Abstract
Chondroitin sulphate (CS) is recommended by the European League Against Rheumatism as a symptomatic slow-acting drug for the treatment of osteoarthritis on the basis of numerous clinical trials and meta-analyses. Furthermore, recent clinical trials have also demonstrated the possible structure-modifying effects of CS. This review focuses on recent experimental results and data available in the scientific literature regarding the anti-inflammatory properties of CS with a view to understanding the molecular basis responsible for its activity. Several animal studies have demonstrated that orally administered CS significantly inhibited hind paw oedema, synovitis and destruction of the articular cartilage in a dose-dependent manner. Furthermore, CS proved to have a beneficial effect in slowing down the development of adjuvant arthritis and in reducing disease markers, findings which support its beneficial activity in humans as a chondroprotective drug. Finally, several in vitro studies have focused on the hypothesis that CS may reduce inflammatory processes by acting on the nuclear translocation of NF-κB, which is closely associated with the blood biomarkers of inflammation, primarily IL-1, IL-6 and C-reactive protein.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
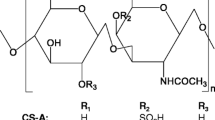
Chondroitin sulphate (CS) belongs to the class of natural complex polysaccharides named glycosaminoglycans (GAGs). It is an unbranched, polydispersed, complex macromolecule extracted and purified from various tissues (Volpi 2007, 2009) composed of alternate disaccharide sequences of differently sulphated residues of d-glucuronic acid (GlcA) and of N-acetyl-d-galactosamine (GalNAc). CS types with different carbohydrate backbones are known, depending on the nature of the disaccharide involved. Chondroitin-4-sulphate, CSA, consists of disaccharides sulphated in position 4 of GalNAc, while chondroitin-6-sulphate, CSC, is principally composed of a disaccharide unit sulphated in position 6 of GalNAc (Fig. 1). However, even if the known CS samples are principally composed of various percentages of these two kinds of disaccharide units, disaccharides with a different number of sulphate groups in a different position can be located, in various percentages, within the polysaccharide chains (Fig. 1). The heterogeneity of sulphation is responsible for the great variability in charge density as well as for the presence of low or highly sulphated sequences inside the carbohydrate backbone. Furthermore, the number of disaccharide units forming the CS polymer is another key factor influencing biological and pharmacological activity. As a consequence, CS represents a widely heterogeneous family of polysaccharides in terms of degree of sulphation, molecular mass and relative amounts of GlcA and iduronic acid, depending on the tissue of origin.
Symptomatic, slow-acting drugs for the treatment of osteoarthritis (SYSADOA) are compounds which have been prescribed in European countries for many years (Uebelhart 2008). Like nonsteroidal anti-inflammatory drugs, these compounds are not rapidly acting agents and their clinical efficacy can be demonstrated only after a relatively long period of regular intake. However, once administration is terminated, they do show carryover effects of various durations, depending on the different formulations. CS belongs to the oral SYSADOA substances, which are able to provide pain relief and increased mobility in osteoarthritis (OA) patients (Uebelhart 2008). In fact, CS is currently recommended by the European League Against Rheumatism (EULAR) as a SYSADOA in Europe for the treatment of knee (Jordan et al. 2003), hip and hand (Zhang et al. 2007) OA. Finally, recent clinical trials have demonstrated that the possible structure-modifying effects of CS as a disease-modifying drug in OA (DiMoDOA) (Reginster et al. 2007. Kahan et al. 2009) are an additional highly important factor to be considered with respect to its well-documented symptom-modifying properties.
The study of the structure and function of GAGs (and CS) has significantly influenced our understanding of a wide variety of biological processes. Historically, these cell surface and extracellular macromolecules were generically designated as “ground substances” or “mucopolysaccharides” and, because of their carbohydrate content and heterogeneity, were extremely difficult to study in relation to proteins. The advent of glycobiology with its tools and techniques for studying GAGs has done much to further the appreciation of how polysaccharides can affect cellular responses to a variety of stimuli. With regard to CS, many studies on cellular and animal models have been used to understand the molecular basis of its efficacy, which has been extensively demonstrated in a high number of clinical trials (Uebelhart 2008). The aim of this review is to focus on recent data regarding the anti-inflammatory properties of CS with the aim of understanding the molecular basis responsible for its activity.
CS biology and interactions
Accumulated evidence implies that CS chains fulfil important biological functions in inflammation, cell proliferation, differentiation, migration, tissue morphogenesis, organogenesis, infection and wound repair (Sugahara et al. 2003; Shriver et al. 2004; Yamada and Sugahara 2008), in addition to its conventional structural roles and its use as a drug for the treatment of OA. These effects are related to the capacity of CS (and CS-proteoglycans) to interact with a wide variety of molecules including (but not limited to) matrix molecules, growth factors, protease inhibitors, cytokines, chemokines, adhesion molecules and pathogen virulence factors via aspecific/specific saccharide domains within the chains (Table 1). Along with aspecific interactions, which are based on both numerous negative charges related to sulphate and carboxyl groups and to its long chains and on particular functional domain structures which are formed by combinations of the various disaccharide units (see Fig. 1), CS may participate in specific binding to bioactive molecules (Table 1).
CS anti-inflammatory activity in cellular models
Inflammation is a protective response initiated after injury caused by physical damage or infection by microorganisms. Consisting of both systemic and local responses, inflammation is an essential biological process with the conserved functions of eliminating the noxious factors, promoting tissue repair and wound healing and establishing memory, which enables the host to mount a faster and more specific response upon a future encounter with the relevant stimulus (Stables and Gilroy 2011). Infections caused by pathogens or by sterile tissue damage activate the pattern-recognition receptors (PRRs) in immune cells by means of cell-membrane molecules and/or of lipopolysaccharides (LPS) producing a defensive response characterized by anti-inflammatory reactions (Cinel and Opal 2009). Along with other receptors, toll-like receptors 1–13 (TLR1–13) are the most frequently activated membrane PRRs. The activation of PRRs along with other receptors will lead to the phosphorylation and activation of extracellular signal-regulated kinases able to initiate a molecular cascade producing the release of a nuclear factor kappa light chain enhancer of activated B cells (NF-κB) and its translocation to the nucleus (Zhong and Kyriakis 2007). The NF-κB dimer regulates the expression of many proteins such as pro- and anti-inflammatory cytokines and enzymes, all of which are able to perpetrate the inflammatory reaction (Ghosh and Hayden 2008).
The administration of nonsteroidal anti-inflammatory drugs is an important tool in the suppression of the inflammatory response in a clinical context and has the ability to inhibit both initial and later manifestations. However, prolonged use of these therapeutics is followed by complications, including gastric perforations, stomach ulcers and bleeding (Iwalewa et al. 2007). On the basis of these considerations, there is intense interest in finding new natural analgesic and anti-inflammatory compounds with few collateral effects (Vanderlei et al. 2010).
In chondrocytes, IL-1β induces the nuclear translocation of NF-κB. Using the chondrocyte stimulated by IL-1β as an experimental model, it has been shown that CS diminishes IL-1β-induced NF-κB nuclear translocation (Jomphe et al. 2008). In another study, the addition of different doses of purified human CS to LPS-stimulated chondrocytes was able to reduce inflammatory cytokines and iNOS, both at mRNA and protein levels by blocking NF-κB activation (Campo et al. 2008). Additionally, CS significantly inhibited NF-κB activation, the production of inflammation cytokines and inducible nitric oxide synthase in a model of LPS-induced inflammatory cytokines in mouse chondrocytes where TLR-4 was a specific target of its action (Campo et al. 2009). Finally, a recent study indicates that CS can potentially reduce neuro-inflammation by inhibition of NF-κB (Cañas et al. 2010), and endogenous polysaccharide could mediate neuro-immunomodulatory actions under neurotoxic conditions.
Besides the studies referred to above, pioneering research (Ronca et al. 1998) demonstrated the capacity of CS to inhibit directional chemotaxis, decrease phagocytosis and the release of lysozyme, and protect the plasma membrane from oxygen reactive species in human leukocytes. CS appeared to be effective in cellular inflammation events and oedema formation.
CS anti-inflammatory activity in animal models
Models of OA and rheumatoid arthritis are useful tools for studying these pathogenic processes. Adjuvant arthritis is one of the most widely used models. Rat adjuvant arthritis is an experimental model of polyarthritis that has been widely used to test numerous anti-arthritis agents either under preclinical or clinical investigation (Bendele et al. 1999). The hallmarks of this model are the reliable onset of robust polyarticular inflammation, marked bone resorption and periosteal bone proliferation. Cartilage destruction occurs, but it is disproportionally mild in comparison to the inflammation and bone destruction that occurs. While no models mimic perfectly the condition of human diseases, the models in question are easily reproducible, well defined and have proven useful for the development of new therapies for arthritis, as exemplified by cytokine blockade therapies. Furthermore, adjuvant arthritis has been extensively used for pharmaceutical testing, which means that a large amount of data is available for comparison with humans (Bendele et al. 1999). Because of these factors, the adjuvant arthritis animal model along with collagen-induced arthritis (CIA) has been used in several studies to evaluate CS efficacy (Table 2).
In a pioneering study (Kingston et al. 1993), different fractions of cartilage proteoglycans, whole proteoglycan, proteoglycan core protein, CS-rich region and keratan sulphate-rich region were prepared and tested in adjuvant-treated rats. Amongst the different fractions, only the CS-rich region was able to produce an inhibition of Mycobacterium tuberculosis-induced arthritis (Kingston et al. 1993).
In a rabbit model of articular cartilage injury, Condrosulf®, a highly pure pharmaceutical-grade CS, was found, during a pre-treatment regimen, to have a protective effect on the damaged cartilage (Uebelhart et al. 1998). In this study, the effect of oral and intramuscular administration of pure pharmaceutical grade CS after chymopapain-induced cartilage injury was investigated. Serum keratan sulphate levels were monitored to ensure degradation of the cartilage PG. Upon killing the animal, cartilage proteoglycan contents were markedly reduced in animals receiving chymopapain but no further treatment. In contrast, oral administration of CS beginning 11 days prior to chymopapain injury resulted in significantly higher cartilage proteoglycan contents. Additionally, intramuscular administration of Condrosulf® resulted in higher even if less significant cartilage proteoglycan amount. These results suggest that daily CS treatment prior to and continuing after chymopapain injury may have a protective effect on the damaged cartilage.
CS at increasing doses was orally administered to mice once daily, beginning 14 days before initial immunization, in type II collagen-induced arthritis (CIA) (Omata et al. 2000). Both the arthritis index and serum anti-collagen II antibody titre were reduced following treatment with CS in a dose-dependent manner. At the higher dose (1,000 mg/kg), CS significantly inhibited hind paw oedema, synovitis and destruction of the articular cartilage.
Comparable results were observed in the same animal model by oral administration of low molecular weight CS (Cho et al. 2004), orally administered to DBA/1J mice once daily for 14 days prior to initial immunization with type II collagen. Low molecular weight CS inhibited elastase activity slightly, but hind paw oedema was significantly diminished. Levels of anti-type II collagen antibody and tumour necrosis factor-α (TNF-α) in sera were also reduced. The low molecular weight CS preparation showed preventive effects on the type II CIA in DBA/1J mice.
In another study (Jin et al. 2007), the oral administration of CS extracted and purified from the cartilage of Raja kenojei was evaluated in relation to its capacity to modulate the progression of rheumatoid arthritis (RA) using CIA mice. The development of arthritis was delayed dose dependently in the CS-treated groups. The pre- and late-treated groups receiving 1,000 mg/kg of CS had clinical scores that were significantly reduced compared to the vehicle-treated groups. Haematoxylin eosin staining and X-ray radiography showed that the CS reduced infiltration of inflammatory cells and prevented joint destruction of the knee and paw. Furthermore, reverse transcription-polymerase chain reaction analysis revealed that CS administration inhibited the expressions of TNF-α, interleukin-1β (IL-1β) and interferon-γ (IFN-γ) in joints to a greater extent than administration of the vehicle. Finally, CS treatment also decreased the production of rheumatoid factors, IgG and IgM, in the serum of CIA mice. These results indicate that CS administration has a protective effect involving the inhibition of pro-inflammatory cytokine production in CIA mice (Jin et al. 2007).
In another recent study (Herrero-Beaumont et al. 2008), CS administration reduced the concentration of the pro-inflammatory molecules, C-reactive protein (CRP) and IL-6, and the nuclear translocation of NF-κB in a rabbit model of atherosclerosis aggravated by chronic arthritis (Herrero-Beaumont et al. 2008). Moreover, CS decreased the percentage of rabbits with atherosclerosis and chronic arthritis, which developed vascular lesions in the aorta.
In a recent research study (Bauerova et al. 2011), the effect of two different doses, 300 or 900 mg/kg daily, of highly purified CS was evaluated in an adjuvant arthritis animal model after oral administration. Adjuvant arthritis was induced by a single intradermal injection of heat-inactivated Mycobacterium butyricum in incomplete Freund’s adjuvant. The experiments included healthy animals, untreated arthritic animals and arthritic animals that had been administered CS from 14 days before adjuvant arthritis induction until the end of the experiment. Arthritic animals had been administered CS daily, from day 1 until the end of the experiment. Several inflammation and oxidative stress parameters were evaluated (Fig. 2). CS was capable of significantly reducing the severity of arthritis along with oxidative stress, a consequence of chronic inflammatory processes occurring in adjuvant arthritis. The CS pre-treatment regimen was effective throughout the whole sub-acute phase, while treatment from day 1 proved effective only in the chronic period. The effects were confirmed by improved total antioxidant status and γ-glutamyltransferase activity. CS administered under a pre-treatment regimen was also able to reduce the production of pro-inflammatory cytokines, C-reactive protein in plasma, phagocytic activity and the intracellular oxidative burst of neutrophils (Bauerova et al. 2011). In this study, CS proved to be effective in slowing down the development of adjuvant arthritis and reducing disease markers, thus supporting its beneficial activity as a drug in humans.
Biochemical and immunologic parameters monitored in an adjuvant arthritis animal model developed to evaluate highly pure CS effect (see Bauerova et al. 2011)
CS activity in relation to its quality
As is well known, CS is derived from animal sources by extraction and purification processes. As a consequence, source material, manufacturing processes, the presence of contaminants and many other factors contribute to the overall biological and pharmacological actions of these agents. Pharmaceutical grade CS formulations possess high and standardized quality, purity and properties, as a result of the stricter regulations to which this drug is subjected by the competent national institutes of health with regard to production and characteristics (Volpi 2007, 2009). In contrast, as several published studies available in the literature indicate, the CS quality of several nutraceuticals is poor (see Volpi 2009 for review). Clinical studies and CS efficacy have been evaluated using a very pure product with specific properties and physicochemical characteristics as approved by the various national institutes of health. For example, a highly purified preparation of standardized structure, not less than 95%, produced from bovine cartilage was used to assess the long-term combined symptom- and structure-modifying effects of CS (Kahan et al. 2009). As a consequence, activities in animal models should be evaluated using high-quality CS tested by quality control laboratories to confirm its purity and by specific and accurate analytical procedures to determine its structure and physicochemical characteristics.
Conclusions
Animal models provide insight into the role of soluble factors in arthritis (and also in rheumatoid arthritis) as not all cellular interactions are carried out through direct cell-to-cell contact. Pro-inflammatory cytokines, such as IL-1β and IL-6, regulate inflammatory and immune responses and play a pivotal role in steering the disease in arthritis models. Time course studies have consistently found IL-1β and IL-6 (and other key pro-inflammatory cytokines and chemokines) expressed in a variety of disease models including adjuvant arthritis (Kannan et al. 2005). In particular, IL-1β promotes cartilage and bone destruction, whereas IL-6 is produced by a multitude of cell types capable of stimulating the inflammatory response. This knowledge has led to current trials of anti-IL-6 therapy in RA (Kannan et al. 2005). CS administered under a pre-treatment regimen was able to reduce significantly the production of these pro-inflammatory cytokines throughout the whole sub-acute phase (from day 14 to 28), while CS administered as treatment at the higher dose was also able to diminish arthritic cytokines at the onset of the chronic period (Bauerova et al. 2011). According to its capacity to reduce pro-inflammatory cytokines, CS was also able to decrease CRP concentration in the plasma significantly, particularly when administered as pre-treatment. Furthermore, neutrophils are considered to be important in models of inflammation, since they have the potential to play a twofold role, both in the initial response and later in the destructive process of the chronic phase (Burmester et al. 1997). In our animal model, adjuvant arthritis triggered a marked enhancement of phagocytic activity and the intracellular oxidative burst of neutrophil functionality. Under the pre-treatment regimen, CS was able to lower both parameters, particularly during the sub-acute phase.
It is likely that inflammatory arthritis events are triggered by the activation of TLRs, either in the secondary lymphoid organs or in the arthritis synovium, since TLRs are known to mediate the activation of NF-κB and mitogen-activated protein kinases (MAPKs) resulting in the production of pro-inflammatory cytokines. Transcription factor NF-κB, which regulates the production of various cytokines, adhesion molecules and anti-apoptotic factors, is a critical mediator of intracellular TLR signalling (Chu et al. 1997; Karin and Lin 2002). As previously reported in detail, several in vitro studies (Vallières and du Souich 2010; Egea et al. 2010) have focused on the hypothesis that CS may reduce inflammatory processes by acting on the nuclear translocation of NF-κB, which is associated with blood biomarkers of inflammation, primarily IL-1, IL-6 and CRP. Bauerova et al. (2011) demonstrated a sharp decrease in these markers in an adjuvant arthritis animal model, confirming in vivo the potential of CS to regulate NF-κB activity. Overall, in the light of this evidence, CS proved to have a beneficial effect in slowing down the development of adjuvant arthritis and reducing disease markers, which is indicative of its beneficial activity in humans. In fact, on the basis of several previous studies, we may reasonably suppose that the effect of CS on the symptomatology and evolution of OA in patients is mainly associated with the inhibition of NF-κB nuclear translocation at the cellular level, in chondrocytes, synovial macrophages and synoviocytes.
Abbreviations
- BDNF:
-
Brain-derived growth factor
- CIA:
-
Collagen-induced arthritis
- CRP:
-
C-reactive protein
- CS:
-
Chondroitin sulphate
- EULAR:
-
European League Against Rheumatism
- FGF:
-
Fibroblast growth factor
- GAGs:
-
Glycosaminoglycans
- GalNAc:
-
N-acetyl-d-galactosamine
- GDNF:
-
Glial-derived growth factor
- GlcA:
-
d-Glucuronic acid
- HGF–FS:
-
Hepatocyte growth factor/scatter factor
- KGF:
-
Keratinocyte growth factor
- IFN-γ:
-
Interferon-γ
- IL:
-
Interleukin
- IP-10:
-
γ-Interferon inducible protein-10
- LPS:
-
Lipopolysaccharides
- MAPK(s):
-
Mitogen-activated protein kinases
- MCP-1:
-
Monocyte chemoattractant protein-1
- MK:
-
Midkine
- MIP-1α:
-
Macrophage inflammatory peptides-1α
- OA:
-
Osteoarthritis
- PDGF:
-
Platelet-derived growth factor
- PF4:
-
Platelet factor 4
- PRR(s):
-
Pattern recognition receptor(s)
- PTN/HB-GAM:
-
Pleiotrophin
- RA:
-
Rheumatoid arthritis
- SDS-1β:
-
Stromal cell-derived factor-1β
- SLC:
-
Secondary lymphoid tissue chemokine
- SYSADOA:
-
Symptomatic slow-acting drugs for the treatment of osteoarthritis
- TNF-α:
-
Tumour necrosis factor-α
- TLR(s):
-
Toll-like receptor(s)
- VEGF:
-
Vascular endothelial growth factor
References
Bauerova K, Ponist S, Kuncirova V, Mihalova D, Paulovicova E, Volpi N (2011) Protective effect of chondroitin sulfate on induced arthritis in rats. Osteoarthr Cartil 19:1373–1379
Bendele A, McComb J, Gould T, McAbee T, Sennello G, Chlipala E, Guy M (1999) Animal models of arthritis: relevance to human disease. Toxicol Pathol 27:134–142
Burmester GR, Stuhlmuller B, Keyszer G, Kinne RW (1997) Mononuclear phagocytes and rheumatoid synovitis. Mastermind or workhorse in arthritis? Arthritis Rheum 40:5–18
Campo GM, Avenoso A, Campo S, D’Ascola A, Traina P, Samà D, Calatroni A (2008) Purified human plasma glycosaminoglycans reduced NF-kappaB activation, pro-inflammatory cytokine production and apoptosis in LPS-treated chondrocytes. Innate Immun 14:233–246
Campo GM, Avenoso A, Campo S, Traina P, D’Ascola A, Calatroni A (2009) Glycosaminoglycans reduced inflammatory response by modulating toll-like receptor-4 in LPS-stimulated chondrocytes. Arch Biochem Biophys 491:7–15
Cañas N, Gorina R, Planas AM, Vergés J, Montell E, García AG, López MG (2010) Chondroitin sulfate inhibits lipopolysaccharide-induced inflammation in rat astrocytes by preventing nuclear factor kappa B activation. Neuroscience 167:872–879
Cho SY, Sim JS, Jeong CS, Chang SY, Choi DW, Toida T, Kim YS (2004) Effects of low molecular weight chondroitin sulfate on type II collagen-induced arthritis in DBA/1J mice. Biol Pharm Bull 27:47–51
Chu ZL, McKinsey TA, Liu L, Gentry JJ, Malim MH, Ballard DW (1997) Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc Natl Acad Sci USA 94:10057–10062
Cinel I, Opal SM (2009) Molecular biology of inflammation and sepsis: a primer. Crit Care Med 37:291–304
Egea J, García AG, Verges J, Montell E, López MG (2010) Antioxidant, antiinflammatory and neuroprotective actions of chondroitin sulfate and proteoglycans. Osteoarthr Cartil 18(Suppl 1):S24–S27
Ghosh S, Hayden MS (2008) New regulators of NF-kappaB in inflammation. Nat Rev Immunol 8:837–848
Herrero-Beaumont G, Marcos ME, Sánchez-Pernaute O, Granados R, Ortega L, Montell E, Vergés J, Egido J, Largo R (2008) Effect of chondroitin sulphate in a rabbit model of atherosclerosis aggravated by chronic arthritis. Br J Pharmacol 154:843–851
Iwalewa EO, McGaw LJ, Naidoo V, Eloff JN (2007) Inflammation: the foundation of disease and disorders. A review of phytomedicines of South African origin used to treat pain and inflammatory conditions. Afr J Biotech 6:2868–2885
Jin C-H, Yang U, Kim S-H, Ryu J-W, Lee J-C, Lee D-S, Lee T-H (2007) The protective effect of chondroitin from Raja kenojei cartilage on collagen-induced arthritis in DBA/1. J Mice Food Sci Biotechnol 16:594–599
Jomphe C, Gabriac M, Hale TM, Héroux L, Trudeau LE, Deblois D, Montell E, Vergés J, du Souich P (2008) Chondroitin sulfate inhibits the nuclear translocation of nuclear factor-kappaB in interleukin-1beta-stimulated chondrocytes. Basic Clin Pharmacol Toxicol 102:59–65
Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JW, Dieppe P et al (2003) EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 62:1145–1155
Kahan A, Uebelhart D, De Vathaire F, Delmas PD, Reginster JY (2009) Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 60:524–533
Kannan K, Ortmann RA, Kimpel D (2005) Animal models of rheumatoid arthritis and their relevance to human disease. Pathophysiology 12:167–181
Karin M, Lin A (2002) NF-kappaB at the crossroads of life and death. Nat Immunol 3:221–227
Kingston AE, Carney SL, Hicks CA, Billingham MEJ (1993) Arthritis and Osteoarthritis. Birkhauser, Basel, pp 75–79
Omata T, Itokazu Y, Inoue N, Segawa Y (2000) Effects of chondroitin sulfate-C on articular cartilage destruction in murine collagen-induced arthritis. Arzneimittelforschung 50:148–153
Reginster JY, Heraud F, Zegels B, Bruyere O (2007) Symptom and structure modifying properties of chondroitin sulfate in osteoarthritis. Mini Rev Med Chem 7:1051–1061
Ronca F, Palmieri L, Panicucci P, Ronca G (1998) Anti-inflammatory activity of chondroitin sulfate. Osteoarthr Cartil 6(Suppl A):14–21
Shriver Z, Raguram S, Sasisekharan R (2004) Glycomics: a pathway to a class of new and improved therapeutics. Nat Rev Drug Discov 3:863–873
Stables MJ, Gilroy DW (2011) Old and new generation lipid mediators in acute inflammation and resolution. Progr Lipid Res 50:35–51
Sugahara K, Mikami T, Uyama T, Mizuguchi S, Nomura K, Kitagawa H (2003) Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr Opin Struct Biol 13:612–620
Uebelhart D (2008) Clinical review of chondroitin sulfate in osteoarthritis. Osteoarthr Cartil 16:S19–S21
Uebelhart D, Thonar EJ, Zhang J, Williams JM (1998) Protective effect of exogenous chondroitin 4,6-sulfate in the acute degradation of articular cartilage in the rabbit. Osteoarthr Cartil 6(Suppl A):6–13
Vallières M, du Souich P (2010) Modulation of inflammation by chondroitin sulfate. Osteoarthr Cartil 18(Suppl 1):S1–S6
Vanderlei ESO, Patoilo KKNR, Lima NA, Lima APS, Rodrigues JAG, Silva LMCM, Lima MEP, Lima V, Benevides NMB (2010) Antinociceptive and anti-inflammatory activities of lectin from the marine green alga Caulerpa cupressoides. Int Immunopharmacol 10:1113–1118
Volpi N (2007) Analytical aspects of pharmaceutical grade chondroitin sulfates. J Pharm Sci 96:3168–3180
Volpi N (2009) Quality of different chondroitin sulfate preparations in relation to their therapeutic activity. Pharm Pharmacol 61:1271–1280
Volpi N (2010) Dermatan sulfate: recent structural and activity data. Carb Polymers 82:233–239
Yamada S, Sugahara K (2008) Potential therapeutic application of chondroitin sulfate/dermatan sulfate. Curr Drug Discov Technol 5:289–301
Zhang W, Doherty M, Leeb BF, Alekseeva L, Arden NK, Bijlsma JW et al (2007) EULAR evidence based recommendations for the management of hand osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 66:377–388
Zhong J, Kyriakis JM (2007) Dissection of a signaling pathway by which pathogen-associated molecular patterns recruit the JNK and p38 MAPKs and trigger cytokine release. J Biol Chem 282:24246–24254
Conflict of interest
The author declares that he has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Volpi, N. Anti-inflammatory activity of chondroitin sulphate: new functions from an old natural macromolecule. Inflammopharmacol 19, 299–306 (2011). https://doi.org/10.1007/s10787-011-0098-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-011-0098-0