Abstract
Background
The macrophage and lymphocyte response to wear debris contributes to the failure of some joint replacements. Costimulatory molecule expression by particle-containing macrophages is an evidence for antigen presentation. The NFκB transcription factors are regulators of costimulatory molecules and are present in tissue near failed joint prostheses. The tissue localisation of NFκB and the expression of these factors and costimulatory molecules by U937 cells stimulated with nano- and microparticles are reported, together with the effects of an NFκB inhibitor (sc514).
Materials and methods
The tissue localisation of RelA, RelB, c-rel, p50, p52 and NF-IL6 was examined by immunohistochemistry in samples from 15 patients with failure of metal against polyethylene total hip replacements. The expression of these NFκB factors by U937 cells stimulated with microparticles (CoCr, diamond) and nanoparticles (diamond) was examined by quantified RT-PCR. Lipopolysaccharide provided positive controls while negative controls had no additions to culture. Inhibition of NFκB activity by sc-514 was studied. The expression of costimulatory molecules (CD80, CD86 and HLA-DR) was evaluated in parallel cell culture studies by tricolour flow cytometry.
Results and discussion
Immunohistochemistry of tissue showed the highest expression for NF-IL6 (32.56 ± 11.61 per cent), RelA (33.66 ± 9.98 per cent) and p52 (32.07 ± 12.90 per cent), then RelB (22.63 ± 7.49 per cent), c-rel (14.07 ± 6.72 per cent) and p50 (13.07 ± 5.99 per cent). NF-IL6 was localised to macrophages, RelB to RFD1+ dendritic cells. U937 cells showed an increased expression of all NFκB factors (p < 0.01) in response to CoCr and diamond microparticles. Only RelA and c-rel (p < 0.01) were increased by one diamond nanoparticle and p52 and c-rel (p < 0.01) by another nanoparticulate diamond. Inhibition by sc-514 of RelA, c-rel and p50 expression occurred with all four particles, p52 was decreased for all diamond particles (but not CoCr) and RelB was not inhibited with any of the particles. CD86 and HLA-DR expression were upregulated by microparticles (CoCr, diamond) (p ≪ 0.01) with lower levels (significant) of these molecules found with diamond nanoparticles. CD80 expression was much less than CD86 and HLA-DR. Costimulatory molecule expression in the bone-implant interface indicates antigen presentation by macrophages. Functional studies with U937 monocytes show the same molecules expressed on exposure to micro- and nanoparticles. Highest values occur with CoCr while the smallest diamond nanoparticles are the least stimulatory. NFκB expression gives an insight into the immunogenic potential of the different particles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
‘There is at bottom only one genuinely scientific treatment for all diseases, and that is to stimulate the phagocytes’ (The Doctor’s Dilemma, George Bernard Shaw, Act 1).
This paper reports research in which there is stimulation of the phagocytes. Sir John Charnley observed that the presence of macrophages is ‘a tissue reaction which no implant surgeon can lightly dismiss’ (Charnley 1979). The macrophage response to foreign particles has considerable significance in the context of joint replacement surgery. It is completely topical as concerns are expressed over the effects of metal wear particles from replaced joints and the possibility of adverse immunological reactions.
Wear occurs at the bearing surfaces of every artificial joint giving rise to particulate debris which may also be formed by abrasion or corrosion of components (Revell 2008). Particles accumulate in the synovial fluid from which they are cleared by the phagocytic cells of the synovium and removed via the lymphatics to local lymph nodes (Vernon-Roberts and Freeman 1976). The actual identification of lymphatic vessels in the synovium and implant interface has recently been achieved (Jell et al. 2006). Dissemination of particles to distant sites including the spleen is well recognised (Revell 1982; Case et al. 1994; Bae et al. 1996). Continuity between the synovial fluid and the tissue fluid surrounding the implant with particle migration by fluid pressure mechanisms has been described (Aspenberg and Van der Vis 1998). Particles are found in the bone-implant interface distant from the articulating surfaces in the absence as well as presence of implant loosening and bone loss (Revell 2008).
Macrophages and the closely related multinucleate giant cells (MNGCs) predominate in the bone-implant interface of failed joint replacements regardless of the type of particle present. At least 24 different pro-inflammatory cytokines and other mediators are produced by these cells in relation to wear debris (Revell 2008, 2012). Apart from physically removing debris from the tissues, macrophages initiate immunological reactions in partnership with lymphocytes. The presence of lymphocytes in the infiltrate related to joint prostheses was noted over 30 years ago by Vernon-Roberts and Freeman (1976). Lalor and her colleagues (Lalor et al. 1990, 1991; Lalor and Revell 1993) were among the first to recognise their significance at the implant interface of metal implants and to suggest an immunological process. It is now clear that a proportion of cases coming to revision surgery, be they metal against polyethylene (M–P) or metal against metal (M–M) articulations, have a significant lymphocytic component to the peri-implant infiltrate (Lalor et al. 1991; Salter et al. 1992; Lalor and Revell 1993; Al-Saffar et al. 1994; Revell and Al-Saffar 1994; Davies et al. 2005; Willert et al. 2005; Park et al. 2005; Milosev et al. 2006; Toms et al. 2008; Mahendra et al. 2009). That the interaction between lymphocytes and macrophages involves interplay through cytokines and other mediators is undoubted, but the possibility of antigen presentation also needs to be considered. The presence of the costimulatory molecules (CD80, CD86, CD28) on these two cells in peri-implant tissues is salient (Bainbridge et al. 2001; Farber et al. 2001; Altaf et al. 2003) as is HLA-DR expression which is indicative of antigen presenting cell (APC) activation.
Costimulatory molecule expression on the cell surface is greatly influenced by activity of the NFκB transcription factors. Ligation of costimulatory molecule cell surface receptors provides an activation signal for NFκB and in turn, NFκB acts as a regulator of costimulatory molecule expression. The interaction between APC and T cells causes NFκB activation in both cell types (Kane et al. 2002). The NFκB family (RelA, RelB, c-rel, p50 and p52) lies at the heart of immune response regulation. These factors exist as heterodimers latently held in the cytoplasm of cells by inhibitory proteins, IκB, which upon activation are phosphorylated by the IκB Kinase (IKK) complex, resulting in the nuclear translocation of NFκB. The IKK complex consists of two catalytic subunits, IKKα and IKKβ, and a regulatory component, called NFκB essential modulator (NEMO) or IKKγ. Both IKKα and IKKβ can phosphorylate IκB. IKK influences NFκB activation by either the canonical or the non-canonical pathway. The canonical pathway plays an important role in the activation of innate immunity and inflammation. It involves the activation of IKKβ in response to inflammatory mediators with the phosphorylation and degradation of IκBα and consequent nuclear translocation of NFκB. RelA, p50 and c-rel are activated via the canonical pathway, whereas the transcriptional activity of p52 and RelB is dependant upon the non-canonical pathway. This pathway leads to the activation of NFκB under the control of IKKα, activation of which leads to the ubiquitination of p100 and consequent generation of p52. This NFκB factor forms a heterodimer with RelB, so that the non-canonical pathway is involved in the nuclear translocation of RelB: p52 complexes.
This paper describes studies of NFκB expression in response to particles, firstly by demonstrating the presence of these transcription factors in the bone-implant interface and secondly by reporting cell culture experiments in which the expression of these molecules by monocytes is examined. The effect of an inhibitor of NFκB activation on the in vitro response to particle ingestion is also described. Evaluation of costimulatory molecule expression by U937 cells serves to complete the link to the in vivo findings. The size and composition of particles is considered, since those generated by M–P and M–M articulations differ. The former are mostly micrometre in size (Yamac 1999; Iwaki et al. 2000), while nanoparticles have been shown to be released by M–M joints (Doorn et al. 1998).
Materials and methods
Tissue localisation of NFκB transcription factors
Immunohistochemistry
Bone-implant interface tissue was obtained from 15 patients (7 M, 8 F, age 41–93 years) undergoing revision of M–P THR (Zimmer CPT) for aseptic loosening after implantation for 36–252 months with ethical committee approval. Eleven cases were first and four were second revisions. There was no microbiological evidence of infection in any of these cases. Samples were anonymised throughout. Tissue [acetabular (n = 8); femoral (n = 7)] was snap frozen in liquid nitrogen/isopentane and stored in liquid nitrogen. Tissue sections were stained for NF-IL6, RelA, RelB, c-rel, p50, p52, CD3, RFD1 and CD68 using the alkaline phosphatase streptavidin (APS) technique. Briefly, cryostat sections (5 μm) were fixed in acetone at rtp for 10 min, blocked with PBS/BSA (0.1 %), then treated with primary antibodies in PBS/BSA at optimum dilutions, followed by biotinylated secondary antibody (1:100) (rtp) (1 h) and APS (1 h). Optimum dilutions (1:50 for all primary antibodies except CD68 (1:100) and CD3 (1:25) were determined by titration studies with known control tissue (lymph node, tonsil). Sections were developed using Fast Red staining before viewing with conventional light microscopy.
Double-labelling immunofluorescence
Acetone-fixed PBS/BSA blocked frozen sections (5 μm) from the same clinical cases were incubated with the primary rabbit antibodies against RelB or NF-IL6 combined with one or other primary mouse anti-human CD68 or RFD1 antibodies. Antibodies from two different species were used to avoid cross-reactivity. Washed sections were incubated (1 h) (rtp) with fluorescent-tagged secondary antibodies [goat anti-rabbit (1:50) (Texas red) and goat anti-mouse (1:50) (FITC)], then Vectashield mounted and viewed by fluorescence (u/v light) and laser confocal microscopy.
Quantitation
The percentage of positive cells in both immunolabelling studies was calculated after counting eight high-power fields (HPF) (X63). Reproducibility was checked (for 8XHPF) by plotting the cumulative mean percentage values for positive cells against the number of HPF counted. Statistical analysis was performed using the Student t test. That cells containing transcription factors were of a particular type was confirmed non-quantitatively by double-labelled immunofluorescence.
In vitro response of U937 cells to CoCr and diamond particles: NFκB expression
Cells of the U937 cell line (LGC Promochem, UK) were cultured in 24 well plates in RPMI 1640 containing 10 % heat inactivated fetal calf serum (FCS), 1 % streptomycin and 5 % l-glutamine, passaged for 7 days, then after washing (HBSS) and viability counting, resuspended to 1 × 106 cells/ml and cultured in 24 well plates. Cells were challenged with CoCr particles (Goodrich, Cambridge) at a concentration of 2.5 particles/cell, diamond microparticles (DB) (2.5 particles/cell) and diamond nanoparticles (D1, D2) at six aggregates of particles/cell (see below). The diamond particles, all synthetic, were a generous gift from Dr. Kasia Bakowicz (Technical University of Lodz, Poland). All particles were washed (X3) in 70 % ethanol and stored in ethanol, then washed in HBSS immediately before use. Positive controls consisting of 1 × 106 cells/ml challenged with 5 μl (10 ng/ml) of lipopolysaccharide (LPS) were included in each experiment. Negative controls were cells maintained without any additions. All samples were analysed by RT-PCR for NFκB transcription factors and observations made in triplicate.
Inhibition of NFκB
Inhibition of NFκB signalling was studied using sc-514 (EMDMillipore) which irreversibly suppresses IKKβ activation in the NFκB pathway (Baxter et al. 2004). Sc-514 (10 μM) was added to U937 cells (1 × 106/ml) in 24 well plates and incubation for 1 h carried out before challenging with particles or LPS as above.
RT-PCR
After incubation with different particles, cells were homogenised using Trizol reagent. RNA isolation was by the addition of chloroform, centrifugation and washing the upper aqueous phase with isopropanol. 2 μg of RNA was converted to cDNA and used in the Superscript III one-step RT-PCR system with platinum Taq (Invitrogen). The mRNA expression of β-actin and NFκB transcription factors (RelA, RelB, c-rel, p50 and p52) was noted.
Quantitation
The pixel density of each band was measured to quantify the expression of factors. The statistical significance of the differences between the values obtained for LPS and each particle compared with the negative control was determined by the Student t test.
Characterisation of particles
CoCr particles were spherical with a mean equivalent circle diameter (ECD) of 2.11 ± 0.86 μm as characterised by transmission electron microscopy (TEM) (Fig. 1). Microparticles of diamond had a mean ECD of 1.05 ± 0.15 μm (DB). Individual nanoparticles of diamond (D1 and D2) were in the 1–5 nm range. Both formed aggregates with ECDs of 0.85 ± 0.26 μm (D1) and 0.12 ± 0.06 μm (D2) No attempt was made to disaggregate the nanoparticles before addition to cell culture.
In vitro response of U937 cells to CoCr and diamond particles: costimulatory molecules
Preparation and culture of U937 cells with CoCr and diamond particles was exactly the same as described above for 24 h, followed by centrifugation, washing with PBS/10 % FBS and resuspension in 100 μl PBS/FBS. Three fluorochrome-conjugated antibodies [anti-CD80-FITC, anti-CD86-PE, anti-HLA-DR-PEcy5 (Vector) (5 μl)] were added to each suspension. After an half-hour incubation (4 °C) with this antibody cocktail, the cells were washed with PBS/FBS (X3) and resuspended in 500 μl PBS/FBS for analysis with a Becton–Dickinson FACScan. Positive controls were cells challenged with LPS (10 ng/ml) and negative controls were cell cultures without additions. A sample for each test group was prepared devoid of any fluorochrome-conjugated antibodies for standardisation of the fluorescence intensity. This provided the negative FACS control against which the test group fluorescence was assessed.
Results
In situ localisation studies: NFκB transcription factors
Immunohistochemical staining for NFκB factors
The expression of NF-IL6 and NFκB in the bone-implant interface tissues was assessed quantitatively by immunohistochemistry. Large percentages of cells expressed NF-IL6 [32.56 ± 11.61 per cent (m ± SD)], RelA (33.66 + 9.98 per cent) and p52 (32.07 ± 12.90 per cent), whereas a smaller proportion stained positively for c-rel (14.07 ± 6.72 per cent) and p50 (13.07 ± 5.99 per cent) with intermediate levels for RelB (22.63 ± 7.49 per cent). Examples of the appearances are shown in Fig. 2.
Double-labelling immunofluorescence
The cell types expressing NF-IL6 and RelB were identified by double-labelling immunofluorescence. Cell counting showed that a mean of 40 % of macrophages (CD68+) were activated as judged by nuclear expression of NF-IL6. 10 % of MNGC (CD68+) and no RFD1+ (antigen-presenting) cells expressed NF-IL6. By contrast, a majority of RFD1+ cells (80 %) showed RelB expression. RelB was also present in a small proportion of macrophages (10 %) and MNGC (5 %).
In vitro cell studies: NFκB
Expression of NFκB by U937 monocytes in response to CoCr and diamond particles
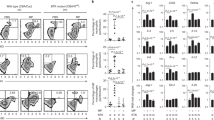
The NFκB expression by U937 cells in response to particles was measured by densitometry of RT-PCR. Stimulation with LPS resulted in the significant upregulation of all NFκB factors compared with the negative controls (p < 0.05). There was a significant increase in expression of all the factors in relation to CoCr and DB compared with negative controls (p < 0.05), while a significant increase occurred for RelA with D2, p52 with D1 and c-rel with both D1 and D2 (p < 0.05). Cells incubated with CoCr showed a significant increase in the expression of RelA, c-rel, and p50 (p < 0.01) compared with the LPS control levels. The results are shown in Fig. 3.
Inhibition of NFκB activity by sc-514, a suppressor of IKKβ activation, caused a reduction in the expression of RelA, c-rel and p50 on incubation with all four particles (D1, D2, DB and CoCr). Downregulation of p52 by sc-514 occurred with all three diamond particles, but not with CoCr. There was no inhibition of RelB expression with any of the particles. Representative results are shown in Fig. 4.
Expression of NFκB factors by U937 monocytes stimulated by incubation with microparticles (CoCr, DB), nano particles (D1,D2) or LPS (positive control). Results show effects of particles in the absence of inhibitor (stimulus) and in the presence of sc-514, an inhibitor of NFκB activation (inhibitor). RelA is an example in which expression was reduced in relation to all the stimuli by sc-514, while no inhibition of RelB expression occurred with any of the particles and there was downregulation of p52 by sc-514 with all three diamond particles, but not with CoCr
In vitro cell studies: costimulatory molecules
Expression of CD80, CD86, HLA-DR by U937 monocytes in response to CoCr and diamond particles
The expression of costimulatory molecules (Fig. 5) was most significantly upregulated in response to stimulation with LPS (CD86, p = 8.36 × 10−7; HLA-DR, p = 1.11 × 10−5) and the two microparticles, namely DB (CD86, p = 5.67 × 10−7; HLA-DR, p = 5.52 × 10−8) and CoCr (CD86, p = 1.11 × 10−5; HLA-DR, p = 1.34 × 10−7). Lower, but still significant, levels of CD86 (p = 0.002) and HLA-DR (p = 1.59 × 10−5) expression occurred with D1. Stimulation with D2 produced the lowest expression of CD80 and CD86, with that of HLA-DR (p = 0.0008) being the only one significantly higher than negative control. CD80 was the least expressed of the molecules, but the low levels detected for LPS, DB and CoCr were nevertheless significantly different from negative controls (p < 0.01).
Discussion
The response to wear debris around failed joint replacements in the absence of infection is characterised by the presence of macrophages and MNGC. Lymphocytes are found in a proportion of cases, be they M–M or M–P devices (Lalor et al. 1991; Salter et al. 1992; Lalor and Revell 1993; Al-Saffar et al. 1994; Revell and Al-Saffar 1994; Davies et al. 2005; Willert et al. 2005; Park et al. 2005; Milosev et al. 2006; Toms et al. 2008; Mahendra et al. 2009). The expression of cytokines, integrins and adhesion molecules by cells in the bone-implant interface suggests an immune-mediated inflammatory response (Al-Saffar et al. 1994; Clarke 1999; Clarke and Revell 2001; McFarlane and Revell 2004; Hercus 2005; review by Revell 2012). Whether there are marked immune reactions to modern M–M hip joints has become of concern recently (Medicines and healthcare products regulatory agency 2010, 2012), but sensitisation to metal was observed with first generation M–M joints (Evans et al. 1974; Benson et al. 1975; Elves et al. 1975; Nater et al. 1976). Some modern M–M hips showed a marked periprosthetic lymphocytic infiltrate, but no definite clinical sensitisation was demonstrated (Davies et al. 2005; Willert et al. 2005; Park et al. 2005; Milosev et al. 2006; Toms et al. 2008; Mahendra et al. 2009). Actual sensitisation to metal has been noted with M–P joints (Nater et al. 1976; Deutman et al. 1977; Lalor et al. 1991; Lalor and Revell 1993). Moreover, metal particles are present in tissues related to M–P joints (Pazzaglia et al. 1985; Kadoya et al. 1996, 1997). The lymphocytic reaction related to M–P joints has notably involved T cells with B cells not found (Al-Saffar et al. 1995; Revell et al. 1997; Revell 2006a) and these lymphocytes are predominantly T helper cells of TH1 sub-type (Weyand et al. 1998; Hercus and Revell 2001; Hercus et al. 2002; Arora et al. 2003; Hercus 2005; Altaf 2007). By contrast, both T and B cells are found in the lymphocytic component related to M–M hip joints (Willert et al. 2005, 2007; Mahendra et al. 2009). Pseudotumour, another M–M associated condition, shows few B cells and might be a type IV immune response (Pandit et al. 2008). The T cells in the interface of M–P joints are primed memory cells expressing CD45RO (Salter et al. 1992; Al-Saffar et al. 1994; Revell and Al-Saffar 1994). That there is active antigen presentation by macrophages to lymphocytes in the implant interface is shown by the presence of costimulatory molecules (CD80, CD86, CD28) on these cells (Bainbridge et al. 2001; Farber et al. 2001; Altaf et al. 2003). The increased expression of HLA-DR in peri-implant tissue is also indicative of antigen presentation by activated macrophages.
Costimulatory molecule expression on the cell surface is influenced by the activity of intracytoplasmic NFκB transcription factors. The ligation of costimulatory molecule cell surface receptors gives an activation signal for NFκB which, in turn, acts as a regulator of costimulatory molecule expression. The NFκB family of factors have been demonstrated in this study in macrophages, MNGC and dendritic cells at the implant interface with NF-IL6, RelA and p52 present in as much as one-third of cells. NF-IL6 was found in macrophages and RelB was located to RFD1 positive dendritic cells in double-labelling studies. NF-IL6 is a marker of activated macrophages. The RelB+ RFD1+ cells point to antigen presentation in the interface tissues. RelB was expressed by 10 % of macrophages and 5 % of MNGC so this factor is not exclusive to dendritic cells, as previously published studies report (Feuillard et al. 1996; Pettit et al. 1997). Dendritic cells and some of the other macrophages are likely to be involved in the presentation of antigen to T cells in relation to wear particles. Polyethylene (PE) wear debris may also play a role in the activation of antigen-presenting cells as there is a correlation between large populations of RelB+ RFD1+ cells and large amounts of PE wear debris (Altaf 2007).
The NF-κB proteins are grouped into two classes. Class I proteins (p50, p52) are synthesised as precursors (inhibitory IκB proteins) which have to be proteolysed to form the mature factors. Class II factors (RelA, RelB, c-rel) have transcriptional transactivation domains (TD) in the C terminal. Since p50 and p52 do not possess TD, they cannot act as transcription activators independently (Li and Verma 2002). The findings from this study have demonstrated varying degrees of cytoplasmic expression of all NFκB factors. It is unclear what precise combinations of NFκB homo- or heterodimers are active in the interface tissues and this area requires further research. The possibilities for the heterodimer combinations present are c-rel: p50, RelA: p52 and RelB: p52, all of which regulate the transcription of pro-inflammatory factors. Activation is by a different pathway for c-rel:p50 than for RelA:p50 and RelB:p52.
Large percentages of cells express RelA, RelB and p52 in the interface tissues. While the expression of p52 is widespread in cells, it is preferentially expressed by the dendritic cells and macrophages and plays an important role in antigen-presenting cell function (Shishodia and Aggarwal 2004). Nuclear translocation of p52 is associated with the regulation of MHC expression. In addition, RelA:p52 and RelB:p52 complexes are known to play an important role in lymphocyte proliferation (Caamano et al. 1998; Coope et al. 2002). Expression of the active nuclear form of RelB is found in APC, including differentiated DC, as well as monocytes that have undergone differentiation to increase their APC function (Pettit and Thomas 1999).
Since NFκB transcription factors translocate to the nucleus in the presence of activating stimuli, evaluation of the mRNA levels of NFκB provides an insight into the inflammatory potential of different particles. The cell culture studies reported here involve the use of U937 cells, a leukaemia cell line. An alternative cell for in vitro studies is the peripheral blood monocyte (PBM), but this varies between individual donor subjects and from day-to-day from the same donor. Higher levels of mRNA expression for NFκB factors in response to particles have been described for PBM compared with U937 cells (Altaf 2007). However, the U937 cell line was deliberately selected for investigations in vitro as results are more reproducible, enabling comparative studies to be made. Integrated density values were very similar between individual experiments for both LPS and particles. Schreiber et al. (2006) showed the expression of all the NFκB factors was upregulated in response to LPS.
The particles used enabled investigation of the effects of constitution and size on cellular response. Microparticles were of different chemistry (CoCr and diamond), while nanoparticles had the same diamond chemistry (D1, D2). Size was expressed as mean equivalent circle diameter (ECD), this being the diameter of a circle with the same area as that of the particle (or aggregate of nanoparticles).
The RT-PCR results show an upregulation of NFκB factors with all particles. The highest values occurred with the largest particles, namely CoCr, with highly significant increases of RelA, c-rel and p50, although there was also lower level expression of RelB and p52. This increased expression of NFκB is a further confirmation that metal wear particles may initiate an inflammatory response, as is known from a large number of other studies in which cytokine production by cultured cells has been shown (Al-Saffar and Revell 1994; Shanbhag et al. 1994, 1995; Rogers et al. 1997; Goodman et al. 1998; Green et al. 1998). The co-existent expression of cytokines and NFκB factors by U937 cells on incubation with particles has been shown by Altaf (2007), but other studies of NFκB expression by cell lines cultured with particles have given conflicting results. Akisue et al. (2002) considered that there was no NFκB expression by THP-1 cells and thought effects were due to the presence of endotoxin. By contrast, Baumann et al. (2005) found activation of NFκB signalling and TNFα expression in THP-1 cells stimulated with TiAlV and polyethylene particles. The latter authors used the ethanol washing method of Ragab et al. (1999) for the removal of adherent endotoxin from their particles. Similar ethanol washing was used for the particles in the present study.
The effect of particle size on NFκB factor production is partly addressed by the present results. Microparticles of diamond (DB) had a similar, though slightly less marked, effect to CoCr. Whether this is related to smaller size or different chemistry cannot be determined. An increased response due the difference in composition would imply that CoCr is more immunogenic than diamond. The inflammatory effect of particles with different chemistry has recently been reported (Kaufman et al. 2008). TiAlV particles caused the greatest production of a numerous pro-inflammatory cytokines, followed by CoCr and alumina, with polyethylene the least stimulatory. Titanium alloy also had the larger effect in an earlier study (Shanbhag et al. 1995) while CoCr was more toxic to rat peritoneal macrophages, but TiAlV caused more cytokine release (Haynes et al. 1993).
Comparison of results in this study for the diamond particles shows that nanoparticles (D1 and D2) are less inflammatory than microparticles, since they result in lower expression of all the NFκB factors. Nanoparticles are strictly defined as being under 100 nm, so that the aggregates are in the microparticle size range. Separation of aggregates into individual nanoparticles is extremely difficult and was not attempted. The literature suggests that nanoparticles have the same effects on cells in aggregated form as they would if present as individual particles (Revell 2006b; Rabolli et al. 2011).
Submicron pure titanium particles with a mean diameter of 0.24 μm, and therefore, not truly nanoparticles, gave rise to cytokine production according to Taira et al. (2010). A comparison of 0.5 and 1.5 μm alumina particles showed the smaller particles to cause greater production of various cytokines (Yagil-Kelmer et al. 2004), but Kranz et al. (2009) did not find a statistically significant difference between true nanoparticles (27–43 nm) and microparticles (1–1.7 μm) of corundum (Al2O3) with respect to the production of numerous cytokines.
Phagocytosis occurs as particles are opsonised (i.e. coated with proteins) (Xia and Triffitt 2006) and it is postulated that particles are opsonised by fetal calf serum present in tissue culture medium. An AFM study by Shukla et al. (2005) on gold nanoparticles suggested cellular uptake by pinocytosis. The D2 nanoparticle aggregates were below the size suggested as optimal for phagocytosis (0.3 μm by Green et al. 1998; 0.5 μm by Shanbhag et al. 1994) and, thus, may have been taken into the cells through pinocytosis. The microparticles (DB and CoCr) and the aggregates of D1 nanoparticles were most likely taken up by phagocytosis.
There are two phases of NFκB activation according to Hohmann et al. (1991). The first phase is associated with the preformed cytosolic pool of NFκB/IκB, the transcription factor and its inhibitor, complexes being induced within minutes to provide the rapid inflammatory response associated with NFκB activation. In the second phase, the pool of pre-existing NFκB becomes exhausted and active NFκB is synthesised de novo for the long-term maintenance of NFκB levels. The NFκB activation in vitro after 24 h demonstrated in this study is the second phase and so likely to be representative of long-term stimulation of cells by wear debris in vivo. Different times of culture are reported in the literature, but 24 h is a point at which optimal changes are found in systematic studies (Altaf 2007; Kranz et al. 2009). This duration gives a model for the transition between an acute and chronic inflammatory response (Curtis 2002). In a kinetics study, the phagocytosis of ceramic particles was initiated within 2 h and ingestion increased with time up to 15 h. After this time, phagocytosis and ingestion were on a plateau, the “maximum phagocytosable volume”, beyond which macrophages were unable to digest particles. Twenty-four hours, when the maximum phagocytosable volume has been reached, is the optimum time to evaluate the response to biomaterial particles when modelling macrophage behaviour (Catelas et al. 1998).
One of two pathways for NFκB activation in response to inflammatory mediators includes a kinase, IKKβ (Monaco et al. 2004). Studies have shown that RelA, c-rel and p50 are activated by the canonical pathway, whereas the transcriptional activity of p52 and RelB depends upon the non-canonical pathway (Lernbecher et al. 1994; Xiao et al. 2006). RelA, c-rel and p50 were downregulated in response to CoCr and DB by an inhibitor of IKKβ (sc514), while the expression of RelB and p52 was not affected. Mordmüller et al. (2003) showed that stimulation of primary human DC with LPS resulted in rapidly induced NFκB activity seen exclusively as p50 and RelA, whereas delayed but persistent activation caused accumulation of RelB and p52 complexes. More detailed research is required to determine the pathways relevant to activation by different particles.
The presence of costimulatory molecules in the bone-implant interface has been established (Bainbridge et al. 2001; Farber et al. 2001; Altaf et al. 2003). Bainbridge et al. (2001) also showed that U937 cells express these molecules in response to stainless steel and TiAlV alloy microparticles using fluorescence microscopy. For the first time, flow cytometry reported here has provided quantitative results for costimulatory molecule expression by cells in response to particle challenge.
The costimulatory molecule CD86 was highly expressed, being significantly upregulated in response to all the particles except D2, while HLA-DR expression was increased with all stimuli. CD86 is found early in the immune response, binds to CD28 with a greater affinity than CD80 and is implicated in the perpetuation of inflammatory responses (Zhang et al. 1997). Little is known of costimulatory molecules in the context of particle phagocytosis. Challenge of cultured human monocytic cells with carbon black nanoparticles did not directly induce HLA-DR expression, but did initiate cytokine release which in turn upregulated HLA-DR (Don Porto Carero et al. 2002). Upregulation of HLA-DR in monocytes by IL-2, IL-4 and TNFα has been shown (Limb et al. 1992). These pro-inflammatory cytokines act as inducers of NFκB and are themselves by-products of a signal transduction cascade in an autoregulatory feedback loop (Perkins 2000). All three are found in the implant interface (Revell 2012). CD80 showed much lower expression compared with CD86 and HLA-DR and was not significantly increased in response to the nanoparticle aggregates although the microparticles (DB and CoCr) caused a small significant increase in this costimulatory molecule. The expression of CD80 by DC may be regulated by RelB, since gene expression was correlated with the presence of RelB and a majority of CD80+ cells co-express RelB (Clarke 1999; Clark et al. 1999; Clarke and Revell 2001). In the present study, an increased expression of RelB was noted in U937 cells stimulated with LPS, DB and CoCr. Stimulation with nanoparticles (D1 and D2) increased the expression of c-rel but not of RelB. These complex inter-relationships have been explored further for NFκB and cytokine expression in response to CoCr and the three diamond particles by Altaf (2007).
Abbreviations
- APC:
-
Antigen presenting cell
- BSA:
-
Bovine serum albumin
- C/EBP-beta:
-
CCAAT enhancer binding protein-beta
- CD:
-
Cluster of differentiation (classification system for cellular surface molecules; as in CD68, CD80)
- CoCr:
-
Cobalt chromium alloy
- D1, D2, DB:
-
Diamond particles according to own laboratory notation
- DC:
-
Dendritic cell
- ECD:
-
Equivalent circle diameter
- FBS:
-
Fetal bovine serum
- FITC:
-
Fluorescein isothiocyanate
- HLA:
-
Human leucocyte antigen (HLA-DR is an MHC class II cell surface receptor)
- IKKβ:
-
IκB (inhibitor of NFβ) kinase beta
- IL1:
-
Interleukin 1
- IL10:
-
Interleukin 10
- LPS:
-
Lipopolysaccharide
- M–M:
-
Joint replacement prosthesis in which metal articulates against metal
- M–P:
-
Joint replacement prosthesis in which metal articulates against polyethylene
- NF-IL6:
-
Nuclear transcription factor for interleukin 6
- NFκB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells, a family of protein factors that control the transcription of DNA
- p value:
-
Probability value
- PBS:
-
Phosphate buffered saline
- PE:
-
R-Phycoerythrin
- PE-cy5:
-
Phycoerythrin coupled with the indotricarbocyanine dye, cy5
- RelA, RelB, c-rel, p50, p52:
-
Individual names given to NFκB proteins which are transcription factors
- RHD:
-
Rel homology domain
- rtp:
-
Room temperature
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- SD:
-
Standard deviation
- THR:
-
Total hip replacement
- TiAlV:
-
Titanium–aluminium–vanadium alloy
- TNFα:
-
Tumour necrosis factor alpha
References
Akisue T, Bauer TW, Farver CF, Mochida Y (2002) The effect of particle wear debris on NFkappaB activation and pro-inflammatory cytokine release in differentiated THP-1 cells. J Biomed Mater Res 59:507–515
Al-Saffar N, Revell PA (1994) Interleukin-1 production by activated macrophages surrounding loosened orthopaedic implants: a potential role in osteolysis. Br J Rheumatol 33:309–316
Al-Saffar N, Kadoya Y, Revell PA (1994) The role of newly formed vessels and cell adhesion molecules in the tissue response to wear products from orthopaedic implants. J Mater Sci Mater Med 5:813–818
Al-Saffar N, Mah JTL, Kadoya Y, Revell PA (1995) Neovascularisation and the induction of cell adhesion molecules in response to degradation products from orthopaedic implants. Ann Rheum Dis 54:201–208
Altaf H (2007) The inflammatory response to particulate wear debris in the context of total hip replacement. PhD thesis, University of London, London
Altaf H, Saeed S, Bhatt R, Revell PA (2003) The assessment of antigen presenting cells in the bone-implant interface. Biomaterialen 4:86–88
Arora A, Song Y, Chun L, Huie P, Trindade M, Lane Smith R, Goodman S (2003) The role of the TH1 and TH2 immune responses in loosening and osteolysis of cemented total hip replacements. J Biomed Mater Res 64A:693–697
Aspenberg P, Van der Vis H (1998) Migration, particles, and fluid pressure. Clin Orthop Rel Res 352:75–80
Bae SC, Park CK, Jun JB, Kim SY, Bae DK (1996) Multiple lymphadenopathy induced by wear debris after total knee replacement. Scand J Rheumatol 25:388–390
Bainbridge JA, Revell PA, Al-Saffar N (2001) Costimulatory molecule expression following exposure to orthopaedic implants wear debris. J Biomed Mater Res 54:328–334
Baumann B, Seufert J, Jakob F, Nöth U, Rolf O, Eulert J, Rader CP (2005) Activation of NF-kappaB signalling and TNFalpha-expression in THP-1 macrophages by TiAlV- and polyethylene-wear particles. J Orthop Res 23:1241–1248
Baxter A, Brough S, Cooper A, Floettmann E, Foster S, Harding C, Kettle J, McInally T, Martin C, Mobbs M, Needham M, Newham P, Paine S, St-Gallay S, Salter S, Unitt J, Xue Y (2004) Hit-to-lead studies: the discovery of potent, orally active, thiophenecarboxamide IKK-2 inhibitors. Bioorg Med Chem Lett 14:2817–2822
Benson MKD, Goodwin PG, Brostoff J (1975) Metal sensitivity in patients with joint replacement arthroplasties Brit Med J 4:374–375
Caamano JH, Rizzo CA, Durham SK, Barton DS, Raventos-Suarez C, Snapper CM, Bravo R (1998) Nuclear factor NFκB2 (p100/p52) is required for normal splenic microarchitecture and B cell-mediated immune responses. J Exp Med 187:185–196
Case CP, Langkamer VG, James C, Palmer MR, Kemp AJ, Heap PF, Solomon L (1994) Widespread dissemination of metal debris from implants. J Bone Jt Surg 76B:701–712
Catelas I, Huk OL, Petit A, Zukor DJ, Marchand R, Yahia L (1998) Flow cytometric analysis of macrophage response to ceramic and polyethylene particles: effects of size, concentration, and composition. J Biomed Mater Res 41:600–607
Charnley J (1979) Low friction arthroplasty of the hip. Springer, Berlin
Clark GJ, Gunningham S, Troy A, Vuckovic S, Hart DN (1999) Expression of the transcription factor RelB correlates with the activation of human dendritic cells. Immunology 98:189–196
Clarke SA (1999) Integrin expression at the bone biomaterial interface. PhD thesis, University of London, London
Clarke SA, Revell PA (2001) Integrin expression at the bone/biomaterial interface. J Biomed Mater Res 57:84–91
Coope HJ, Atkinson PGP, Huhse B, Belich MP, Janzen J, Holman M, Klaus GGB, Johnston LH, Ley SC (2002) CD40 regulates the processing of NFκB2 p100 to p52. EMBO J 21:5375–5385
Curtis PE (2002) Signalling in macrophages following exposure to retrieved wear particles. PhD thesis, University of London, London
Davies AP, Willert HG, Campbell PA, Learmonth ID, Case CP (2005) An unusual lymphocytic perivascular infiltration in tissues around contemporary metal-on-metal joint replacements. J Bone Jt Surg 87A:18–27
Deutman R, Mulder T, Brian R, Nater JP (1977) Metal sensitivity before and after total hip arthroplasty. J Bone Jt Surg 59A:862–865
Don Porto Carero A, Hoet PH, Nemery B, Schoeters G (2002) Increased HLA-DR expression after exposure of human monocytic cells to air particulates. Clin Exp Allergy 32:296–300
Doorn PF, Campbell PA, Worrall J, Benya PD, McKellop HA (1998) Metal wear particle characterization from metal on metal total hip replacements: transmission electron microscopy study of periprosthetic tissues and isolated particles. J Biomed Mater Res 42:103–111
Elves MW, Wilson N, Scales T, Kemp HBS (1975) Incidence of metal sensitivity in patients with total joint replacements. Brit Med J 4:376–378
Evans EM, Freeman MAR, Miller AJ, Vernon-Roberts B (1974) Metal sensitivity as a cause of bone necrosis and loosening of the prosthesis in total joint replacement. J Bone Jt Surg 56B:626–642
Farber A, Chin R, Song Y, Huie P, Goodman S (2001) Chronic antigen-specific immune-system activation may potentially be involved in the loosening of cemented acetabular components. J Biomed Mater Res 55:433–441
Feuillard J, Korner M, Israel A, Vassy J, Raphael M (1996) Differential localisation of p50, p52 and RelB proteins in human accessory cells in the immune response in situ. Eur J Immunol 26:2547–2555
Goodman SB, Lind M, Song Y, Smith RL (1998) In vitro, in vivo, and tissue retrieval studies on particulate debris. Clin Orthop 352:25–34
Green TR, Fisher J, Stone M, Wroblewski BM, Ingham E (1998) Polyethylene particles of a ‘critical size’ are necessary for the induction of cytokines by macrophages in vitro. Biomaterials 19:2297–2302
Haynes DR, Rogers SD, Hay S, Pearcy MJ, Howie DW (1993) The differences in toxicity and release of bone-resorbing mediators induced by titanium and cobalt-chromium-alloy wear particles. J Bone Jt Surg 75A:825–834
Hercus B (2005) Modelling T lymphocyte reactions to biomedical materials. PhD thesis, University of London, London
Hercus B, Revell PA (2001) Phenotypic characteristics of T lymphocytes in the interfacial tissue of aseptically loosened prosthetic joints. J Mater Sci Mater Med 12:1063–1067
Hercus B, Saeed S, Revell PA (2002) Expression profile of T cell associated molecules in the interfacial tissue of aseptically loosened prosthetic joints. J Mater Sci: Mater Med 13:1153–1156
Hohmann HP, Remy R, Scheidereit C, van Loon AP (1991) Maintenance of NF-kappa B activity is dependent on protein synthesis and the continuous presence of external stimuli. Mol Cell Biol 11:259–266
Iwaki H, Miyaguchi M, Kobayashi A, Kadoya Y, Yamac T, Revell PA, Freeman MAR, Yamano Y (2000) The size, shape and number if three kinds of wear particles in cemented hip arthroplasty. In: Transactions of the 6th World Congress on Biomaterials, Hawaii, p 1434
Jell G, Kerjaschki D, Revell P, Al-Saffar N (2006) Lymphangiogenesis in the bone–implant interface of orthopedic implants: importance and consequence. J Biomed Mater Res 77A:119–127
Kadoya Y, Revell PA, Al-Saffar N, Kobayashi A, Scott G, Freeman MAR (1996) The bone formation and bone resorption in failed total joint arthroplasties. Histomorphometric analysis with histochemical and immunohistochemical technique. J Orthop Res 14:473–482
Kadoya Y, Revell PA, Kobayashi A, AL-Saffar N, Scott G, Freeman MAR (1997) Wear particulate species and bone loss in failed total joint arthroplasties. Clin Orthop Rel Res 340:118–129
Kane LP, Lin J, Weiss A (2002) It’s all Rel-ative: NFκB and CD28 costimulation of T-cell activation. Trends Immunol 23:413–420
Kaufman AM, Alabre CI, Rubash HE, Shanbhag AS (2008) Human macrophage response to UHMWPE, TiAlV, CoCr, and alumina particles: analysis of multiple cytokines using protein arrays. J Biomed Mater Res A 84:464–474
Kranz I, Gonzalez JB, Dörfel I, Gemeinert M, Griepentrog M, Klaffke D, Knabe C, Osterle W, Gross U (2009) Biological response to micron- and nanometer-sized particles known as potential wear products from artificial hip joints: part II: reaction of murine macrophages to corundum particles of different size distributions. J Biomed Mater Res A 89:390–401
Lalor P, Revell PA (1993) T-lymphocytes and titanium–aluminium–vanadium (TiAlV) alloy. Evidence for immunological events associated with debris deposition. Clin Mater 12:57–62
Lalor PA, Gray AB, Wright S, Railton B, Freeman MAR, Revell P (1990) Contact hypersensitivity to titanium hip prosthesis? A preliminary report. Contact Dermat 23:193–194
Lalor PA, Revell PA, Gray AB, Wright SG, Railton GT, Freeman MAR (1991) Sensitivity to titanium. A cause of implant failure? J Bone Jt Surg 73B:25–28
Lernbecher T, Kistler B, Wirth T (1994) Two distinct mechanisms contribute to the constitutive activation of RelB in lymphoid cells. EMBO J 13:4060–4069
Li Q, Verma IM (2002) NFκB regulation in the immune system. Nat Rev Immunol 2:725–734
Limb GA, Hamblin AS, Wolstencroft RA, Dumonde DC (1992) Rapid cytokine up-regulation of integrins, complement receptor 1 and HLA-DR on monocytes but not on lymphocytes. Immunology 77:88–94
Mahendra G, Pandit H, Kliskey K, Murray D, Gill HS, Athanasou N (2009) Necrotic and inflammatory changes in metal-on-metal resurfacing hip arthroplasties. Relation to implant failure and pseudotumor formation. Acta Orthop 80:653–659
McFarlane T, Revell PA (2004) The expression of CD44 in archival paraffin embedded interface tissues of failed orthopaedic implants. J Mater Sci Mater Med 15:315–319
Medicines and healthcare products regulatory agency (2010) Medical device alert: all metal-on- metal (MoM) hip replacements (MDA/2010/03). http://www.mhra.gov.uk/Publications/Safetywarnings/MedicalDeviceAlerts/CONO79157.pdf. Accessed 10 Oct 2011
Medicines and healthcare products regulatory agency (2012) Medical device alert: all metal-on-metal (MoM) hip replacements (MDA/2012/036). http://www.mhra.gov.uk/home/groups/dts-bs/documents/medicaldevicealert/con155767.pdf. Accessed 21 Jan 2013
Milosev I, Trebse R, Kovac S, Cor A, Pisot V (2006) Survivorship and retrieval analysis of Sikomet metal-on-metal total hip replacements at a mean of seven years. J Bone Jt Surg 88A:1173–1182
Monaco C, Andreakos E, Kiriakidis S, Mauri C, Bicknell C, Foxwell B, Cheshire N, Paleolog E, Feldmann M (2004) Canonical pathway of nuclear factor kappa B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc Natl Acad Sci USA 101:5634–5639
Mordmüller B, Krappmann D, Esen M, Wegener E, Scheidereit C (2003) Lymphotoxin and lipopolysaccharide induce NF-kappaB-p52 generation by a co-translational mechanism. EMBO Rep 4:82–87
Nater JP, Brian RG, Deutman R, Mulder T (1976) The development of metal hypersensitivity in patients with metal-to-plastic hip arthroplasties. Contact Dermat 2:259–261
Pandit H, Vlychou M, Whitwell D, Crook D, Luqmani R, Ostlere S, Murray DW, Athanasou NA (2008) Necrotic granulomatous pseudotumours in bilateral resurfacing hip arthoplasties: evidence for a type IV immune response. Virchows Arch 453:529–534
Park Y-S, Moon Y-W, Lim S-J, Yang J-M, Ahn G, Choi Y-L (2005) Early osteolysis following second-generation metal-on-metal hip replacement. J Bone Jt Surg 87A:1515–1521
Pazzaglia UE, Ceciliani L, Wilkinson MJ, Dell’Orbo C (1985) Involvement of metal particles in loosening of metal-plastic total hip prostheses. Arch Orthop Trauma Surg 104:164–174
Perkins ND (2000) The Rel/NFκB family: friend and foe. Trends Biochem Sci 25:434–440
Pettit AR, Thomas R (1999) Dendritic cells: the driving force behind autoimmunity in rheumatoid arthritis. Immunol & Cell Biol 77:420–427
Pettit AR, Quinn C, MacDonald KP, Cavanagh LL, Thomas G, Townsend W, Handel M, Thomas R (1997) Nuclear localisation of RelB is associated with effective antigen presenting function. J Immunol 26:2547–2555
Rabolli V, Thomassen LC, Uwambayinema F, Martens JA, Lison D (2011) The cytotoxic activity of amorphous silica nanoparticles is mainly influenced by surface area and not by aggregation. Toxicol Lett 206:197–203
Ragab AA, Van De Motter R, Lavish SA, Goldberg V, Ninomiya JT, Carlin CR, Greenfield EM (1999) Measurement and removal of adherent endotoxin from titanium particles and implant surfaces. J Orthop Res 17:803–809
Revell PA (1982) Tissue reactions to joint prostheses and the products of wear and corrosion. Curr Top Pathol 71:73–101
Revell PA (2006a) Characterization of the cells and immunological reactions adjacent to aseptically loosened orthopaedic implants. J Histotechnol 29:287–295
Revell PA (2006b) The biological effects of nanoparticles. Nanotechnol Percept 2:283–298
Revell PA (2008) Biological causes of prosthetic joint failure. In: Revell PA (ed) Joint replacement technology. Woodhead, Cambridge, pp 349–396
Revell PA (2012) Biological response to artificial discs. In: Ambrosio L, Tanner E (eds) Biomaterials for spinal surgery. Woodhead, Oxford, pp 313–361
Revell PA, Al-Saffar N (1994) Inflammatory mediators in aseptic loosening of prostheses. In: Downes S, Dabestani N (eds) Failure of joint replacement. A biological, mechanical or surgical problem. Institute of Orthopaedics, London, pp 89–96
Revell PA, Al-Saffar N, Kobayashi A (1997) Biological reaction to debris in relation to joint prostheses. Proc Inst Mech Eng 211H:187–197
Rogers SD, Howie DW, Graves SE, Pearcy MJ, Haynes DR (1997) In vitro human monocyte response to wear particles of titanium alloy containing vanadium or niobium. J Bone Jt Surg 79B:311–315
Salter DM, Krajewski AS, Robertson S (1992) Lymphocytes in pseudomembranes of late prosthetic joint failure. J Pathol 166:271–275
Schreiber J, Jenner RG, Murray HL, Gerber GK, Gifford DK, Young RA (2006) Coordinated binding of NF-kappaB family members in the response of human cells to lipopolysaccharide. Proc Natl Acad Sci USA 103:5899–5904
Shanbhag AS, Jacobs JJ, Black J, Galante JO, Glant TT (1994) Macrophage/particle interactions: effect of size, composition and surface area. J Biomed Mater Res 28:81–90
Shanbhag AS, Jacobs JJ, Black J, Galante JO, Glant TT (1995) Human monocyte response to particulate biomaterials generated in vivo and in vitro. J Orthop Res 13:792–801
Shishodia S, Aggarwal BB (2004) NFκB: a friend or foe in cancer? Biochem Pharmacol 68:1071–1080
Shukla R, Bansal V, Chaudhary M, Basu A, Bhonde RR, Sastry M (2005) Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: a microscopic overview. Langmuir 21:10644–10654
Taira M, Kagiya T, Harada H, Sasaki M, Kimura S, Narushima T, Nezu T, Araki Y (2010) Microscopic observations and inflammatory cytokine productions of human macrophage phagocytising submicron titanium particles. J Mater Sci Mater Med 21:267–275
Toms AP, Marshall TJ, Cahira J, Darrah C, Nolan J, Donell ST, Barker T, Tucker JK (2008) MRI of early symptomatic metal-on-metal total hip arthroplasty: a retrospective review of radiological findings in 20 hips. Clinical Radiol 63:49–58
Vernon-Roberts B, Freeman MAR (1976) Morphological and analytical studies of the tissues adjacent to joint prostheses: investigations into the causes of loosening of prostheses. In: Shaldach M, Hohmann M (eds) Advances in artificial hip and knee joint technology. Springer, Berlin, pp 148–185
Weyand CM, Geisler A, Brack ME, Bolander ME, Goronzy JJ (1998) Oligoclonal T-cell proliferation and interferon-gamma production in periprosthetic inflammation. Lab Invest 78:677–685
Willert H-G, Buchhorn GH, Fayyazi A, Flury R, Windler M, Koster G, Lohmann CH (2005) Metal-on-metal beaings and hypersensitivity in patients with artificial hip joints: a clinical and histomorphological study. J Bone Jt Surg 87A:28–36
Witzleb W-C, Hanisch U, Kolar N, Krummenauer F, Guenther K-P (2007) Neo-capsule tissue reactions in metal-on-metal hip arthroplasty. Acta Orthop 78:211–220
Xia Z, Triffitt JT (2006) A review on macrophage response to biomaterials. Biomed Mater 1:R1–R9
Xiao G, Rabson AB, Young W, Qing G, Qu Z (2006) Alternative pathways of NF-kappaB activation: a double-edged sword in health and disease. Cytokine Growth Factor Rev 17:281–293
Yagil-Kelmer E, Kazmier P, Rahaman MN, Bal BS, Tessman RK, Estes DM (2004) Comparison of the response of primary human blood monocytes and the U937 human monocytic cell line to two different sizes of alumina ceramic particles. J Orthop Res 22:832–838
Yamac T (1999) The extraction and characterisation of wear particles from tissues around failed orthopaedic implants of different designs. PhD thesis, University of London, London
Zhang YQ, Joost van Neerven RJ, Van Gool SW, Coorevits L, De Boer M, Ceuppens JL (1997) B7-CD28 interaction is a late acting costimulatory signal for human T cell responses. Int Immunol 9:1095–1102
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Altaf, H., Revell, P.A. Evidence for active antigen presentation by monocyte/macrophages in response to stimulation with particles: the expression of NFκB transcription factors and costimulatory molecules. Inflammopharmacol 21, 279–290 (2013). https://doi.org/10.1007/s10787-013-0170-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-013-0170-z