Abstract
Background
Botulinum toxin type A (BoNTA) is a neurotoxin that acts by inhibiting the release of neurotransmitters acetylcholine at neuromuscular junctions, thus reducing muscular contractions. Recent evidence suggests that BoNTA can reduce nociceptive activities of sensory neurons in animal models by inhibiting release of certain neuropeptides. Despite the therapeutic benefit of BoNTA in alleviating painful muscle spasms, its efficacy in other musculoskeletal pain conditions is less clear.
Objective
We aim to examine the efficacy of BoNTA in reducing chronic musculoskeletal pain.
Methods
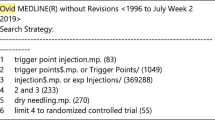
Studies for inclusion in our report were identified using MEDLINE, EMBASE, PUBMED, Cochrane Central Register of Controlled Trials, CINAHL, and reference lists of relevant articles. Studies were considered eligible for inclusion if they were randomized controlled trials (RCTs), evaluating the efficacy of BoNTA injections in pain reduction. All studies were assessed and data were abstracted independently by paired reviewers. The outcome measures were baseline and final pain scores as assessed by the patients. The internal validity of trials was assessed with the Jadad scale. Disagreements were resolved through discussions.
Main results
Twenty-one studies were included in the systematic review and 15 of them were included in the final meta-analysis. There was a total of 706 patients in the meta-analysis, represented from trials of plantar fasciitis (n = 1), tennis elbow (n = 2), shoulder pain (n = 1), whiplash (n = 3), and myofascial pain (n = 8). Overall, there was a small to moderate pain reduction among BoNTA patients when compared to control (SMD = −0.27, 95% CI: −0.44 to −0.11). When the results were analyzed in subgroups, only tennis elbow (SMD = −0.44, 95% CI: −0.86 to −0.01) and plantar fasciitis (SMD = −1.04, 95% CI: −1.68 to −0.40) demonstrated significant pain relief. Although not in the meta-analysis, one back pain study also demonstrated positive results for BoNTA. Lastly, BoNTA was effective when used at ≥25 units per anatomical site or after a period ≥5 weeks.
Conclusion
In our meta-analysis, BoNTA had a small to moderate analgesic effect in chronic musculoskeletal pain conditions. It was particularly effective in plantar fasciitis, tennis elbow, and back pain, but not in whiplash or shoulder pain patients. However, more evidence is required before definitive conclusions can be drawn. On the other hand, there is convincing evidence that BoNTA lacks strong analgesic effects in patients with myofascial pain syndrome. A general dose-dependent and temporal response with BoNTA injections was also observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Musculoskeletal disorders are the most common cause of long-term chronic pain, affecting people worldwide in the range of hundreds of millions (Woolf and Pfleger 2003). In the United States alone, it has been estimated that more than 50 million Americans are suffering from chronic pain conditions with almost half due to ailments in the musculoskeletal system (Lang 2003). Without proper treatments, chronic pain can have a strong disruptive impact on an individual’s physical, psychological and social well-being. For example, decreased physical activity (Tuzun 2007), depression (Magni et al. 1990; Herr et al. 1993), and impaired cognitive function (Eccleston et al. 1997) have all been found to associate with chronic pain. At the societal level, pain creates a tremendous economic and workplace burden. The annual cost of chronic pain in the United States, including health care expenditures, lost productivity, and absenteeism, is estimated to total $100 billion (National Institute of Health 1998). As the prevalence of musculoskeletal pain is projected to rise with a greyer and longer living worldwide population (Woolf and Pfleger 2003), it calls for greater effort in development and evaluation of new ways of managing these patients.
Currently, pharmacotherapy plays an important role in alleviating pain for these patients. Non-steroidal anti-inflammatory drugs (NSAIDs), muscle relaxants, and opioid analgesics are some of the most common classes of drugs provided. Unfortunately, these drugs may not be effective in many patients (Charles 2004) and can also lead to serious and occasionally fatal complications (Singh and Triadafilopoulos 1999). In light of these problems, the search for complementary or alternative therapies has received much attention. The emergence of botulinum toxin type A (BoNTA) is one such example of a potential new therapy aimed at musculoskeletal pain management.
Botulinum toxin type A is a neurotoxin that acts by inhibiting the release of the neurotransmitter, acetylcholine, at neuromuscular junctions, thus reducing muscular contractions (Borodic et al. 2001). Because of this muscle relaxing property, BoNTA was first used in humans to treat strabismus and blepharospasm (Flanders et al. 1987). Its clinical implications have since expanded as a treatment for focal dystonia and various types of muscle spasms (Hallett 1999). Additionally, pain caused by the muscle overactivity in these disorders can also be alleviated. Recently, Cui (2004) proposed another mechanism of action for BoNTA based on their work with the rat formalin model. Their observations suggest that BoNTA may reduce nociceptive activities of sensory neurons by inhibiting release of certain neuropeptides, thus decreasing perception of pain. The exact mechanisms are yet to be clarified.
Despite the promising therapeutic potential of BoNTA in alleviating painful muscle spasms, its efficacy in other musculoskeletal pain conditions are not well established, including its use in myofascial pain (Lew 2002). With this meta-analysis, we set out to examine the evidence regarding the usefulness of BoNTA in treating musculoskeletal pain conditions.
Objectives
The primary objective of this review is to examine the efficacy of BoNTA versus non-active injection or other treatments in reducing chronic musculoskeletal pain as assessed by a series of pain scales. We hypothesized a priori that BoNTA would be more effective, reflected by an improvement in patients’ pain scores. Subgroup analyses were then proposed to explore whether the analgesic effects of BoNTA varies across different musculoskeletal disorders, doses, and time periods.
Criteria for considering studies for this review
Types of studies
We only considered randomized controlled trials (RCTs), investigating BoNTA as a single or complementary therapy.
Types of participants
Patients of all ages, genders, and degrees of severity were included in the review, provided that they were experiencing chronic musculoskeletal pain.
Types of interventions
Intramuscular or subcutaneous BoNTA injections (from any commercially available preparations) were compared to placebos or other non-active therapies, including exercise. We allowed all techniques and schema of administration.
Types of outcomes
The primary outcome for our study is the reduction in pain severity through the period of follow-up. Only self-assessments of pain from patients were included because it is commonly reported. Other forms of pain assessment, such as those used by the health care providers, were known to be not readily available in every study. Patient characteristics, such as the disease of interest, dosing regimen, and length of follow-up were also recorded.
Exclusion criteria
Observational studies, case reports, and other non-randomized studies were not included. Studies comparing BoNTA with other active medical injections or studies without measures of pain were excluded. Additionally, because of the mixed pathogenesis for conditions of localised head pain, referred pain to the head, and intrinsic headache, we did not consider these types of conditions in our study.
Search methods for identification of studies
We identified relevant trials from the following sources:
-
1.
MEDLINE (1950–November week 3 2008).
-
2.
EMBASE (1980–2008 week 50).
-
3.
PUBMED (date of search: December 18, 2008).
-
4.
Cochrane Central Register of Controlled Trials (4th quarter 2008).
-
5.
CINAHL (1982–December 2008).
-
6.
Reference lists of relevant articles.
The following search terms and their MESH equivalents were used: BoNTA, BTX, BoNT, musculoskeletal diseases, arthritis, whiplash, shoulder pain, neck pain, back pain, limb pain, and joint pain. Another search was conducted on August 25, 2009. An additional article was retrieved (Singh et al. 2009).
Methods of review
Four reviewers (AA, MM, WZ, and AV), paired into two groups, independently reviewed all studies identified by our search strategy. Using the criteria described above, they first assessed titles and abstracts to determine relevant studies using standardized forms. Full-texts of these articles were then retrieved to ascertain if all inclusion criteria have been met. Upon inclusion of a study, our reviewers (TZ and AA) then performed data extraction in an independent duplicate manner using pilot-tested forms. Any disagreements were resolved through discussions. Authors were contacted through email to retrieve or clarify data when necessary. The qualities of included trials were independently graded by two reviewers (AA and WZ) using the Jadad scale (Jadad et al. 1996), which takes into account the method of randomization, blinding, and loss to follow-up. Each study received a grade of 0–5. Higher grades indicate higher methodological quality.
Statistical analysis
We used kappa statistics to evaluate agreement between reviewers on study selection. We used the standardized mean difference (SMD) with two-sided 95% confidence interval to assess the effect sizes of BoNTA because of the different pain scales employed in the selected studies. The included scales are both 10 and 100 points pain visual analog scales, 50 points neck pain and disability scale, as well as a Biobehavioural Questionnaire. When multiple BoNTA groups with varying dosages were evaluated in a single study, we employed the inverse variance weighting method to estimate the overall analgesic effect of BoNTA before comparing to the placebo. For cross-over trials, only data from the first period was incorporated. Where it was not reported, we calculated the standard deviation (SD) for the change score using estimated SDs of pre- and post-treatment pain scores and zero correlation (Wiebe et al. 2006).
We employed a random-effects model suggested by DerSimonian and Laird (1986) to pool data across studies, accounting for both within- and between-study variability. We used Cochran’s chi-square test to examine heterogeneity with statistical significance at α = 0.10. We calculated the I 2 statistic to quantify the degree of inconsistency between studies due to heterogeneity rather than sampling error. We performed subgroup analyses primarily to generate hypotheses regarding three factors: patient’s presenting disease, dosage per injection site, and treatment period. Studies examining multiple BoNTA groups with varying dosages were not included in the dosage subgroup analysis. However, sensitivity analysis was performed to evaluate the effect of incorporating the low dose or high dose group. We applied a conversion ratio of 3:1 Units to equate Dysport® potency with that of Botox® (Odergrena et al. 1998; Wohlfarth et al. 2009). We generated funnel plot to investigate the extent of publication bias. We performed all meta-analyses in RevMan 5.0 (Review Manager 2008).
Description of studies
With a broad based search strategy, we identified a total of 1,427 articles (Fig. 1). 253 citations were duplicates. Upon an initial screening of the titles and abstracts alone, all articles were found to be unsuitable except for 26 of them. These articles were retrieved and a total of 21 studies were eventually included.
Assessment of study selection agreement between the reviewers resulted in a κ = 0.70 (95% CI: 0.51–0.90) and 0.72 (95% CI: 0.56–0.88) between the two groups of reviewers.
Excluded studies
Five of the 26 articles were excluded (Jabbari 2007; Porta 1999; Kamanli et al. 2005; Szczepanska-Szerej et al. 2003; Sohling 2002). For detailed characteristics, please see Table 1. One study was a review article (Jabbari 2007) and thus was excluded. Two trials (Porta 1999; Kamanli et al. 2005) were RCTs but active medical therapies were used as controls, which did not meet our inclusion criteria. The last two studies (Szczepanska-Szerej et al. 2003; Sohling 2002) were excluded as the abstract/full text could not be located and attempts to contact the authors were unsuccessful.
Included studies
Twenty-one studies have been included in this review. 15 of them were selected for the meta-analysis. They are summarized below with details provided in Tables 2, 3.
Participants
Plantar fasciitis
There was only one trial retrieved examining plantar fasciitis (Babcock et al. 2005). Patients were recruited with bilateral symptoms consistent with plantar fasciitis. Patients were excluded if they had other pain or neurological conditions, including fibromyalgia.
Tennis elbow
Two of the three trials included patients who were previously diagnosed with tennis elbow and who already tried some conservative therapies (Placzeck et al. 2007; Hayton et al. 2005). The other trial recruited similar patients (Wong et al. 2005). However, subjects were excluded if they had any previous local injection treatments.
Shoulder pain
The included trial studied patients with osteoarthritis or rheumatoid arthritis induced shoulder pain and who were not responsive to corticosteroid injections (Singh et al. 2009).
Chronic low back pain
The single study in this category had participants with lateral pain between L1 and S1 that lasted more than 6 months (Foster et al. 2001).
Whiplash associated disorder
The three studies all recruited patients with a history of a whiplash injury and subsequent localized neck pain (Braker et al. 2008; Carroll et al. 2008; Padberg et al. 2007). However, the duration of symptoms prior to trial enrolment was not consistent among the studies, ranging from 2 weeks to a year.
Myofascial pain syndrome
There are 12 studies in this category (Ferrante et al. 2005; Guarda-Nardini et al. 2008; Lew et al. 2008; Kurtoglu et al. 2008; Nixdorf et al. 2002; Ojala et al. 2006; Qerama et al 2006; Wheeler et al. 2001b; Cheshire et al. 1994; Esenyel et al. 2007; Gobel et al. 2006; Wheeler et al. 1998). A broad spectrum of patients were therefore represented in those trials with differences in affected anatomical sites, trigger points, previous exposure to conservative therapies, and presenting pain.
Types of interventions
BoNTA used in our studies were one time injections. They were either Botox® from Allergan Inc., USA or Dysport®, from Ipsen Limited, UK.
Types of outcomes
The outcomes documented in included trials range from pain scores using visual analog scale, global assessment scores, physician rating scores to regional pain scales (Maryland foot score, neck pain and disability scale). We decided a priori to use only patient’s self rating scores.
Methodological quality
The Jadad scores of included studies ranged from 1 to 5 but the mean Jadad score assigned was 4.1, indicating the general high quality of studies included. In addressing our particular clinical question, double blinding is especially important since our outcome of interest (pain as measured on various scales) is patient-determined and thus subjective. All of the studies were conducted in a double-blind manner. However, in Esenyel’s study (2007), reporting of blinding was not found. For the most part, concealment of the allocation sequences was also described and carried out adequately in the studies. All studies were examined for the possibility of selective outcome reporting, and none was noted.
Results
A total of 15 studies with 706 patients were included in the meta-analysis—390 in BoNTA group and 316 in non-active group.
A total of 21 studies (Foster et al. 2001; Hayton et al. 2005; Cheshire et al. 1994; Esenyel et al. 2007; Gobel et al. 2006; Wheeler et al. 1998) were not included in the statistical analysis because of inadequate data reporting. Among the 15 trials included, the musculoskeletal conditions studied were plantar fasciitis (n = 1), tennis elbow (n = 2), shoulder pain (n = 1), whiplash (n = 3), and myofascial pain (n = 8). The mean age of patients ranged from 25 to 71. The percentage of females across the studies was wide-ranging from 2 to 100%. Most of these patients had experienced pain for at least 3 months with little or no response to traditional pain-modulating therapies (e.g., NSAIDS, steroids).
Based on Cohen’s (1988) difference index, there was a significant small to moderate pain reduction among BoNTA patients comparing to controls (SMD = −0.27, 95% Cl: −0.44 to −0.11) (Fig. 2). No significant heterogeneity was present in the overall analysis (p = 0.34, I 2 = 10%).
The results of disease subgroup analysis are presented in Fig. 3. In the myofascial pain group, BoNTA resulted in small pain relief which was not statistically significant (SMD = −0.16, 95% CI: −0.39 to 0.06). Among patients with tennis elbow, two studies demonstrated significant pain relief (SMD = −0.44, 95% CI: −0.86 to −0.01). Three other trials recruited patients with prior whiplash injuries. The usage of BoNTA did not seem to lead to greater pain relief for patients (SMD = 0.00, 95% CI: −0.41 to 0.40). Lastly, plantar fasciitis and shoulder pain were each examined by a study. BoNTA was shown to be effective in plantar fasciitis (SMD = −1.04, 95% CI: −1.68 to −0.40) but not in shoulder pain (SMD = −0.45, 95% CI: −1.06 to 0.16).
When patients were stratified on the basis of dosage (Fig. 4), only those who received an injection of 25 units or more per anatomical site benefited (SMD = −0.57, 95% Cl: −0.92 to −0.22). Moreover, the study done by Ferrante et al. 2005 was not included in the subgroup analysis since it had multiple intervention arms with varying BoNTA dosages. However, including the results from either the 50 U/site (n = 31) or 10 U/site (n = 32) group as part of a sensitivity analysis did not significantly change our outcome. By incorporating the low dose arm, the 1–10 U/site subgroup analysis showed a comparable result as before (SMD = −0.08, 95% CI: −0.29 to 0.14, heterogeneity p = 0.75, I 2 = 0%). Similarly, including the high dose arm results did not lead to any deviation in original outcomes (SMD = −0.45, 95% CI: −0.76 to −0.13, heterogeneity p = 0.31, I 2 = 17%).
As shown in Fig. 5 studies with short-term follow-up showed no significant pain relief effect with BoNTA (SMD = −0.15, 95% Cl: −0.50 to 0.2). For the 5–8 weeks group, BoNTA group experienced significantly greater pain reduction (SMD = −0.94, 95% CI: −1.49 to −0.39,). A similar analgesic effect was also observed for long-term follow-up group although to a lesser degree (SMD = −0.24, 95% CI: −0.41 to −0.06,).
There was no major publication bias as assessed by the funnel plot (Fig. 6).
Funnel plot assessing publication bias of the meta-analysis. The graph plots effect estimates on the horizontal axis against standard error of intervention effect estimates. This places larger or most powerful studies towards the top and smaller studies scattering more widely at the bottom. There seems to be missing some small sized studies that strongly favour or disfavour BoNTA. Nevertheless, included studies are plotted symmetrically around the pooled estimate
Studies not in the statistical analysis
Among the 6 studies that were not included in the statistical analysis, four followed patients with myofascial pain syndrome (Cheshire et al. 1994; Esenyel et al. 2007; Gobel et al. 2006; Wheeler et al. 1998). In a study of 33 patients with refractory cervicothoracic paraspinal myofascial pain syndrome, Wheeler et al. (1998) reported no statistically significant benefit of 50/100 U BoNTA over placebo after a follow-up of 16 weeks. The trial by (Gobel et al. 2006) included 145 patients with moderate to severe myofascial pain syndrome. Seventy-five patients received Dysport® treatments with an average of 40 U/site. At week 5, significantly more people in the Dysport® group experienced mild or no pain. Another trial (Hayton et al. 2005) studied six patients with chronic myofascial pain syndrome. Botox® was used in four patients with a total of 50 U divided between two and three sites while the rest received saline solution. At follow-up, all Botox® patients reported at least 30% pain reduction comparing to no pain relief in placebo group. In the last study, Esenyel et al. (2007) compared BoNTA group to a number of interventions, including stretching exercise and lidocaine. There were a total of 90 subjects—18 in BoNTA group and 18 with stretching exercise. Each of the BoNTA subjects received a 10 U injection per trigger point. After 4 weeks, BoNTA and lidocaine were found to be statistically more effective in relieving pain than other modalities.
Out of the other two trials that could not be incorporated in the statistical analysis, one (Foster et al. 2001) studied the usefulness of Botox® injections (40 U/site) in patients with chronic low back pain. In this randomized, double-blind study, 15 patients in the Botox® group and 16 in the control group were evaluated. This trial revealed a significant increase in Botox® patients having at least 50% pain relief in comparison to control at 8 weeks (60 vs. 12.5%). The other study (Hayton et al. 2005) involved a total of 40 patients with diagnosed tennis elbow. No significant differences were observed between the two groups at 12 weeks.
It was not the purpose of this study to address the toxicity or adverse effects of BoNTA use. However, it is important to state that the adverse reactions identified in the studies reported did not influence the outcome measures in any major way. Most studies report none or only transient side effects that resolved spontaneously. There were two studies where dropout occurred due to side effects. In the Gobel et al. (2006) study, there were no serious events. However, one patient from the BoNTA group (n = 75) and one from the control group (n = 70) dropped out due to sore muscles. In a cross-over trial by Nixdorf et al. (2002), there were a total of 15 patients and 5 dropped out before completion of the study. Three were receiving BoNTA, and their reasons for withdrawal were paralysis (n = 2), and increased pain (n = 1). The other two patients who dropped out were receiving placebo and their reasons for withdrawal were increased pain. A detailed review of BoNTA’s toxicity is provided in the Compendium of Pharmaceuticals and Specialties (2010).
Discussion
The primary objective finding of this meta-analysis is that BoNTA treatments resulted in small to moderate pain relief. This beneficial effect was especially noted in patients with tennis elbow, plantar fasciitis, and low back pain. The effect was also noticeable when the injection was >25 U (Botox®) per anatomical site or when the post-injection period was equal to or greater than 5 weeks.
MSK pain disorders
Myofascial pain syndrome is a regional condition of muscle pain and stiffness. It is also characterized by trigger points that generate local twitch response and referred pain on palpation. Its etiology is still unclear but muscle hyperactivity and inflammatory processes have been associated with this phenomenon (Simons et al. 1999; Borg-Stein and Simons 2002). A number of open label and retrospective studies have been done to assess the usefulness of BoNTA in treating this condition. The preliminary results were encouraging as Botox was shown to be effective in relieving pain (Lang 2000; De Andrés et al. 2003; Wheeler et al. 2001a; Royal et al. 2001). However, these findings have not been confirmed by RCTs. Out of the 12 myofascial pain trials included in our study, only 3 were positive with respect to alleviating pain intensity. Cheshire (1994) showed that Botox reduced experience of pain significantly at follow-up but the trial was rather small with 6 chronic myofascial pain patients. Similarly, Esenyel (2007) concluded with favourable results for Botox® in terms of pain alleviation when comparing to stretching exercises. Lastly, Gobel (2006) reported significantly greater pain reduction with Dysport® among patients of myofascial pain syndrome in the upper back. Despite these positive studies, the majority of trials have not demonstrated BoNTA’s analgesic effects in treating myofascial pain syndrome.
Plantar fasciitis, tennis elbow, shoulder pain, whiplash injuries, and back pain were also studied although to a more limited extent. Given the inflammatory pathophysiology of these conditions, BoNTA was shown to have antinociceptive effects in the plantar fasciitis trial (Babcock et al. 2005) and the two tennis elbow studies (Porta 1999; Placzeck et al. 2007). However, the other tennis elbow study by Hayton et al. (2005) did not demonstrate the usefulness of BoNTA in reducing pain. In addition, BoNTA did not significantly improve pain scores among the small number of shoulder pain and whiplash injury studies (Singh et al. 2009; Braker et al. 2008; Carroll et al. 2008; Padberg et al. 2007). Back pain was evaluated in one RCT in our systematic review (Foster et al. 2001). It demonstrated significant pain relief with BoNTA injections. On the basis of this result, the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology concluded in its 2008 report that BoNTA is “possibly effective” for low back pain (Naumann et al. 2008). Nevertheless, more robust evidence is required for a more definitive understanding.
Dosing
In this meta-analysis, there were two studies that specifically examined the dose–response relationship. In one trial, Ferrante used 10, 25, or 50 U Botox® injections per site in the assigned patients (Ferrante et al. 2005). When compared to placebo group, no differences in pain scores were found. In the other study by Wheeler et al. (1998), a total of 50 or 100 U Botox® injections were used in each subject. Injection dosage per site, however, could not be determined. Again, no differences between saline and intervention groups were observed. This seemingly lack of dose–response relationship from these two studies needs to be considered in the context of the studies’ population. Both trials involved patients with myofascial pain, which BoNTA has shown based on our results not to be consistently effective. In our subgroup analysis, however, studies with >25 U Botox® injections reported significant pain reduction with the treatment (Fig. 4). Furthermore, dose response relationships have been observed in other clinical applications of BoNTA, such as its usage in spastic hypertonia (Francisco 2004). Further research is certainly warranted to discern the painkilling effects of BoNTA in MSK pain disorders when used in various doses.
Duration of effectiveness
It is well known that Botox can decrease muscular tone and associated symptoms of pain by inhibiting the release of acetylcholine at neuromuscular junctions (Borodic et al. 2001). The onset of action and duration of this mechanism can vary from days to weeks. However, little is known about the temporality of its anti-nociceptive effect. In rats, the effect was found to last about 12 days (Cui et al. 2004). Clinically, there have been observations that pain reduction precedes muscular improvements but no accurate scientific documentation has been established (Aoki 2003).
Based on our analysis, the period of significant analgesic effect took place during the median and long term post-injection timeframe. The greatest effect was noted during the median-term follow-up (5–8 weeks).
Limitations
Our meta-analysis has a number of limitations. First, the study is limited in making reliable conclusions with regards to BoNTA’s efficacy in treating non-myofascial related musculoskeletal pain due to limited number of patients included. More RCTs in those clinical areas would be valuable. Second, we did not explore other factors, such as variation in injection techniques or gender differences because of the existing clinical heterogeneity of the population.
Conclusions
Overall, we show that BoNTA treatments can result in a small to moderate pain relief when injected in patients with musculoskeletal pain. It was especially noted in the small number of plantar fasciitis, tennis elbow, and back pain studies but not in the whiplash or shoulder pain studies. More RCTs are required to further understand the role of BoNTA in these conditions. On the other hand, our results from a convincing number of RCTs suggested that BoNTA injections do not result in any significant pain relief for patients with myofascial pain syndrome. Finally, we noted an increased analgesic effect of BoNTA with increased dosage or longer follow-up period. These observations should warrant closer examination in future studies.
References
Aoki RK (2003) Evidence for antinociceptive activity of botulinum toxin type A in pain management. Headache 43(Suppl 1):S9–S15
Babcock MS, Foster L, Pasquina P, Jabbari B (2005) Treatment of pain attributed to plantar fasciitis with botulinum toxin A. Am J Phys Med Rebabil 84(9):649–654
Borg-Stein J, Simons DG (2002) Focused review: myofascial pain. Arch Phys Med Rehabil 83(3 suppl 1):S40–S49
Borodic GE, Acquadro M, Johnson EA (2001) Botulinum toxin therapy for pain and inflammatory disorders: mechanisms and therapeutic effects. Expert Opin Investig Drugs 10:1531–1544
Braker C, Yariv S, Adler R, Badarny S, Eisenberg E (2008) The analgesic effect of botulinum-toxin A on post-whiplash neck pain. Clin J Pain 24(1):5–10
Carroll A, Barnes M, Comiskey C (2008) A prospective randomized controlled study of the role of botulinum toxin in whiplash-associated disorder. Clin Rehabil 22:513–519
Charles PD (2004) Botulinum neurotoxin serotype A: a clinical update on non-cosmetic uses. Am J Health Syst Pharm 61(Suppl 6):S11–S23
Cheshire WP, Abashian SW, Mann DJ (1994) Botulinum toxin in the treatment of myofascial pain syndrome. Pain 59:65–69
Cohen J (1988) Statistical Power Analysis in the Behavioral Sciences, 2nd edn Lawrence Erlbaum Associates, Inc., Hillsdale (NJ)
Compendium of Pharmaceuticals and Specialties (2010) Webcom inc, Toronto 407 –410, Online http://www.e-cps.ca
Cui M, Khanijou S, Rubino J, Aoki KR (2004) Subcutaneous administration of Botulinum toxin A reduces formalin-induced pain. Pain 107:125–133
De Andrés J, Cerda-Olmedo G, Valía JC, Monsalve V, Lopez-Alarcon MinguezA (2003) Use of botulinum toxin in the treatment of chronic myofascial pain. Clin J Pain 19(4):269–275
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Eccleston C, Crombez G, Aldrich S, Stannard C (1997) Attention and somatic awareness in chronic pain. Pain 72:209–215
Esenyel M, Aldemir T, Gursoy E, Esenyel CZ, Demir S, Durmusoglu G (2007) Myofascial pain syndrome: efficacy of different therapies. J Back Musculoskelet Rehabil 20:43–47
Ferrante FM, Bearn L, Rothrock R, King L (2005) Evidence against trigger point injection technique for the treatment of cervicothoracic myofascial pain with botulinum toxin type A. Anaesthesiology 103(2):377–383
Flanders M, Tischler A, Wise J, Williams F, Beneish R, Auger N (1987) Injection of type A botulinum toxin into extraocular muscles for correction of strabismus. Can J Ophtalmol 22(4):212–217
Foster L, Clapp L, Erickson M, Jabbari B (2001) Botulinum toxin A and chronic low back pain. Neurology 56:1290–1293
Francisco GE (2004) Botulinum toxin: dosing and dilution. Am J Phys Med Rehabil 83(suppl):S30–S37
Gobel H, Heinze A, Reichel G, Hefter H, Benecke R (2006) Efficacy and safety of a single botulinum type A toxin complex treatment (Dysport) for the relief of upper back myofascial pain syndrome: results from a randomized double-blind placebo-controlled multicentre study. Pain 125(1–2):82–88
Guarda-Nardini L, Manfredini D, Salamone M, Salmaso L, Tonello S, Ferronato G (2008) Efficacy of botulinum toxin in treating myofascial pain in bruxers: a controlled placebo pilot study. J Craniomandibular Pract 26(2):126–135
Hallett M (1999) One man’s position: clinical applications of botulinum toxin. N Engl J Med 341:118–120
Hayton MJ, Santini AJA, Hughes PJ, Frostick SP, Trail IA, Stanley JK (2005) Botulinum toxin injection in the treatment of tennis elbow: A double blind, randomized, controlled, pilot study. J Bone Joint Surg Am 87:503–507
Herr KA, Mobily PR, Smith C (1993) Depression and the experience of chronic back pain: a study of related variables and age differences. Clin J Pain 9:104–114
Jabbari B (2007) Treatment of chronic low back pain with botulinum neurotoxins. Curr Pain Headache Rep 11(5):352–358
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomised controlled trials: is blinding necessary? Control Clin Trials 17:1–12
Kamanli A, Kaya A, Ardicoglu O, Ozgocmen S, Zengin FO, Bayik Y (2005) Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol Int 25(8):604–611
Kurtoglu C, Gur OH, Kurkcu M, Sertdemir Y, Guler-Uysal F, Uysal H (2008) Effect of botulinum toxin A in myofascial pain patients with or without functional disc displacement. J Oral Maxillofac Surg 66:1644–1651
Lang AM (2000) A pilot study of botulinum toxin type A (Botox), administered using a novel injection technique, for the treatment of myofascial pain. Am J Pain Manag 10:108–112
Lang AM (2003) Botulinum toxin type A therapy in chronic pain disorders. Arch Phys Med Rehabil 84(Suppl 1):S69–S73
Lew MF (2002) Review of the FDA-approved uses of botulinum toxins, including data suggesting efficacy in pain reduction. Clin J Pain 18:S142–S146
Lew HL, Lee EH, Castaneda A, Klima R, Date E (2008) Therapeutic use of botulinum toxin type A in treating neck and upper-back pain of myofascial origin: A pilot study. Arch Phys Med Rehabil 89(1):75–80
Magni G, Caldieron C, Rigatti-Luchini S et al (1990) Chronic musculoskeletal pain and depressive symptoms in the general population: an analysis of the 1st national health and nutrition examination survey data. Pain 43:299–307
National Institute of Health (1998) NIH Guide: new directions in pain research I. Sept 4, available from http://www.grants.nih.gov/grants/guide/pa-files/PA-98-102.html
Naumann M, So Y, Argoff CE, Childers MK, Dykstra DD, Gronseth GS, Kaufmann HC, Schurch B, Silberstein SD, Simpson DM (2008) Assessment: botuinum neurotoxin in the treatment of autonomic disorders and pain (an evidence-based review). Neurology 70:1707–1714
Nixdorf DR, Heo G, Major PW (2002) Randomized controlled trial of botulinum toxin A for chronic myogenous orofacial pain. Pain 99:465–473
Odergrena T, Hjaltasona H, Kaakkolab H, Soldersc G, Hankod J, Fehlingd C, Marttilae RJ, Lundhf H, Geding S, Westergreng I, Richardsonh A, Dotth C, Cohenh H (1998) A double blind, randomised, parallel group study to investigate the dose equivalence of Dysport® and Botox® in the treatment of cervical dystonia. J Neurol Neurosurg Psychiatr 64:6–12
Ojala T, Arokoski JPA, Partanen J (2006) The effect of small doses of botulinum toxin A on neck-shoulder myofascial pain syndrome: a double-blind, randomized, and controlled crossover trial. Clin J Pain 22:90–96
Padberg M, de Bruijn SFTM, Tavy DLJ (2007) Neck pain in chronic whiplash syndrome treated with botulinum toxin. A double-blind, placebo-controlled clinical trial. J Neurol 254:290–295
Placzeck R, Drescher W, Deuretzbacher G, Hempfing A, Meiss L (2007) Treatment of chronic radial epicondylitis with botulinum toxin A. A double-blind, placebo-controlled, randomized multicenter study. J Bone Joint Surg Am 89:255–260
Porta M (1999) Botulinum toxin type A injections for myofascial pain syndrome and tension-type headache. Eur J Neurol 6(S4):s103–s109
Qerama E, Fuglsang-Frederiksen A, Kasch H, Bach FW, Jensen TS (2006) A double-blind, controlled study of botulinum toxin A in chronic myofascial pain. Neurology 67:241–245
Review Manager (RevMan) (2008) (Computer program). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration
Royal MA, Gunyea I, Bhakta B, Movva V, Ward S, Jenson M (2001) Botulinum toxin type A in the treatment of refractory myofascial pain (abstract). Neurology 56(Suppl 3):A350
Simons DG, Travell JG, Simons LS (1999) Myofascial pain and dysfunction: The trigger point manual. 2nd edn. Upper half of body, vol. 1, Williams & Wilkins, Baltimore (MD)
Singh G, Triadafilopoulos G (1999) Epidemiology of NSAID induced gastrointestinal complications. J Rheumatol 26(Suppl 56):18–24
Singh JA, Mahowald ML, Noorbaloochi S (2009) Intra-articular botulinum toxin A for refractory shoulder pain: a randomized, double-blinded, placebo-controlled trial. Transl Res 153:205–221
Sohling M (2002) Treatment of myofascial pain of the shoulder and neck region with botulinum toxin A. Naunyn-Schmiedeberg’s Arch Pharmacol 365(2):R42
Szczepanska-Szerej A, Stepniak C, Szczepanski L (2003) Clinical evaluation of botulinum toxin A in the treatment of pain in patients with fibromyalgia. Reumatologia 41(4):335–340
Tuzun EH (2007) Quality of life in chronic musculoskeletal pain. Best Pract Res Clin Rheumatol 21:567–579
Wheeler AH, Goolkasian P (2001a) Open label assessment of botulinum toxin A for pain treatment in a private outpatient setting. J Musculoskeletal Pain 9:67–82
Wheeler AH, Goolkasian P, Gretz SS (1998) A randomized, double-blind, prospective pilot study of botulinum toxin injection for refractory, unilateral, cervicothoracic, paraspinal, myofascial pain syndrome. Spine 23(15):1662–1666
Wheeler AH, Goolkasian P, Gretz SS (2001b) Botulinum toxin A for the treatment of chronic neck pain. Pain 94:255–260
Wiebe N, Vandermeer B, Platt RW, Klassen TP, Moher D, Barrowman NJ (2006) A systematic review identifies a lack of standardization in methods for handling missing variance data. J Clin Epidemiol 59:342–353
Wohlfarth K, Sycha T, Ranoux D, Naver H, Caird D (2009) Dose equivalence of two commercial preparations of botulinum neurotoxin type A: time for a reassessment? Curr Med Res Opin 25(7):1573–1584
Wong SM, Hul ACF, Tong P, Poon DWF, Yu E, Wong LKS (2005) Treatment of lateral epicondylitis with botulinum toxin. Ann Intern Med 143:793–797
Woolf AD, Pfleger B (2003) Burden of major musculoskeletal conditions. Bull World Health Organ 81:646–656
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, T., Adatia, A., Zarin, W. et al. The efficacy of botulinum toxin type A in managing chronic musculoskeletal pain: a systematic review and meta analysis. Inflammopharmacol 19, 21–34 (2011). https://doi.org/10.1007/s10787-010-0069-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-010-0069-x