Abstract
Fluctuations in resource availability occur in all ecosystems. To survive, species must alter their foraging strategies according to the quantity, quality, and distribution of available food. The rhesus macaque (Macaca mulatta), a commensal primate, is considered a generalist omnivore and very few studies have addressed how its feeding strategies change with respect to resource availability. We examined dietary diversity and frugivory levels in a group of rhesus macaques at the Buxa Tiger Reserve in northern India across one year. Using behavioural observations of diet and phenological monitoring, we found that although rhesus macaques fed on 107 food items including leaves, flowers, fruits, seeds, and insects, fruits made up ca. 74% of their diet. Fruit consumption correlated positively with fruit availability, but fruit preference appeared to play an important role; 16% of all the fruit species they fed on accounted for >50% of all fruit feeding observations. We suggest that afforestation programs involving preferred fruit species at the agricultural land–forest interface would prevent forest groups of rhesus macaques from gravitating toward human habitations and reduce conflict over anthropogenic resources. We further propose that the movement of certain primates in the direction of human habitations may be contingent on resource availability and food preference rather than an inherent propensity to gravitate to anthropogenic areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The quantity and quality of food available across habitats is one of the principal drivers of primate distribution and abundance (Bracebridge et al. 2012; Brugiere et al. 2002; O’Driscoll-Worman and Chapman 2006). The feeding ecology of primates is thus central to an understanding of primate population dynamics and socio-ecology (Marshall et al. 2009; Robbins and Hohmann 2006). Resource availability varies in natural ecosystems and the existence of distinct dry and wet seasons regulates the availability of plant parts such as immature leaves, flowers, fruits, and seeds, thereby inducing periods of food abundance as well as those of scarcity (Brugiere et al. 2002; Janson and Chapman 1999; van Schaik et al. 1993). Depending on varying resource availability, species change their foraging strategies to survive (Felton et al. 2008; van Schaik et al. 1993). In times of resource scarcity, primates may increase foraging effort and home range sizes to locate specific food items such as fruits (Krishnadas et al. 2011; Mourthé 2014; Wallace 2005). Alternatively, they may depend on “fallback foods,” i.e., foods of comparatively low quality that are available all the year round but are fed on only in times of food scarcity (Marshall et al. 2009).
Researchers have studied dietary modifications in response to resource fluctuations in primate frugivores such as spider monkeys (Ateles: Terborgh 1983; Wallace 2005), folivores such as colobus monkeys (Colobus: Bocian 1997; Oates 1977), and seed eaters such as uakaris (Cacajao: Bowler and Bodmer 2011). Frugivores are generally known to fall back on figs as well as leaves, flowers, unripe fruits, and seeds in times of fruit scarcity (Terborgh 1983; Wallace 2005). Folivores feed on fruits and seeds when the availability of young leaves is low (Bocian 1997; Oates 1977) and some seed-eating primates shift to fruit pulp, leaves, insects, and flowers when seeds are scarce (Boubli 1999; Cunningham and Janson 2006).
Several studies have investigated the feeding ecology of various omnivorous primates such as baboons (Papio) and macaques (Macaca) (Altmann 1998; Codron et al. 2006; Hill and Dunbar 2002; Kunz and Linsenmair 2008; Swedell et al. 2008; Tang et al. 2016; Zhou et al. 2014). Little, however, is known about food preference in omnivorous primates, which are known to include a wide range of foods in their diets such as fruits, seeds, flowers, leaves, buds, shoots, twigs, stems, roots, bark, pith, and resin of a large number of plant species, as well as fungi, various invertebrates, fish, bird eggs, and honey combs (Fooden 2000). Preferred foods form a subset of all the food items consumed and such preference is determined by taking into account consumption of foods with respect to their availability (sensu Russo et al. 2005; Stevenson and Link 2010).
The rhesus macaque (Macaca mulatta) has the widest geographic distribution among nonhuman primates (primates henceforth) and can adapt to a range of habitats including temperate coniferous forests, moist and dry deciduous forests, bamboo thickets, mixed forests, mangroves, scrub vegetation, rainforests, and areas in and around human settlements (IUCN 2016; Srivastava and Mohnot 2001). The rhesus macaque also forages on crops in many parts of their geographic range (Radhakrishna and Sinha 2011). The feeding ecology of rhesus macaques has been studied mostly in commensal populations (Goldstein and Richard 1989; Lindburg 1977); in contrast, the number of studies addressing the diet of completely wild rhesus macaques is much smaller. Largely described as a generalist omnivore (Clymer 2006; Johnson 2000; Zhou et al. 2014), rhesus macaque diet may vary strongly across different habitats (Tang et al. 2016). For example, in the temperate forests of northwestern Pakistan, and in the limestone forests of China, rhesus macaques are mostly folivorous, whereas in tropical forests, they are generally frugivorous (Goldstein and Richard 1989; Lindburg 1977; Tang et al. 2016). Studies of rhesus macaque feeding ecology also report extremely variable levels of frugivory: from 6.2% in China (Zhou et al. 2009) to 70% in Uttar Pradesh, India (Lindburg 1977).
Our previous studies at the Buxa Tiger Reserve, West Bengal, India, revealed that a group of rhesus macaques in the Checko Block of the Reserve were completely dependent on natural resources and that an unusually high percentage of their diet (79%) was accounted for by fruits and seeds (Sengupta et al. 2014). These findings led us to investigate how rhesus macaques adapt to changing fruit availability at the study site. More specifically, we addressed the following questions: 1) How does fruit consumption in rhesus macaques vary with changing fruit availability? 2) How does dietary fruit diversity vary with respect to fruit availability? We predicted that the percentage of fruit included in the diet of the rhesus macaques as well as the dietary fruit diversity would increase with increasing fruit availability, given the dietary flexibility of the species.
Methods
Study Area
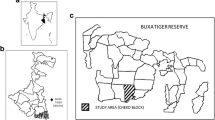
We carried out the study at the Buxa Tiger Reserve (BTR, 26°30′–23°50′N, 89°25′–89°55′E) located at the foothills of the Eastern Himalayas (see Sengupta et al. 2014 for map) from July 2012 to June 2013. Located in the Alipurduar district, West Bengal, India, BTR is adjacent to the Phibsoo Wildlife Sanctuary in Bhutan, Manas National Park, and Jaldapara Wildlife Sanctuary to its north, east, and west. BTR has a core and a buffer zone spanning 385 and 376 km2 respectively (Sukumar et al. 2003). The northern tracts are hilly; the elevation ranges from 60 to 1750 m, and the mean annual rainfall is 4100 mm with temperatures ranging between 12 and 32 °C. A larger portion of the reserve lies within the plains (Sukumar et al. 2003). Tropical moist deciduous forest is the main forest type alongside regions of evergreen, semievergreen, scrub and riverine forests, grasslands, and plantations (Sivakumar et al. 2006). We collected rainfall data between July 2012 and June 2013, from the Rajabhat Tea Estate located in the vicinity of the Reserve. The wet season (rainfall >100 mm, following Bracebridge et al. 2012) lasted from April to October while November to March constituted the dry season (rainfall <100 mm). Rainfall peaked in July 2012 (1245 mm) while there was no rainfall between November 2012 and January 2013. The mean monthly rainfall during the wet season was 622.9 mm (SD ± 350.3 mm) while that during the dry season was 5.2 mm (SD ± 6.5 mm).
We followed and observed a group of rhesus macaques in the Checko Block within the buffer zone of BTR. The group comprised 41 individuals (9 adult males, 11 adult females, 9 juvenile males, 10 juvenile females, and 2 infants; age classes assigned in accordance with National Research Council 1981) and was nonprovisioned and solely dependent on natural resources. The group had a home range of 45 ha (range: 25.5–70 ha, based on monthly means over the year; N = 12 months). This site was a mosaic of natural forest and mixed‐species plantation and the dominant species were Terminalia chebula, Terminalia belerica, Terminalia crenulata, Terminalia myriocarpa, Tectona grandis, Shorea robusta, Lagerstroemia speciosa, Gmelina arborea, Syzygium cumini, and Michelia champaca.
Fruit Availability
We assessed fruit availability along seven transects in the home range of the study group. Three of the transects were oriented in the north–south direction (one of them 1 km long, the remaining 500 m each) and four were oriented in the east–west direction (each 500 m in length). The width of each transect was 20 m, and together the seven transects covered 18% of the home range area. We tagged all trees with diameter at breast height (DBH) ≥10 cm and lianas present on trees along the transects. We conducted measurements on 2439 trees (of which 134 were lianas) belonging to 107 species (of which nine were lianas). We calculated the basal area of a tree (B) with the following formula:
Once a month across the year, we visually estimated the percentage of crown area covered by fruit of all the trees marked in the transect, and on that basis, we ranked trees on a 5-point scale where a score of 0 implied no fruit and 1, 2, 3, and 4 implied 1–25%, 26–50%, 51–75%, and ≥ 76% of the crown area covered by fruit respectively (Albert et al. 2013). We calculated indices to quantify overall fruit availability (FAI) and dietary fruit availability (DFAI; Table I).
Dietary Observations
We followed the macaques for 10 days every month from their waking sites to the sleeping trees and collected data from 06:00 to 18:00 h. We used scan sampling at intervals of 30 min (Altmann 1974; Giraldo et al. 2007; Robinson 1986) and in each sample, we scanned the group for 15 min and noted the following activities: moving, resting, social interactions, and feeding. We included all male and female adult and juvenile individuals in scans. When we observed a macaque feeding (defined as the actual manipulation or intake of food items, as per Menon and Poirier 1996), we noted the food species as well as the food class (fruit, seed, leaf, flower of plant species, insects) fed on. We defined a food item as food species × food class, calculated time spent feeding on each food item from scan data as a proportion of all feeding observations and summarized this as monthly percentages (Bracebridge et al. 2012). When we observed the macaques feeding on fruits, we used focal sampling for up to 30 min or until the macaques stopped feeding on fruits on randomly chosen adult individuals to understand which parts of the fruit they fed on (whole fruit, only pulp, only seed). We also studied the remnants of fruits/seeds beneath the feeding trees to confirm the exact part consumed and collected fresh fecal samples to check the number and status (intact/crunched) of seeds within. When macaques crunched seeds or consumed unripe fruits, we considered those plant species to be specifically targeted for seeds; we considered the remaining species to be targeted for fruit pulp.
We calculated dietary diversity (h′) and evenness indices (e′) (Table I). We additionally calculated diversity of fruit in the rhesus macaque diet (dietary fruit diversity index, Fruit h′) taking into account number of fruit species consumed as well as their evenness index (Fruit e′). From Fruit h′, we also calculated the Effective Number of Species (ENS; Table I).
Statistical Analyses
We used Spearman’s rank correlation coefficients (Zar 2010) to understand the relationships between FAI and 1) the percentage of diet constituted by each of the food classes, 2) h′, and 3) e′ (ɑ = 0.05). We used the same statistical measure to assess the relationship between DFAI and 1) Fruit h′, (ii) Fruit e′, and 3) ENS. We conducted all the analyses using R version 3.2.0 (R Core Team 2015).
Results
Fruit Availability and Dietary Fruit Availability Indices
The mean FAI was 470,001 (± SD 443,939, N = 12 months) and ranged from 99,765 in April to 1,266,063 in June (Tables I and II). The DFAI ranged between 28,898 (December) to 629,858 (June; mean = 238,959 ± SD 256,398, N = 12 months, Tables I and II).
Dietary Observations
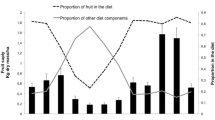
We collected 2865 scans (amounting to 716.25 observation hours) and 600 focal animal protocols (amounting to 300 observation hours) of macaque feeding behavior. We collected data on 26 (mean ± SD 4) individuals (range: 20–39 individuals) in each scan, which included adult males (mean = 6 ± SD 2, range: 5–9), adult females (mean = 8 ± SD 3, range: 6–11), juvenile males (mean = 6 ± SD 2, range: 4–9), and juvenile females (mean = 6 ± SD 3, range: 5–10). Individuals spent 58% of their active time feeding. Macaques fed on 107 food items, including leaves, flowers, fruits, and seeds of 77 species, insects, and fungi (since fungi accounted for just 0.1% of the diet, we excluded it from the rest of the analysis; Fig. 1 and Electronic Supplementary Material [ESM] Table SI). They consumed fruits, leaves, flowers, and seeds of 72% of the species present in the phenology transects (N = 107 tree species in the phenology transects). Fruits comprised 73.6% of the diet with leaves, flowers, seeds, and insects accounting for 12.5, 5.8, 5.7, and 2.4% of the diet respectively (N = 1667 dietary scans).
The dietary diversity index (h′) ranged between 0.48 in June and 1.15 in November (mean = 0.84 ± SD 0.25, N = 12 months; Tables I and II) and negatively correlated with Fruit Availability Index (FAI; r = −0.94, P < 0.001; Fig. 2, Table III). The dietary evenness index (e′) ranged from 0.37 (August) to 0.71 (November) (mean = 0.55 ± SD 0.12, N = 12 months) and also had a negative correlation with FAI (r = −0.90, P < 0.001; Fig. 2, Table III). Fruit consumption ranged from 60.4% (November) to 86.3% (June) across the year (mean = 73.6%, SD = 9.06%, N = 12 months; Fig. 1) and increased with increasing FAI (r = 0.94, P < 0.001; Fig. 3, Table III). While consumption of leaves (r = −0.36, P = 0.30, Table III) and insects (r = 0.25, P = 0.40, Table III) did not correlate with FAI, consumption of seeds and flowers negatively correlated with FAI (seeds: r = −0.77, P < 0.001; flowers: r = −0.69; P < 0.001, Table III).
The dietary fruit diversity index (Fruit h′) ranged between 1.72 (September) and 2.44 (June; mean = 2.07 ± SD 0.19, N = 12 months; Tables I and II) and did not significantly correlate with DFAI (r = 0.37, P = 0.21, Table III). The Fruit e′ ranged between 0.67 (August) and 0.99 (March; mean = 0.85 ± SD 0.12, N = 12 months) and had a negative correlation with DFAI (r = −0.88, P < 0.001; Fig. 4, Table III). Thus, some fruit species were consumed more irrespective of the availability of other species. Although the actual number of fruit species consumed varied from 6 to 25, the ENS consumed each month ranged from 6 (March and September) to 11 (June; Tables I and II). ENS did not significantly correlate with DFAI (r = 0.41, P = 0.11; Table III). During the entire study period, just seven species—Artocarpus chaplasha, Elaeocarpus varuna, Premna bengalensis, Beilschmiedia gammaeiana, Ziziphus mauritiana, Chisocheton paniculatus, Anthocephalus chinensis—accounted for 51.1% of the fruit feeding scans (N = 1226 fruit feeding scans; Table IV). Every month, just two to four species accounted for >50% (mean = 57.1%, SD = 3.7%, N = 12 months) of time spent on fruit consumption (Table IV).
Discussion
Rhesus macaques included leaves, flowers, fruits, or seeds of 77 plant species in their diet. However, fruits accounted for almost 74% of their time spent feeding, indicating that this population was primarily frugivorous. This degree of frugivory is comparable to those reported for several other macaque species such as the Tonkean macaque (Macaca tonkeana), southern pig-tailed macaque (M. nemestrina), Celebes crested macaque (M. nigra), Gorontalo macaque (M. nigrescens), Siberut macaque (M. siberu), northern pig-tailed macaque (M. leonina), lion-tailed macaque (M. silenus), Formosan rock macaque (M. cyclopis), bonnet macaque (M. radiata), and the long-tailed macaque (M. fascicularis: Richter et al. 2013; Tsuji et al. 2013). However, many studies have reported low levels of frugivory in rhesus macaques (6.2%: Zhou et al. 2009; 8%: Goldstein and Richard 1989; 27.3–28.7%: Tang et al. 2016). In limestone forests, young leaves seem to be their main food item (Huang et al. 2015; Zhou et al. 2009, 2011), similar to reports for the species in the temperate forests of Pakistan (Goldstein and Richard 1989). The low levels of frugivory of rhesus macaques in these habitats can also be explained by differing levels of overall fruit availability and seasonal fruit scarcity (Tang et al. 2016).
Fruits were the preferred foods of rhesus macaques. In this study, fruits were available throughout the year, but availability was greater in May to September. Supporting our predictions, fruit consumption by rhesus macaques at this study site positively correlated with fruit availability; contrary to our predictions though, Fruit e′ negatively correlated with fruit availability. Seed consumption and flower consumption were negatively related to the availability of the preferred food class, i.e., fruit, implying that these may be fallback foods (Marshall et al. 2009) for rhesus macaques. However, this hypothesis needs further examination as fallback foods, by definition, are food items available throughout the year but consumed only when preferred food is scarce (Altmann 1998). We did not measure flower and seed availability throughout the year. Hence we cannot confirm that these are indeed fallback foods for rhesus macaques.
An increase in fruit consumption with higher fruit availability has been noted in other species of primates such as the black-faced black spider monkey (Ateles chamek: Symington 1987), variegated spider monkey (A. hybridus: Link et al. 2012), Humboldt’s woolly monkey (Lagothrix lagotricha: Peres 1994), northern muriqui (Brachyteles hypoxanthus: Strier 1991), and the Japanese macaque (Macaca fuscata: Hanya 2004). Even during the months of low fruit availability at the study site, fruits accounted for 60.4–70.5% of the diet of rhesus macaques. This may be attributed to the lack of competition from other primate species for the same resource, as has been observed for variegated spider monkeys in Colombia (Link et al. 2012). However, the study site is home to a host of arboreal frugivores such as common palm civet (Paradoxurus hermaphrodites, which can be both arboreal and terrestrial), Malayan giant squirrel (Ratufa bicolor), Oriental pied hornbill (Anthracoceros albirostris), Alexandrine parakeet (Psittacula eupatria), rufous necked hornbill (Aceros nipalensis), great hornbill (Buceros bicornis), and red breasted parakeet (Psittacula alexandri) (Sekar and Sukumar 2013; N. P. Sharma and S. Roy pers. comm.). Further studies that investigate the degree of dietary overlap between rhesus macaques and any of these species would shed more light on the effects of interspecific competition on frugivory in rhesus macaques.
Our results underline the importance of food preference in the rhesus macaques, an aspect of feeding ecology that is often ignored in generalist species. An animal might feed on a particular food material not just because it is abundantly available but also because it chooses to do so (McConkey et al. 2002). The general notion is that omnivorous primates can be dietarily flexible and can include in their diet a vast range of food items (Milton 1987). In fact, their success as crop foragers has been attributed to the fact that they can potentially feed on any crop at any stage of its maturity (Sillero-Zubiri and Switzer 2001). We found that the dietary evenness index of rhesus macaques had a negative correlation with FAI, suggesting that when overall fruit availability increased, dietary diversity went down and the macaques ate fewer food types and thus became more selective. Also, Fruit e′ negatively correlated with FAI. This implies that even among the fruit species that were included in the diet, some species were consumed in greater proportions than the others. For example, although the actual number of species included in the diet in June was 25, the ENS amounted to 11. Such dependence on a relatively small number of fruit species has been noted in other primates such as spider monkeys, mangabays (Lophocebus), kipunjis (Rungwecebus), gibbons (Hylobates), and howlers (Alouatta: Ahsan 1994; Bracebridge et al. 2012; Dew 2005; Julliot 1996; Poulsen et al. 2001) and questions the notion of omnivory as it is usually used. Altmann’s (1998, 2009) concept of eclectic omnivory, used to describe the Amboseli baboons, rests on three central tenets: dietary preference, dietary flexibility, and dietary diversity. Our study reveals that 1) rhesus macaques consumed as many as 107 food items from 77 sources, 2) fruit consumption in rhesus macaques was driven by fruit availability, and 3) rhesus macaques exhibit preference for certain fruit species. As these findings support dietary diversity, dietary flexibility, and dietary preference in the species, we suggest that rhesus macaques may also be eclectic omnivores.
Across the year, >50% of time spent consuming fruits by rhesus macaques was accounted for by just seven species. All of these species had juicy edible tissue—the main fruit trait that rhesus macaques prefer (Sengupta and Radhakrishna 2015). Three of these species— Elaeocarpus varuna, Beilschmiedia gammeiana, and Chisocheton paniculatus—were characterized by all the fruit and seed traits that are preferred by rhesus macaques, i.e., external covers that can be easily pierced by a fingernail, juicy soft edible tissue, and medium to large true stone-like seeds (Sengupta and Radhakrishna 2015). Thus, the preference for these species may be explained by their traits.
Several generalist primate species such as macaques and baboons have become problem primates because they forage on crops, and a better understanding of how generalist species adapt to resource scarcity in natural ecosystems may help in devising appropriate management options to mitigate human–wildlife conflict over shared resources (Naughton-Treves et al. 1998; Riley et al. 2013). Richard et al. (1989) suggested that some “weed” macaques (including rhesus macaques) have a natural propensity to gravitate toward human habitations and thrive in anthropogenic areas. However, such dependence on human-generated food resources may also be because cultivated food resources are usually high-calorie, easily digestible, have a spatiotemporally predictable distribution, and are available in greater proportions than natural resources in any given area (Saj et al. 1999). Indeed, many studies have reported that crop raiding occurs during periods of low food availability in the forests (Agetsuma 2007; Dove 1993; Siex and Struhsaker 1999). However, in Uganda, foraging on crops by baboons, red-tailed monkeys (Cercopithecus ascanius), and chimpanzees (Pan troglodytes) was not related to lower food availability as a whole, but to the reduced availability of a particular tree species: Mimusops bagshawei (Naughton-Treves et al. 1998).
Based on our findings, we propose that rhesus macaques’ gravitation toward anthropogenic areas in many situations may be in response to lack of or decline of preferred tree species. We therefore suggest that 1) afforestation programs involving preferred tree species at the forest–agricultural land interface, may prevent infiltration of rhesus macaque groups into human-dominated areas and that 2) it is critical to ensure the natural regeneration and recruitment of these species by not disturbing forests any further. Similar measures have also been suggested to prevent Tonkean macaques from raiding cacao plantations in Indonesia (Riley et al. 2013). This mitigation measure has also been reported to be successful in Costa Rica, where buffer plantations of plantain restricted the movement of capuchins toward other cash crops (Baker and Shutt 2005). We further recommend long-term studies of primate diets across years and habitats to improve the understanding of the influence of spatiotemporal variation in food availability on their foraging and ranging behaviors, and consequently, on their interactions with humans.
References
Agetsuma, N. (2007). Ecological function losses caused by monotonous land use induce crop raiding by wildlife on the island of Yakushima, southern Japan. Ecological Research, 22(3), 390–402.
Ahsan, M. D. F. (1994). Behavioural ecology of the hoolock gibbon (Hylobates hoolock) in Bangladesh. Ph.D. dissertation, University of Cambridge, Cambridge.
Albert, A., Hambuckers, A., Culot, L., Savini, T., & Huynen, M.-C. (2013). Frugivory and seed dispersal by northern pigtailed macaques (Macaca leonina) in Thailand. International Journal of Primatology, 34(1), 170–193.
Altmann, J. (1974). Observational study of behavior: sampling methods. Behaviour, 49(3), 227–267.
Altmann, S. A. (1998). Foraging for survival: Yearling baboons in Africa. Chicago: University of Chicago Press.
Altmann, S. A. (2009). Fallback foods, eclectic omnivores, and the packaging problem. American Journal of Physical Anthropology, 140(4), 615–629.
Baker, M., & Shutt, A. (2005). Managing monkeys and mangos. In J. D. Paterson & J. Wallis (Eds.), Commensalism and conflict: The human-primate interface (pp. 445–463). Norman: American Society of Primatologists.
Bocian, C. M. (1997). Niche separation of black-and-white colobus monkeys (Colobus angolensis and C. guereza) in the Ituri Forest. Ph.D. dissertation, City University of New York.
Boubli, J. P. (1999). Feeding ecology of black-headed uakaris (Cacajao melanocephalus melanocephalus) in the Pico de Neblina National Park, Brazil. International Journal of Primatology, 20(5), 719–749.
Bowler, M., & Bodmer, R. E. (2011). Diet and food choice in Peruvian red uakaris (Cacajao calvus ucayalii): selective or opportunistic seed predation? International Journal of Primatology, 32(5), 1109–1122.
Bracebridge, C. E., Davenport, T. R., & Marsden, S. J. (2012). The impact of forest disturbance on the seasonal foraging ecology of a Critically Endangered African primate. Biotropica, 44(4), 560–568.
Brugiere, D., Gautier, J.-P., Moungazi, A., & Gautier-Hion, A. (2002). Primate diet and biomass in relation to vegetation composition and fruiting phenology in a rain forest in Gabon. International Journal of Primatology, 23(5), 999–1024.
Chapman, C., Chapman, L. J., Wrangham, R., Hunt, K., Gebo, D., & Gardner, L. (1992). Estimators of fruit abundance of tropical trees. Biotropica, 24, 527–531.
Clymer, G. A. (2006). Foraging responses to nutritional pressures in two species of Cercopithecines: Macaca mulatta and Papio ursinus. Ph.D. dissertation, Georgia State University.
Codron, D., Lee‐Thorp, J. A., Sponheimer, M., de Ruiter, D., & Codron, J. (2006). Inter‐and intra-habitat dietary variability of chacma baboons (Papio ursinus) in South African savannas based on fecal δ13C, δ15N, and% N. American Journal of Physical Anthropology, 129(2), 204–214.
Cunningham, E. P., & Janson, C. H. (2006). Pithecia pithecia’s behavioral response to decreasing fruit abundance. American Journal of Primatology, 68(5), 491–497.
Dew, J. L. (2005). Foraging, food choice, and food processing by sympatric ripe-fruit specialists: Lagothrix lagotricha poeppigii and Ateles belzebuth belzebuth. International Journal of Primatology, 26(5), 1107–1135.
Dove, M. R. (1993). The responses of Dayak and bearded pig to mast-fruiting in Kalimantan: An analysis of nature-culture analogies. In C. M. Hladik, A. Hladik, O. F. Linares, H. Pagezy, A. Semple, & M. Hadley (Eds.), Tropical forests, people and food: Biocultural interactions and application to development (pp. 113–123). Paris: Parthenon Publishing.
Felton, A. M., Felton, A., Wood, J. T., & Lindenmayer, D. B. (2008). Diet and feeding ecology of the Peruvian spider monkey (Ateles chamek) in a Bolivian semihumid forest: the importance of Ficus as a staple food resource. International Journal of Primatology, 29(2), 379–403.
Fooden, J. (2000). Systematic review of the rhesus macaque, Macaca mulatta (Zimmermann, 1780). Fieldiana Zoology, New Series, 96, 1–180.
Giraldo, P., Gómez-Posada, C., Martinez, J., & Kattan, G. (2007). Resource use and seed dispersal by red howler monkeys (Alouatta seniculus) in a Colombian Andean Forest. Neotropical Primates, 14(2), 55–64.
Goldstein, S. J., & Richard, A. F. (1989). Ecology of rhesus macaques (Macaca mulatta) in northwest Pakistan. International Journal of Primatology, 10(6), 531–567.
Hanya, G. (2004). Diet of a Japanese macaque troop in the coniferous forest of Yakushima. International Journal of Primatology, 25(1), 55–71.
Hill, R. A., & Dunbar, R. I. M. (2002). Climatic determinants of diet and foraging behaviour in baboons. Evolutionary Ecology, 16(6), 579–593.
Huang, Z., Huang, C., Tang, C., Huang, L., Tang, H., et al. (2015). Dietary adaptations of Assamese macaques (Macaca assamensis) in limestone forests in southwest China. American Journal of Primatology, 77(2), 171–185.
IUCN (2016). IUCN red list of threatened species. Version. 2016.2. Available at: www.iucnredlist.org. Accessed 1 Apr 2016.
Janson, C. H., & Chapman, C. A. (1999). Resources and primate community structure. In J. G. Fleagle, C. Janson, & K. E. Reed (Eds.), Primate communities (pp. 237–267). Cambridge: Cambridge University Press.
Johnson, E. (2000). Food neophobia in semi-free ranging rhesus macaques: the effects of food limitation and food source. American Journal of Primatology, 50(1), 25–35.
Julliot, C. (1996). Seed dispersal by red howling monkeys (Alouatta seniculus) in the tropical rain forest of French Guiana. International Journal of Primatology, 17(2), 239–258.
Krishnadas, M., Chandrasekhara, K., & Kumar, A. (2011). The response of the frugivorous lion-tailed macaque (Macaca silenus) to a period of fruit scarcity. American Journal of Primatology, 73(12), 1250–1260.
Kunz, B. A., & Linsenmair, K. E. (2008). The role of olive baboons as seed dispersers in the savannah‐forest mosaic of West Africa. Journal of Tropical Ecology, 24, 235–246.
Lindburg, D. G. (1977). Feeding behavior and diet of rhesus macaques (Macaca mulatta) in a Siwalik forest in norther India. In T. H. Clutton-Brock (Ed.), Primate ecology: Studies of feeding and ranging behaviour in lemurs, monkeys and apes (pp. 223–249). London: Academic.
Link, A., Galvis, N., Marquez, M., Guerrero, J., Solano, C., & Stevenson, P. R. (2012). Diet of the critically endangered brown spider monkey (Ateles hybridus) in an inter‐Andean lowland rainforest in Colombia. American Journal of Primatology, 74(12), 1097–1105.
Marshall, A. J., Boyko, C. M., Feilen, K. L., Boyko, R. H., & Leighton, M. (2009). Defining fallback foods and assessing their importance in primate ecology and evolution. American Journal of Physical Anthropology, 140(4), 603–614.
McConkey, K. R., Aldy, F., Ario, A., & Chivers, D. J. (2002). Selection of fruit by gibbons (Hylobates muelleri × agilis) in the rain forests of Central Borneo. International Journal of Primatology, 23(1), 123–145.
Menon, S., & Poirier, F. E. (1996). Lion-tailed macaques (Macaca silenus) in a disturbed forest fragment: activity patterns and time budget. International Journal of Primatology, 17(6), 969–985.
Milton, K. (1987). Primate diets and gut morphology: implications for hominid evolution. Food and evolution: toward a theory of human food habits, pp. 93–115.
Mourthé, I. (2014). Response of frugivorous primates to changes in fruit supply in a northern Amazonian forest. Brazilian Journal of Biology, 74(3), 720–727.
National Research Council. (1981). Techniques for the study of primate population ecology. Washington, DC: National Research Council (NRC), National Academy Press.
Naughton-Treves, L., Treves, A., Chapman, C., & Wrangham, R. (1998). Temporal patterns of crop-raiding by primates: linking food availability in croplands and adjacent forest. Journal of Applied Ecology, 35(4), 596–606.
O’Driscoll-Worman, C., & Chapman, C. A. (2006). Densities of two frugivorous primates with respect to forest and fragment tree species composition and fruit availability. International Journal of Primatology, 27(1), 203–225.
Oates, J. F. (1977). The guereza and its food. In T. H. Clutton-Brock (Ed.), Primate ecology: Studies of feeding and ranging behaviour in lemurs, monkeys and apes (pp. 275–321). London: Academic.
Peres, C. A. (1994). Diet and feeding ecology of gray woolly monkeys (Lagothrix lagotricha) in Central Amazonia: comparisons with other atelines. International Journal of Primatology, 15(3), 333–372.
Poulsen, J. R., Clark, C. J., & Smith, T. B. (2001). Seed dispersal by a diurnal primate community in the Dja Reserve, Cameroon. Journal of Tropical Ecology, 17(6), 787–808.
Radhakrishna, S., & Sinha, A. (2011). Less than wild? Commensal primates and wildlife conservation. Journal of Biosciences, 36, 1–5.
R Core Team (2015). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
Richard, A., Goldstein, S., & Dewar, R. (1989). Weed macaques: the evolutionary implications of macaque feeding ecology. International Journal of Primatology, 10(6), 569–594.
Richter, C., Taufiq, A., Hodges, K., Ostner, J., & Schülke, O. (2013). Ecology of an endemic primate species (Macaca siberu) on Siberut Island, Indonesia. SpringerPlus 2, 137, http://www.springerplus.com/content/2/1/137.
Riley, E. P., Tolbert, B., & Farida, W. R. (2013). Nutritional content explains the attractiveness of cacao to crop raiding Tonkean macaques. Current Zoology, 59, 160–169.
Robbins, M. M., & Hohmann, G. (2006). Primate feeding ecology: An integrative approach. In G. Hohmann, M. M. Robbins, & C. Boesch (Eds.), Feeding ecology in apes and other primates (pp. 1–13). Cambridge: Cambridge University Press.
Robinson, J. G. (1986). Seasonal variation in use of time space by wedge capuchin monkey, Cebus olivaceus: implications for foraging theory. Smithsonian Contributions to Zoology, 431, 1–60.
Russo, S. E., Campbell, C. J., Dew, J. L., Stevenson, P. R., & Suarez, S. A. (2005). A multi-forest comparison of dietary preferences and seed dispersal by Ateles spp. International Journal of Primatology, 26(5), 1017–1037.
Saj, T., Sicotte, P., & Paterson, J. D. (1999). Influence of human food consumption on the time budget of vervets. International Journal of Primatology, 20(6), 977–994.
Sekar, N., & Sukumar, R. (2013). Waiting for Gajah: an elephant mutualist’s contingency plan for an endangered megafaunal disperser. Journal of Ecology, 101(6), 1379–1388.
Sengupta, A., & Radhakrishna, S. (2015). Fruit trait preference in rhesus macaques (Macaca mulatta) and its Implications for Seed Dispersal. International Journal of Primatology, 36(5), 999–1013.
Sengupta, A., McConkey, K. R., & Radhakrishna, S. (2014). Seed dispersal by rhesus macaques Macaca mulatta in northern India. American Journal of Primatology, 76(12), 1175–1184.
Siex, K. S., & Struhsaker, T. T. (1999). Colobus monkeys and coconuts: a study of perceived human-wildlife conflicts. Journal of Applied Ecology, 36, 1009–1020.
Sillero-Zubiri, C., & Switzer, D. (2001). Crop-raiding primates: Searching for alternative, humane ways to resolve conflict with farmers in Africa. Oxford: People and Wildlife Initiative. Wildlife Conservation Research Unit, Oxford University.
Sivakumar, S., Varghese, J., & Prakash, V. (2006). Abundance of birds in different habitats in Buxa Tiger Reserve, West Bengal, India. Forktail, 22, 128–133.
Srivastava, A., & Mohnot, S. (2001). Distribution, conservation status and priorities for primates in Northeast India. ENVIS Bulletin, 1, 102–108.
Stevenson, P. R., & Link, A. (2010). Fruit preferences of Ateles belzebuth in Tinigua Park, Northwestern Amazonia. International Journal of Primatology, 31(3), 393–407.
Strier, K. (1991). Diet in one group of woolly spider monkeys, or muriquis (Brachyteles arachnoides). American Journal of Primatology, 23(2), 113–126.
Sukumar, R., Venkataraman, A., Cheeran, J. V., & Mujumdar, P. P. (2003). Study of elephants in Buxa Tiger Reserve and adjoining areas in Northern West Bengal and preparation of conservation action plan. Final Report. Bangalore: Centre for Ecological Sciences, Indian Institute of Science.
Swedell, L., Hailemeskel, G., & Schreier, A. (2008). Composition and seasonality of diet in wild hamadryas baboons: preliminary findings from Filoha. Folia Primatologica, 79(6), 476–490.
Symington, M. M. (1987). Ecological and social correlates of party size in the black spider monkey, Ateles paniscus chamek, Ph.D. Dissertation, New Jersey: Princeton University.
Tang, C., Huang, L., Huang, Z., Krzton, A., Lu, C., & Zhou, Q. (2016). Forest seasonality shapes diet of limestone-living rhesus macaques at Nonggang, China. Primates, 57(1), 83–92.
Terborgh, J. (1983). Five new world primates. Princeton: Princeton University Press.
Tsuji, Y., Hanya, G., & Grueter, C. C. (2013). Feeding strategies of primates in temperate and alpine forests: comparison of Asian macaques and colobines. Primates, 54(3), 201–215.
van Schaik, C. P., Terborgh, J. W., & Wright, S. J. (1993). The phenology of tropical forests: adaptive significance and consequences for primary consumers. Annual Review of Ecology and Systematics, 24, 353–377.
Wallace, R. B. (2005). Seasonal variations in diet and foraging behavior of Ateles chamek in a southern Amazonian tropical forest. International Journal of Primatology, 26(5), 1053–1075.
Zar, J. H. (2010). Biostatistical analysis (4th ed.). Upper Saddle River: Pearson Prentice-Hall.
Zhou, Q., Tang, H., Wei, C., & Huang, C. (2009). Diet and seasonal changes in rhesus macaques (Macaca mulata) at Seven-star Park, Guilin. Acta Theriologica Sinica, 29, 419–426.
Zhou, Q., Wei, H., Huang, Z., & Huang, C. (2011). Diet of the Assamese macaque Macaca assamensis in limestone habitats of Nonggang, China. Current Zoology, 57(1), 18–25.
Zhou, Q., Wei, H., Tang, H., Huang, Z., Krzton, A., & Huang, C. (2014). Niche separation of sympatric macaques, Macaca assamensis and M. mulatta, in limestone habitats of Nonggang, China. Primates, 55(1), 125–137.
Acknowledgements
The authors thank the West Bengal Forest Department for necessary permits. Suresh Roy and Netra Prasad Sharma provided invaluable assistance in the field. The manuscript benefitted immensely from discussions with Dr. Kim R. McConkey. The authors also thank Dr. Joanna Setchell, Dr. Oliver Schülke, and two anonymous reviewers for their comments/suggestions that helped improve the manuscript considerably.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Oliver Schülke
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 27 kb)
Rights and permissions
About this article
Cite this article
Sengupta, A., Radhakrishna, S. Influence of Fruit Availability on Fruit Consumption in a Generalist Primate, the Rhesus Macaque Macaca mulatta . Int J Primatol 37, 703–717 (2016). https://doi.org/10.1007/s10764-016-9933-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-016-9933-x