution potential experiment
Rat duodenum was cut longitudinally, rinsed gently, mounted in a Ussing chamber (World Precision Instrument, Sarasota, FL, USA), and bathed on both sides with normal bathing solution containing (in mmol/mL) 118 NaCl, 4.7 KCl, 1.1 MgCl2, 1.25 CaCl2, 23 NaHCO3, 12 d-glucose, 2.5 l-glutamine, and 2 d-mannitol. The solution was maintained at 37 °C, pH of 7.4, osmolality of 290–295 mmol/kg H2O, and continuously gassed with 5% CO2 in 95% O2. Transepithelial potential difference (PD) and short-circuits current (Isc) were determined by Ag/AgCl electrodes and an epithelial voltage/current clamp apparatus (model ECV-4000; World Precision Instrument) as previously described [41]. Transepithelial resistance (TER) was calculated from PD and Isc by Ohm’s law.
To determine paracellular charge selectivity by measuring absolute sodium permeability (P Na) and chloride permeability P Cl and relative P Na/P Cl [16], dilution potential experiment was performed by modified method of Thongon et al. [41]. In brief, duodenal tissue was equilibrated for 10 min within Ussing chamber in a normal bathing solution containing 145 mmol/l NaCl before the apical solution was replaced with 72.5 mmol/l NaCl-containing solution. Difference between the PD before and after fluid replacement (i.e., dilution potential) was recorded. The P Na/P Cl was calculated by using the Goldman-Hodgkin-Katz equation, whereas P Na and P Cl were calculated by using Kimizuka-Koketsu equations.
Magnesium flux measurement
The duodenum (10 cm) of each rat was dissected into four pieces, which then were rapidly mounted onto four individual modified Ussing chamber setups with an exposed surface area of 0.69 cm2. The tissues were equilibrated for 10 min as mentioned above. To study total Mg2+ flux, the apical solution of one Ussing chamber setup was substituted with Mg-bathing solution containing (in mmol/l) 40 MgCl2, 2.5 CaCl2, 4.5 KCl, 12 d-glucose, 2.5 l-glutamine, 115 mannitol, and 10 HEPES pH 7.4, while the basolateral solution was substituted with Mg-free bathing solution containing (in mmol/l) 1.25 CaCl2, 4.5 KCl, 12 d-glucose, 2.5 l-glutamine, 250 d-mannitol, and 10 HEPES pH 7.4. To investigate the Mg2+ channel-independent Mg2+ transport, mucosal sites of duodenal tissues in other setups were pre-incubated for 10 min with Co(III)hexaammine (1 mmol/l; Sigma), ruthenium red (20 μmol/l; Sigma), or Co(III)hexaammine + ruthenium red. The Mg2+ channel blocker Co(III)hexaammine, which is competing the Mg2+-hexadydrate molecules, and noncompetitive pan-specific TRP channel inhibitor ruthenium red had been reported to completely inhibit epithelial Mg2+ influx [46, 49]. Suppression of mucosal Mg2+ influx in enterocyte epithelium should impede transcellular Mg2+ absorption. After pretreatment, the apical and basolateral solutions were substituted with Mg-bathing solution and Mg-free bathing solution, respectively. At 30, 60, and 120 min after solution replacements, 100-μl solution was collected from the basolateral side, as well as from the apical side. The Mg2+ concentration and the rate Mg2+ flux were determined by the method of Thongon and Krishmanra [42]. The difference between the rate of total Mg2+ transport and Mg2+ channel-independent Mg2+ transport was calculated to be the rate of Mg2+ channel-driven Mg2+ transport.
To study the involvement of basal duodenal HCO3 − secretion on omeprazole-affected Mg2+ transport, mucosal site of duodenal tissues was pre-incubated for 10 min with 500 μmol/l 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS; Sigma) or 50 μmol/l N-(2-naphthalenyl)-((3,5-dibromo-2,4-dihydroxyphenyl)methylene)glycine hydrazide (GlyH-101; Calbiochem, San Diego, CA, USA). After inhibitor pre-incubations, Mg2+ flux study was performed as mentioned above.
Western blot analysis
Caco-2 monolayers were lysed in Piece® Ripa Buffer (Thermo Fisher Scientific Inc., Rockford, IL, USA) with 10% v/v protease inhibitor cocktail (Sigma) and gentle shaking (seesaw mode) for 20 min at 4 °C. Cells were collected by scraping with an ice-cold Cell Scraper (Corning). The duodenal segment was cut longitudinally to expose the mucosa. Duodenal epithelial cells were collected by scraping the mucosal surface with an ice-cold glass slide and lysed in Piece® Ripa Buffer (Thermo Fisher Scientific Inc.) with 10% v/v protease inhibitor cocktail (Sigma). Cell and tissue lysates were sonicated, centrifuged at 12,000g for 15 min, and then heated for 5 min at 95 °C. Proteins (50 μg) or Cruz Marker™ Molecular Weight Standards (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were loaded and separated on SDS-PAGE gel, and then transferred to a polyvinylidene difluoride membrane (PVDF; Amersham, Buckinghamshire, UK). Membranes were blocked with 5% nonfat milk overnight at 4 °C and probed overnight at 4 °C with 1:1000 primary antibodies (Santa Cruz Biotechnology) raised against CNNM4 (sc-68437), Cldn-2 (sc-55617), Cldn-7 (sc-33532), Cldn-12 (sc-98608), Cldn-15 (sc-25712), and TRPM6 (sc-98695). Membranes were also reprobed with 1:5000 anti-β-actin monoclonal antibodies (Santa Cruz Biotechnology). Subsequently, membranes were incubated with 1:10,000 HRP-conjugated secondary antibodies (Santa Cruz Biotechnology) for 2 h at 25 °C, visualized by Thermo Scientific SuperSignal® West Pico Substrate (Thermo Fisher Scientific Inc.) and captured on CL-XPosure Film (Thermo Fisher Scientific Inc.). Densitometric analysis was performed using ImageJ for Mac Os X.
H&E staining
Mouse duodenal tissues were dissected and preserved overnight at 4 °C in 4% w/v paraformaldehyde in phosphate-buffered saline (PBS) (Sigma-Aldrich). After being dehydrated and cleared by graded ethanol and xylene, respectively, they were embedded in paraffin, then cut cross-sectionally into 3-μm thick section. The deparaffinized sections were stained with hematoxylin and eosin and examined under a light microscope (model BX51; Olympus, Tokyo, Japan).
SEM
Femurs were dried at 85 °C for 72 h in an incubator. Fractured head of femur was coated with an ultra-thin gold layer by a sputter coater (Polaron SC7620; Quorum Technologies Ltd., Kent, UK). Surface structure of the trabeculae of femur was captured using scanning electron microscope (SEM) (LEO1450 VP; LEO Electron Microscopy Ltd., Clifton Road, UK).
Statistical analysis
Results were expressed as means ± SE. Two sets of data were compared using unpaired Student’s t test. One-way analysis of variance (ANOVA) with Dunnett’s posttest was used for comparison of multiple sets of data. All data were analyzed by GraphPad Prism for Mac Os (GraphPad Software Inc., San Diego, CA, USA).
Results
Effect of omeprazole administration on gastric and duodenal pH
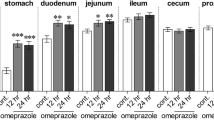
These experiments were performed to demonstrate the effect of 20 mg/kg oral gavage and subcutaneous omeprazole administrations on gastric acid secretion by means of gastric and duodenal pH measurements. After 2 h of omeprazole administration, gastric and duodenal pH of omeprazole-treated rats were significantly higher than that of sham-treated control rats (Fig. 1a, b). Gastric and duodenal pH of omeprazole-treated rats remained higher after 24 h of the last dose when compared with control rats. These results suggested that omeprazole administration (20 mg/kg, daily) effectively inhibited gastric acid secretion. Therefore, we chose subcutaneous omeprazole injection to treat the rats throughout 24 weeks of experiment.
Effect of oral gavage (OG) or subcutaneous injection (SC) of omeprazole on rat gastric and duodenal pH. Gastric (a) and duodenal (b) pH were measured by using test strips at 2 or 24 h after omeprazole administration. Body weight of control (white circles), 4-week omeprazole-treated (gray circles), and 24-week omeprazole-treated (black circles) throughout 24 week of experiment (c). **P < 0.01, ***P < 0.001 compared with the control group (n = 6)
Effect of omeprazole on Mg2+ homeostasis
These experiments aimed to observe the effect of short-term and long-term omeprazole treatment on Mg2+ homeostasis in the rats. Throughout 24 weeks of experiment, all rats were healthy and showed equal increase of body weight (Fig. 1c). The mean body weight, food and water intake, and fecal output at last week of experiment of omeprazole-treated groups were not different from those of sham-treated control group (Table 1). However, 24-week omeprazole-treated groups showed statistically higher diuresis than control group.
Plasma Mg2+ concentration (in mmol/l) of 24-week omeprazole-treated group (0.69 ± 0.05), but not 4-week omeprazole-treated group (0.93 ± 0.07), was significantly decreased in comparison to sham-treated group (1.12 ± 0.09) (Fig. 2a). The 24-week omeprazole-treated rats also had significantly lower 24-h urinary excretion compared to control rats (Fig. 2b). Fecal Mg2+ excretion of all experimental groups did not differ (Fig. 2c). Bone Mg2+ contents of 4- and 24-week omeprazole-treated groups (126.91 ± 3.79 and 95.03 ± 4.46 mmol/100 g dry weight, respectively) were significantly decreased in comparison to control group (143.71 ± 2.20 mmol/100 g dry weight) (Fig. 2d). Muscle Mg2+ content of 24-week omeprazole-treated group, but not 4-week omeprazole-treated group, was significantly lower than that of control group (Fig. 2e). These results indicated hypomagnesemia and depletion of Mg2+ storage in prolonged omeprazole-treated rats.
Effect of omeprazole on Mg2+ homeostasis in male Sprague-Dawley rats. Plasma Mg2+ level (a), 24-h urinary Mg2+ excretion (b), 24-h fecal Mg2+ excretion (c), bone Mg2+ content (d), and muscle Mg2+ content (e) of control (white bars), 4-week omeprazole-treated (gray bars), and 24-week omeprazole-treated (black bars) group. *P < 0.05, **P < 0.01, ***P < 0.001 compared with the control group (n = 6)
Effect of omeprazole on plasma total 25-OH vitamin D, PTH, Ca2+, and PO4 3−, as well as urine Ca2+ and PO4 3−, levels
Since PTH and vitamin D had been reported to regulate plasma Mg2+ level [24, 40, 45], plasma 25-OH vitamin D and PTH levels in PPIH rats were determined. As demonstrated in Table 2, total plasma 25-OH vitamin D of 24-week omeprazole-treated rats, but not 4-week omeprazole-treated rats, was significantly higher than that of control rats. However, plasma PTH level of all experimental groups was not statistically different.
Plasma Ca2+ level of 24 week omeprazole-treated rats was significantly lower than that of control rats. Plasma phosphate and urine Ca2+ levels were not different among all experimental groups. Urinary phosphate excretion of 24-week omeprazole-treated rats was significantly higher than that of control rats.
Omeprazole suppressed duodenal Mg2+ absorption
Intestinal epithelium absorbs Mg2+ through transcellular active and paracellular passive mechanisms. Transcellular active Mg2+ absorption depends on the activity of apical TRPM6 and basolateral CNNM4 proteins [9]. Paracellular passive Mg2+ uptake was modulated by the expression and function of tight junction-associated Cldns [9, 16, 20, 43]. It has been previously showed that mice small intestine absorbed Mg2+ through passive, but not active, mechanism [24]. However, TRPM6 and CNNM4 had been identified in duodenum [46] and small intestine [51], respectively. In the present study, we observed the expression of duodenal TRPM6 (Fig. 3a) and CNNM4 proteins (Fig. 3b) by Western blot analysis. The expressions of TRPM6 and CNNM4 were significantly increased in omeprazole-treated groups compared with the control group (Fig. 3a, b). Therefore, rat duodenum might absorb Mg2+ through both paracellular and transcellular mechanisms. Since the expression of TRPM6 protein is modulated by extracellular Mg2+ concentration [49], we further observed the expression of TRPM6 protein in human epithelial Caco-2 cells that were exposed to culture medium containing low- or high-Mg2+. As demonstrated in Fig. 3c, TRPM6 protein expression was significantly lower in high Mg2+- and higher in low Mg2+-exposed cells compared to the control cells. Our results agreed with previous report that TRPM6 protein expression was significantly decreased in high Mg2+-and increased in low Mg2+-exposed mammary epithelial cells [49]. However, omeprazole had no effect on TRPM6 protein expression in Caco-2 cells (Fig. 3d). In omeprazole-treated Caco-2 monolayers, TRPM6 protein expression was also significantly lower in high Mg2+- and higher in low Mg2+-exposed cells (Fig. 3d).
Effect of omeprazole on duodenal TRPM6 and CNM4 expressions. The quantitative immunobloting and representative densitometric analysis of duodenal TRPM6 (a) and CNNM4 (b) in control and omeprazole-treated groups. The quantitative immunobloting and representative densitometric analysis of TRPM6 protein in normal, high-Mg2+-, and low-Mg2+-exposed Caco-2 monolayers under control (c) or 400 ng/ml omeprazole-treated (d) condition. *P < 0.05, ***P < 0.001 compared with control group (n = 5)
These experiments aimed to observe the direct effect of short-term and long-term omeprazole treatments on duodenal Mg2+ channel-independent and Mg2+ channel-driven Mg2+ absorptions. Figure 4 demonstrated the rates of Mg2+ absorptions, i.e., total, Mg2+ channel-independent, and Mg2+ channel-driven Mg2+ transport, of sham-treated control (Fig. 4a), 4-week (Fig. 4b), and 24-week omeprazole-treated groups (Fig. 4c). In the same experimental group, Mg2+ channel-independent Mg2+ transports, as well as Mg2+ channel-driven Mg2+ transport, upon various inhibitor pretreatments were not statistically different (Fig. 4a–c). Both 4- and 24-week omeprazole-treated groups had statistically lower total Mg2+ absorption than the control group (Fig. 4d). The Mg2+ channel-independent Mg2+ transport, which was referred to paracellular Mg2+ transport, of 4- and 24-week omeprazole-treated groups were statistically lower than that of Co(III)hexaammine-treated control group (Fig. 4e). In addition, Mg2+ channel-driven Mg2+ transport, which was referred to transcellular Mg2+ transport, of 4- and 24-week omeprazole-treated groups were also statistically lower than that of Co(III)hexaammine-treated control group (Fig. 4e). These results suggested that omeprazole suppressed duodenal paracellular and transcellular Mg2+ absorptions.
Effect of omeprazole on rat duodenal Mg2+ absorption. Rate of total (total: white bars), Mg2+ channel-independent (para: gray bars), and Mg2+ channel-driven (trans: black bars) Mg2+ transport in control (a), 4-week omeprazole-treated (b), and 24-week omeprazole-treated (c) groups. Comparison of total (d), Mg2+ channel-independent (e), and Mg2+ channel-driven (f) Mg2+ transport of control (white bars), 4-week omeprazole-treated (gray bars), and 24-week omeprazole-treated (black bars) groups. *P < 0.05, **P < 0.01, ***P < 0.001 compared with the Co(III)hexaammine-treated control group (n = 5). Co Co(III)hexaammine, RR ruthenium red (n = 5)
Omeprazole had no effect on duodenal PD of all groups (Table 3). The Isc of 24-week, but not 4 week, omeprazole-treated group was significantly decreased in comparison to control group. On the other hand, 24-week omeprazole-treated group had higher TER compared to control group. These results indicated lower net ionic movement across omeprazole-treated duodenum.
Omeprazole suppressed duodenal paracellular cation selectivity
Since omeprazole impeded duodenal paracellular Mg2+ absorption, then, we observed epithelial charge selectivity which modulated paracellular permeability [16, 41, 42]. By using the dilution potential technique, we observed P Na, P Cl, and P Na/P Cl. The results showed that 4- and 24-week omeprazole-treated rats had significantly lower P Na/P Cl and P Na compared to sham-treated control group (Table 3). The P Cl was equal in all experimental groups. Therefore, omeprazole suppressed duodenal paracellular cation selectivity.
Omeprazole altered ultrastructure of duodenum
From the observation of 108 slides (9 slides per rat, 4 rats per group), we found blunted and shortened duodenal villi in 24-week omeprazole-treated rats (Fig. 5a–c). Moreover, villous to crypt (V/C) ratio of 24-week omeprazole-treated rats was significantly lower than that of control groups (Fig. 5d). This suggested shortened of villi or crypt hyperplasia [37] which was indicated lower absorption or higher secretion.
Effect of omeprazole on rat duodenum. Representative H&E stained sections of duodenum in control (a), 4-week omeprazole-treated (b), and 24-week omeprazole-treated (c) groups. Villous to crypt (V/C) ratio of control (white bars), 4-week omeprazole-treated (gray bars), and 24-week omeprazole-treated (black bars) groups. *P < 0.05 compared with the control group
Contribution of duodenal HCO3 − secretion on omeprazole-suppressed Mg2+ transport
Mertz-Nielsen et al. [28] reported that omeprazole promoted human duodenal HCO3 − secretion. Previous in vitro study showed that omeprazole enhanced apical basal and HCl-stimulated HCO3 − secretion which led to a lower Mg2+ transport across intestinal-liked Caco-2 monolayers [44]. The Cl−/HCO3 − exchanger and cystic fibrosis transmembrane conductance regulator (CFTR) in the brush-border membranes of duodenum provide important routes for duodenal HCO3 − secretion [4]. In this experiment, we pre-incubated mucosal site of duodenal tissue with Cl−/HCO3 − exchanger inhibitor DIDS and CFTR inhibitor GlyH-101 prior to perform Mg2+ transport study in Ussing chamber setups. As demonstrated in Fig. 6, DIDS and GlyH-101 had no effect on Mg2+ transport in control duodenum. However, both DIDS and GlyH-101 significantly increased Mg2+ transport in 4- and 24-week omeprazole-treated groups when compared to its corresponding vehicle-treated group. These results indicated that omeprazole impeded duodenal Mg2+ absorption due partly to mucosal HCO3 − secretion.
Contribution of mucosal HCO3 − secretion on omeprazole-suppressed duodenal Mg2+ absorption. The total Mg2+ transport of control (white bars), 4-week omeprazole-treated (gray bars), and 24-week omeprazole-treated (black bars) groups. **P < 0.01, ***P < 0.001 compared with its corresponding vehicle-treated group (n = 5)
Omeprazole enhanced duodenal Cldn-2, Cldn-7, Cldn-12, and Cldn-15 expressions
It is widely accepted that tight junction-associated Cldn protein modulates epithelial paracelullar permeability and charge selectivity [16]. Since omeprazole suppressed duodenal paracellular Mg2+ absorption and cation selectivity, we further observed the expression of Cldn proteins. Cldn-16 and -19 had been proposed as paracellular channels for Mg2+ in the kidney [19]. However, Cldn-16 and -19 were not detected along the small intestine [13], suggesting that other Cldns might be involved in paracellular intestinal Mg2+ absorption. In the present study, we observed the expressions of cation selective Cldn, i.e., Cldn-2, -7, -12, and -15, which were expressed in small intestine [13, 14]. Unexpectedly, the expressions of Cldn-2, -7, -12, and -15 were significantly increased in omeprazole-treated groups compared to control group (Fig. 7). These results demonstrated compensatory responds of duodenal epithelium for systemic Mg2+ deficit.
Effect of omeprazole on rat duodenal Cldn-2, -7, -12, and -15 expressions. The quantitative immunobloting analysis of duodenal Cldn-2, -7, -12, and -15 expressions in control and omeprazole-treated groups (a). Representative densitometric analysis of Cldn-2, -7, -12, and -15 (b) expression in control (white bars), 4-week omeprazole-treated (gray bars), and 24-week omeprazole-treated (black bars) groups. ***P < 0.001 compared with the control group (n = 5)
Omeprazole altered structure of trabeculae bone
In human, chronic PPIs administration led to increased risk of fracture and significant suppression of the trabecular bone density [1, 26]. By using SEM, we investigated the structure of trabeculae bone in the head of femurs of omeprazole-treated rats. The 24-week omeprazole-treated rats had thinner and longer trabeculae compared to sham-treated control group (Fig. 8). Wider spaces between trabeculae were also observed in omeprazole-treated rats.
Discussion
There is a growing body of evidence suggesting that severe hypomagnesemia is a side effect of PPIs in chronic users [6–8, 11, 25]. About 18, 29, and 61% of PPIH cases, respectively, had PPIs prescription for at least 2, 10, and 5 years [7]. In the present study, we found that 24-week omeprazole-treated rats had hypomagnesemia. Comparing to human’s age, approximately 16.7 rat days equal to 1 human year [32], thus, 168-day (24 weeks) omeprazole administration in rats equal to 10 years in human. On the other hand, 28-day (4 weeks) omeprazole administration in mice [23] and rats, which is less than two human years, had no effect on the plasma Mg2+ level. In addition, Denziger et al. [8] reported an association of PPIH with loop and thiazide diuretics use, which agreed with our results that PPIH rats had higher diuresis. Previous studies reported that 1,25-OH vitamin D promoted renal Mg2+ excretion [22, 24]. In the present study, the level of total 25-OH vitamin D markedly increased in omeprazole-treated rats. In addition, loop and thiazide diuretics suppresses renal tubular Mg2 reabsorption [3]. Thus, higher renal Mg2+ excretion is probably involved in development of hypomagnsemia in chronic PPIs users. However, lower urinary Mg2+ had been reported in PPIH in human [6, 11, 38] and our rat model, suggesting that hypomagnesemia should be of concern in person who continuously use PPIs for more than 2 years with diuretic administration.
Previous reports suggested that suppression of intestinal Mg2+ absorption and depletion of Mg2+ storage could be involved in the pathophysiology of PPIH [6–8, 11, 25, 38]. Mg2+ retention test demonstrated severe Mg2+ storage depletion in PPIH patients [6]. Intravenous Mg2+ but not high-dose oral Mg2+ supplement rapidly cured hypomagnesemia [11, 38], indicating that PPIs impeded intestinal Mg2+ absorption [6, 25]. Our results supported these case reports that long-term omeprazole administration suppressed plasma Mg2+ level, duodenal Mg2+ absorption, and bone and muscle Mg2+ levels in PPIH rat model. The proposed pathophysiology of PPIH in prolonged users is that PPIs continuously suppress small intestinal Mg2+ absorption which subsequently stimulates Mg2+ releasing from its storage pool. The later development of hypomagnesemia is due to the time required for depletion of Mg2+ from its storage [6].
The exact mechanism of PPIs that suppressed intestinal Mg2+ absorption is currently under debate. Previous in vivo studies indicated that PPIs mainly affected an important active Mg2+ absorption and induced magnesiotropic genes expressions in mouse colon [17, 23]. On the other hand, our previous in vitro studies proposed that PPIs affected passive Mg2+ absorption in the small intestine [42–44]. Previous in vivo study reported that omeprazole enhanced human duodenal HCO3 − secretion [28], which probably suppressed small intestinal Mg2+ absorption [44]. Our data suggested that omeprazole enhanced basal duodenal HCO3 − secretion and suppressed duodenal Mg2+ channel-driven transcellular and Mg2+ channel-independent paracellular Mg2+ absorption under an un-physiological condition of concentration gradient of MgCl2 about 40 mmol/l. Moreover, after feeding, duodenal HCO3 − secretion markedly increased by several activating factors, e.g., gastric acid, CO2, and neurohumeral factors [4]. Secreted HCO3 − suppressed luminal proton and subsequently increased intra-duodenal pH. Since luminal proton enhanced Mg2+ absorptions [18, 23, 43], this impeded large amount Mg2+ uptake by the small intestine. In addition, in human small intestine, luminal acidic environment varied between pH 5.5 and 7.0 [29] which is necessary for mineral absorption by stabilizing their solubility [12]. Elevation of luminal pH led to a lower soluble Mg2+, which decreased from 79.61% at pH 4.4–5.15 to 8.71% at pH 7.8–8.15 [5], that affected intestinal Mg2+ absorption. Our recent results agreed with previous study that omeprazole induced duodenal HCO3 − secretion [28, 44] and effectively increased duodenal pH from 5.38 to 7.50. Therefore, PPI-suppressed small intestinal uptake is due partly to less soluble Mg2+ in small intestine.
As reported previously, omeprazole enhanced TRPM6 mRNA expression in mice colon [23], and in this study, it enhanced TPRM6, CNNM4, Cldn-2, -7, -12, and -15 protein expressions in rat duodenum. On the other hand, extracellular Mg2+, but not omeprazole, regulated TRPM6 protein expression in Caco-2 monolayers. We hypothesized that the expression of duodenal TRPM6 was the compensatory mechanism for systemic Mg2+ deficiency in PPIH rats. Although plasma 25-hydroxyvitamin D level increased in omeprazole-treated rats, the regulation of TRPM6 expression was vitamin D-independent mechanism [24]. Alternatively, vitamin D enhanced small intestinal Cldn-2 and -12 but not Cldn-7 and -15 expressions [14]. Thus, omeprazole probably enhanced duodenal Cldn-2 and -12 expressions through vitamin D-dependent mechanism. Previous in vitro study revealed that omeprazole suppressed Cldn-7 expression in Caco-2 cells [43]. However, our recent study demonstrated that omeprazole enhanced Cldn-7 expression, which is probably due to the difference in humoral factors. However, the regulatory factors and mechanism of how omeprazole affects intestinal TPRM6, CNNM4, Cldn-2, -7, -12, and -15 protein expressions required further study.
Based on our results, a critical question “why the expression of TRPM6 and those cation selective Cldns could not counteract PPIH in our rat model” has been raised. TRPM6 function required an interaction with membrane-associated phosphatidylinositol 4,5-bisphosphate (PIP2), whose hydrolysis through activation of G q protein-coupled receptor-phospholipase C (PLC)-dependent pathway fully inactivated TRPM6 channels [50]. Therefore, omeprazole might induce PIP2 degradation through G q protein-PLC-dependent pathway and then inactivated TRPM6 channels in duodenum of PPIH rats. In addition, TRPM6 mutation caused severe hypomagnesemia [39, 47]. Although mutation of TRPM6 has not been reported in PPIH, this might be involved in development of hypomagnesemia in our rat model. However, the role of PIP2 degradation and TRPM6 mutation in intestinal Mg2+ absorption of PPIH rat requires further study.
It is widely accepted that Cldn modulates paracellular ion permeability [16]. Tight junctions (TJ) are a series of anastomosing membrane strands that occluded the intercellular space between epithelium cells [15, 16]. Dynamic reorganization of TJ strands, i.e., breaking, resealing, and branching, enables paracellular transport without interfering the barrier integrity [15]. Previous in vitro study revealed that over-expression of Cldn-8 or Cldn-15 markedly increased number of TJ strands and decreased paracellular permeability [36, 52]. Therefore, simultaneously, over-expressions of Cldn-2, -7, -12, and -15 in PPIH rats probably led to large increase in number of tight junction strands in duodenal epithelium which impeded tight junction dynamic and paracellular Mg2+ transport. In addition, elevation of extracellular pH was found to increase the sensitivity of Ca2+ sensing receptor (CaSR) [10]. The activation of epithelium-associated CaSR induced Cldn-16 trans-localization from TJ to cytosol, which then suppressed paracellular passive Mg2+ transport [20]. Although Cldn-16 was not detected in duodenum [13], hypersensitivity of duodenal-related CaSR might be involved in the inhibition of Cldn-dependent paracellular Mg2+ absorption in PPIH rats.
As major Mg2+ storage pool, during Mg2+ depletion, bone Mg2+ content gradually declined due to activation of osteoclastic bone resorption and suppression of osteoblastic bone formation [34]. Chronic omeprazole user had lower plasma Mg2+ level that led to increased risk of fractures [1]. Maggio et al. [26] reported a suppression of trabecular bone density in prolonged PPIs users, which agreed with our results that thinner and longer trabeculae had been observed in PPIH rats. However, the effect of prolonged PPIs administration on bone physiology remains unknown.
In conclusion, the present study revealed the potential role of duodenum in handling Mg2+ and regulating of Mg2+ homeostasis and pathophysiology of PPIH. Duodenal HCO3 − secretion might be one of the critical factors of PPIs-impeded intestinal Mg2+ absorption. Reduction of duodenal Mg2+ absorption was shown in omeprazole-treated rats, whether hypomagnesemia was presented or not. Hypomagnesemia occurred only if Mg2+ storage pool was depleted in prolonged omeprazole-treated rats. Therefore, stimulation of intestinal Mg2+ absorption and/or Mg2+ supplement should be considered to avoid PPIH in person who continuously uses PPIs
References
Abrahamsen B, Vestergaard P (2013) Proton pump inhibitor use and fracture risk—effect modification by histamine H1 receptor blockade. Observational case-control study using National Prescription Data. Bone 57(1):269–271
Agar M, Webster R, Lacey J, Donovan B, Walker A (2004) The use of subcutaneous omeprazole in the treatment of dyspepsia in palliative care patients. J Pain Symptom Manag 28(6):529–531
Agus ZS (1999) Hypomagnesemia. J Am Soc Nephrol 10(7):1616–1622
Allen A, Flemström G (2005) Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am J Physiol Cell Physiol 288(1):C1–C19
Ben-Ghedalia D, Tagari H, Zamwel S, Bondi A (1975) Solubility and net exchange of calcium, magnesium and phosphorus in digesta flowing along the gut of the sheep. Br J Nutr 33(1):87–94
Cundy T, Dissanayake A (2008) Severe hypomagnesemia in long-term users of proton-pump inhibitors. Clin Endocrinol 69:338–341
Cundy T, Mackay J (2011) Proton pump inhibitors and severe hypomagnesemia. Curr Opin Gastroenterol 27(2):180–185
Danziger J, William JH, Scott DJ, Lee J, Lehman LW, Mark RG, Howell MD, Celi LA, Mukamal KJ (2013) Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int 83(4):692–699
de Baaij JHF, Hoenderop JG, Bindels RJM (2015) Magnesium in man: implications for health and disease. Physiol Rev 95(1):1–46
Doroszewicz J, Waldegger P, Jeck N, Seyberth H, Waldegger S (2005) pH dependence of extracellular calcium sensing receptor activity determined by a novel technique. Kidney Int 67(1):187–192
Epstein M, McGrath S, Law F (2006) Proton-pump inhibitors and hypomagnesemic hypoparathyroidism. N Engl J Med 355:1834–1836
Evenepoel P (2001) Alteration in digestion and absorption of nutrients during profound acid suppression. Best Pract Res Clin Gastroenterol 15:539–551
Fujita H, Chiba H, Yokozaki H, Sakai N, Sugimoto K, Wada T, Kojima T, Yamashita T, Sawada N (2006) Differential expression and subcellular localization of claudin-7, −8, −12, −13, and −15 along the mouse intestine. J Histochem Cytochem 54:933–944
Fujita H, Sugimoto K, Inatomi S, Maeda T, Osanai M, Uchiyama Y, Yamamoto Y, Wada T, Kojima T, Yokozaki H, Yamashita T, Kato S, Sawada N, Chiba H (2008) Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol Biol Cell 19:1912–1921
Furuse M, Tsukita S (2006) Claudins in occluding junctions of humans and flies. Trends Cell Biol 16(4):181–188
Günzel D, Yu AS (2013) Claudins and the modulation of tight junction permeability. Physiol Rev 93(2):525–569
Hess MW, de Baaij JHF, Gommers LMM, Hoenderop JGJ, Bindels RJM (2015) Dietary inulin fibers prevent proton-pump inhibitor (PPI)-induced hypocalcemia in mice. PLoS One 10(9):e0138881
Heijnen AM, Brink EJ, Lemmens AG, Beynen AC (1993) Ileal pH and apparent absorption of magnesium in rats fed on diets containing either lactose or lactulose. Br J Nutr 70(3):747–756
Hou J, Renigunta A, Gomes AS, Hou M, Paul DL, Waldegger S, Goodenough DA (2009) Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci U S A 106(36):15350–15355
Ikari A, Okude C, Sawada H, Sasaki Y, Yamazaki Y, Sugatani J, Degawa M, Miwa M (2008) Activation of a polyvalent cation-sensing receptor decreases magnesium transport via claudin-16. Biochim Biophys Acta 1778(1):283–290
Im WB, Blakeman DP, Davis JP (1985) Irreversible inactivation of rat gastric (H+-K+)-ATPase in vivo by omeprazole. Biochem Biophys Res Commun 126(1):78–82
Kladnitsky O, Rozenfeld J, Azulay-Debby H, Efrati E, Zelikovic I (2015) The claudin-16 channel gene is transcriptionally inhibited by 1,25-dihydroxyvitamin D. Exp Physiol 100(1):79–94
Lameris ALL, Hess MW, van Kruijsbergen I, Hoenderop JGJ, Bindels RJM (2013) Omeprazole enhances the colonic expression of the Mg2+ transporter TRPM6. Pflugers Arch Eur J Physiol 465(11):1613–1620
Lameris AL, Nevalainen PI, Reijnen D, Simons E, Eygensteyn J, Monnens L, Bindels RJ, Hoenderop JG (2015) Segmental transport of Ca2+ and Mg2+ along the gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 308(3):G206–G216
Luk CP, Parsons R, Lee YP, Hughes JD (2013) Proton pump inhibitor-associated hypomagnesemia: what do FDA data tell us? Ann Pharmacother 47(6):773–780
Maggio M, Lauretani F, Ceda GP, De Vita F, Bondi G, Corsonello A, Cattabiani C, Lattanzio F, Ruggiero C, Nouvenne A, Meschi T, Bandinelli S, Ferrucci L (2013) Use of proton pump inhibitors is associated with lower trabecular bone density in older individuals. Bone 57(2):437–442
Mejia A, Kraft WK (2009) Acid peptic diseases: pharmacological approach to treatment. Expert Rev Clin Pharmacol 2(3):295–314
Mertz-Nielsen A, Hillingsø J, Bukhave K, Rask-Madsen J (1996) Omeprazole promotes proximal duodenal mucosal bicarbonate secretion in humans. Gut 38:6–10
Nugent SG, Kumar D, Rampton DS, Evans DF (2001) Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with aminosalicylates and other drugs. Gut 48:571–577
Patterson Burdsall D, Flores HC, Krueger J, Garretson S, Gorbien MJ, Iacch A, Dobbs V, Homa T (2013) Use of proton pump inhibitors with lack of diagnostic indications in 22 Midwestern US skilled nursing facilities. J Am Med Dir Assoc 14(6):429–432
Quamme GA (2008) Recent developments in intestinal magnesium absorption. Curr Opin Gastroenterol 24(2):230–235
Quinn R (2005) Comparing rat’s to human ’s age: how old is my rat in people years? Nutrition 21(6):775–777
Rondón LJ, Rayssiguier Y, Mazur A (2008) Dietary inulin in mice stimulates Mg2+ absorption and modulates TRPM6 and TRPM7 expression in large intestine and kidney. Magnes Res 21(4):224–231
Rude RK, Gruber HE (2004) Magnesium deficiency and osteoporosis: animal and human observations. J Nutr Biochem 15(12):710–716
Serfaty-Lacrosniere C, Wood RJ, Voytko D, Saltzman JR, Pedrosa M, Sepe TE, Russell RR (1995) Hypochlorhydria from short-term omeprazole treatment does not inhibit intestinal absorption of calcium, phosphorus, magnesium or zinc from food in humans. J Am Coll Nutr 14(4):364–368
Sengoku A, Inai T, Shibata Y (2008) Formation of aberrant TJ strands by overexpression of claudin-15 in MDCK II cells. Histochem Cell Biol 129(2):211–222
Serra S, Jani PA (2006) An approach to duodenal biopsies. J Clin Pathol 59(11):1133–1150
Shabajee N, Lamb EJ, Sturgess I, Sumathipala RW (2008) Omeprazole and refractory hypomagnesemia. BMJ 337:a425
Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D, Syrrou M, Nielsen S, Sassen M, Waldegger S, Seyberth HW, Konrad M (2002) Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet 31:166–170
Swaminathan R (2003) Magnesium metabolism and its disorders. Clin Biochem Rev 24(2):47–66
Thongon N, Nakkrasae LI, Thongbunchoo J, Krishnamra N, Charoenphandhu N (2008) Prolactin stimulates transepithelial calcium transport and modulates paracellular permselectivity in Caco-2 monolayer: mediation by PKC and ROCK pathways. Am J Physiol Cell Physiol 294:C1158–C1168
Thongon N, Krishnamra N (2011) Omeprazole decreases magnesium transport across Caco-2 monolayers. World J Gastroenterol 17(12):1574–1583
Thongon N, Krishnamra N (2012) Apical acidity decreases inhibitory effect of omeprazole on Mg2+ absorption and claudin-7 and -12 expression in Caco-2 monolayers. Exp Mol Med 44(11):684–693
Thongon N, Ketkeaw P, Nuekchob C (2014) The roles of acid-sensing ion channel 1a and ovarian cancer G protein-coupled receptor 1 on passive Mg2+ transport across intestinal epithelium-like Caco-2 monolayers. J Physiol Sci 64(2):129–139
Vetter T, Lohse MJ (2002) Magnesium and the parathyroid. Curr Opin Nephrol Hypertens 11(4):403–410
Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, Bindels RJ, Hoenderop JG (2004) TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem 279:19–25
Walder RY, Landau D, Meyer P, Shalev H, Tsolia M, Borochowitz Z, Boettger MB, Beck GE, Englehardt RK, Carmi R, Sheffield VC (2002) Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet 31:171–174
Wang J, Barbuskaite D, Tozzi M, Giannuzzo A, Sørensen CE, Novak I (2015) Proton pump inhibitors inhibit pancreatic secretion: role of gastric and non-gastric H+/K+-ATPases. PLoS One 10(5):e0126432
Wolf FI, Trapani V, Simonacci M, Mastrototaro L, Cittadini A, Schweigel M (2010) Modulation of TRPM6 and Na+/Mg2+ exchange in mammary epithelial cells in response to variations of magnesium availability. J Cell Physiol 222(2):374–381
Xie J, Sun B, Du J, Yang W, Chen H-C, Overton JD, Runnels LW, Yue L (2011) Phosphatidylinositol 4,5-bisphosphate (PIP2) controls magnesium gatekeeper TRPM6 activity. Sci Rep 1:146
Yamazaki D, Funato Y, Miura J, Sato S, Toyosawa S, Furutani K, Kurachi Y, Omori Y, Furukawa T, Tsuda T, Kuwabata S, Mizukami S, Kikuchi K, Miki H (2013) Basolateral Mg2+ extrusion via CNNM4 mediates transcellular Mg2+ transport across epithelia: a mouse model. PLoS Genet 9(12):e1003983
Yu AS, Enck AH, Lencer WI, Schneeberger EE (2003) Claudin-8 expression in Madin-Darby canine kidney cells augments the paracellular barrier to cation permeation. J Biol Chem 278(19):17350–17359
Acknowledgements
This study was supported by the research grants from the Thailand Research Fund (RSA5680005), Burapha University through National Research Council of Thailand (33/2559), and the Faculty of Allied Health Sciences, Burapha University (AHS08/2558) to N. Thongon. We express our gratitude to Emeritus Prof. Dr. Prasert Sobhon of the Facuty of Allied Health Sciences, Burapha University, for his helpful suggestions and proofreading. We also thank Asst. Prof. Dr. Siriporn Chamniansawat, Ms. Maneerat Sakuntang, Ms. Sariya Ragsanit, Ms. Warintip Wetkama of the Facuty of Allied Health Sciences, Burapha University, Ms. Pornpun Seelaphong of the Microscopic Center, Faculty of Science, Burapha University, and Mr. Sakorn Praiwijarn and Ms. Dokerug Suwanchalong of Medical Laboratory Center, Burapha University Hospital, Burapha University for their excellent technical assistance.
Author contributions
Thongon N designed and performed experiments, analyzed and interpreted the results, wrote and edited the manuscript. Penguy J, Kulwong S, Khongmueang K, and Thongma M performed experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statement
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Thongon, N., Penguy, J., Kulwong, S. et al. Omeprazole suppressed plasma magnesium level and duodenal magnesium absorption in male Sprague-Dawley rats. Pflugers Arch - Eur J Physiol 468, 1809–1821 (2016). https://doi.org/10.1007/s00424-016-1905-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-016-1905-7