Abstract
The release of wild or captive-bred mammals within their historical ranges typically aims to reestablish populations in areas where they have become extinct or extirpated, to reinforce natural populations, or to resolve human–wildlife conflicts. Such programs, which also typically in parallel help foster the protection of the release site, concern a wide range of endangered mammalian species, including our closest living relatives: chimpanzees. In June 2008, the Chimpanzee Conservation Center (CCC), which is located in the High Niger National Park (HNNP) in Guinea, released a group of 12 chimpanzees (Pan troglodytes verus) comprised of 6 females and 6 males (8–20 yr old). The selected release site lies 32 km from the sanctuary in the Mafou, a core area of HNNP where wild chimpanzees are also known to occur. The purpose of this release was therefore to reinforce the natural chimpanzee population within the Mafou core area and to promote the protection of the HNNP. Nearly 2 yr postrelease, 9 chimpanzees still remain free-living. Two thirds of the release chimpanzees were equipped with VHF-GPS store-on-board tracking collars. We used data from retrieved collars to explore the release chimpanzees’ habitat use, individual day range, and core area use (50% and 80%) during the first year of the release. Males traveled significantly further than females. Although minimum day range did not differ between the sexes or vary seasonally, some release males were active for longer during the day than the females. Males also ranged over larger areas and used a wider network of core areas than the females. Habitat use was similar to that recorded in wild chimpanzees in the HNNP. As of September 2010, 2 males and 3 females form a group at the release site. Two of these females gave birth to healthy offspring respectively 16 and 20 mo postrelease. Another female successfully immigrated into a wild chimpanzee community. We suggest that the success of this chimpanzee release can be attributed to the CCC’s lengthy rehabilitation process and the savanna-mosaic habitat of the HNNP. This release demonstrates that under special socioecological circumstances, the release of wild-born adult chimpanzees of both sexes is a viable strategy, which can also function as an effective conservation tool.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The release of wild or captive-bred mammals within their historical ranges typically aims to reestablish populations in areas where they have become extinct or extirpated, to reinforce natural populations, or to solve human–wildlife conflicts (Seddon et al. 2007). It not only helps promote the continued prevalence of endangered species in their natural habitat and ensure their genetic viability, but can also serve as an effective education and awareness raising tool, in addition to promoting the protection of release zones concerned. Such programs concern a wide range of endangered mammalian species, ranging from bird species, large ungulates, and carnivores to nonhuman primates, including our closest living relatives: chimpanzees (Pan troglodytes).
Throughout most of their range, chimpanzees are threatened with extinction due to habitat destruction, disease, and unsustainable levels of hunting and capture (IUCN 2008). The capture of young chimpanzees is often a byproduct of hunting (Ghobrial et al. 2010; Tutin et al. 2001), and the enforcement of national and international laws protecting chimpanzees has entailed the confiscation of these orphan apes. By 2010, concern for their welfare and their well-being led to the creation of 13 Pan African Sanctuary Alliance (PASA)-accredited chimpanzee sanctuaries caring for as many as 859 rescued individuals and several other facilities caring for >200 individuals throughout Africa (Cress pers. comm.; Farmer 2002). The number of incoming orphans into sanctuaries or other facilities in Africa has swelled in recent years and is predicted to increase in the future (Faust et al. 2007). This increase in numbers is in part due to the implementation of confiscation procedures and the development of awareness-raising programs in some range countries, in conjunction with the persistence in the illegal capture of wild chimpanzees. The chimpanzee population currently cared for in sanctuaries or related facilities in Africa represents today in size ca. 1% of the total wild population. For each sale of a young chimpanzee, it is estimated that between 10 and 29 others have perished during the process of capture or transportation (Carter 2003; Goodall 1986; Teleki 1989). It can therefore be estimated that the pet trade has potentially directly affected as much as 7–20% of the wild population in recent years, assuming a total wild population size of 150,000, i.e., lower limit reported by Butynski (2001). The indirect impact is all the more significant given that wild chimpanzees typically present low population densities; a long interbirth interval, i.e., ca. 5 yr (Emery-Thompson et al. 2007); and a complex social organization.
As wild chimpanzee populations are rapidly declining in numbers throughout their ranges (Campbell et al. 2008; IUCN 2008), the release of captive individuals into their natural habitat is increasingly perceived as an acceptable conservation option (Beck 2010; Beck et al. 2007). Clearly, however, releasing chimpanzees is no solution to overcrowding in sanctuaries or to alleviating the financial pressures on sanctuaries caring for individuals of a species with a life span of ca. 50 yr (Beck 2010). Releasing chimpanzees is a complex and still controversial endeavor that requires much careful planning, financial commitment, and maximum adherence to the best practice guidelines issued by the Great Apes Specialist Group of the IUCN (Beck et al. 2007).
Finding a suitable release site is a key step in the preparation process. However, locating a suitable site can be a challenging affair, as few areas in habitat countries permit full compliance with great ape reintroduction guidelines (Beck et al. 2007). In addition to locating a suitable site, the planning and the preparation of a release requires the selection of suitable candidates. Only a sample of chimpanzees currently in sanctuaries may be considered suitable for release. Only rehabilitated individuals that are equipped with the necessary skills to survive in the wild and who show no abnormal or inappropriate social behavioral patterns can be considered for release. Rehabilitation in this context is “the process by which captive great apes are treated for medical and physical disabilities until they regain health, are helped to acquire natural social and ecological skills, and are weaned from human contact and dependence, such that they can survive independently (or with greater independence) in the wild” (Beck et al. 2007, p.5). Further, selected individuals need to be tested for disease before release to ensure their well-being upon release and to prevent disease transmission to wild conspecifics should release occur in an area harboring a wild population (Beck et al. 2007). Finally, release candidates need to be genetically screened before release to ensure that they are being released in the natural historical or current range of the subspecies to which they belong.
Since the late 1960s, there have been several chimpanzee reintroductions. Reintroductions in this sense represent “an attempt to establish a species in an area which was once part of its historic range [including islands in river or freshwater lakes and within 1 km from the shoreline], but from which it has been extirpated or become extinct” (Beck et al. 2007, p. 4). So far only 1 such reintroduction on a 240-km2 island on the southwestern shore of Lake Victoria in Tanzania known as the Rubondo National Park has succeeded in establishing a self-sustaining population (Borner 1985; Moscovice et al. 2007). Seventeen wild-born chimpanzees between the ages of 4 and 12 yr from European zoos were released onto the island over a period of 3 yr (1966–1969; Borner 1985). The success of this reintroduction has been attributed to the highly favorable habitat available to chimpanzees on the island, which provides them with a year-round abundance of high-quality foods, i.e., fruit (Moscovice et al. 2007). This reintroduction project demonstrates that wild-born released chimpanzees can under exceptional circumstances thrive with limited prerelease preparation and postrelease monitoring and support onto large islands presenting favorable ecological conditions. However, as highlighted in the great apes reintroduction guidelines, the lack of prerelease training and postrelease monitoring is not a recommended model to be emulated (Beck et al. 2007).
The Habitat Ecologique et Liberté des Primates (HELP) project in the Republic of Congo released in separate cohorts 37 chimpanzees (Pan troglodytes troglodytes) between 1996 and 2001 in the forest-dominated Conkouati-Douli National Park (Farmer et al. 2006; Goossens et al. 2005; Le Hellaye et al. 2010; Tutin et al. 2001). As opposed to a reintroduction, this release project represents a population reinforcement or supplementation as it took place in an area already harboring wild chimpanzees (Beck et al. 2007). The health and genetic status, as well as the behavioral and psychological well-being of released individuals and the suitability of the release site, were all carefully evaluated during the preparation stages of this release program (Tutin et al. 2001). Lessons learnt from the HELP-Congo project have revealed that wild-born captive chimpanzees can successfully be released back to the wild, although the risk of aggression on rehabilitants by wild conspecifics can be relatively high, especially for males (Goossens et al. 2005). Nevertheless, as of 2004, the survival rate of the HELP-Congo’s released chimpanzees was 62% and 5 births were also recorded among released females (Goossens et al. 2005).
The Chimpanzee Conservation Center (CCC) is the only sanctuary caring for orphans, victims of the pet trade, confiscated by the national authorities in Guinea, West Africa. The CCC has been rehabilitating chimpanzees since 1997 with the purpose of releasing suitable candidates. The first challenge was to locate a suitable release site. The CCC evaluated several sites in Guinea on the basis of 5 major selection criteria: 1) habitat suitability; 2) distance from human habitation and settlement, i.e., distance to villages and settlements had to exceed 20 km, or if less, access had to be obstructed by natural boundaries, e.g., a river; 3) the protection status of the release area and current and future anthropic pressures on the local fauna, chimpanzees, if present, and the habitat; 4) the presence or absence, distribution, and status of wild conspecifics if present; and 5) the potential for long-term survival independently from human assistance. Finally, based on surveys conducted in 4 areas and evaluation of the above-mentioned criteria, an area within the core area of the Mafou in the Haut Niger National Park, 32 km by road from the CCC facility, was selected as the most suitable release site (Humle et al. in prep. A; Lucas 2004; Raballand 2004). Thus, after many years of preparation, the CCC finally released a first group of 12 chimpanzees (Pan troglodytes verus) in the High Niger National Park on June 27, 2008. This release has been innovative in its use of sophisticated radiotracking collar systems equipped with GPS store-on-board and ARGOS for satellite tracking (Humle et al. in prep. b). We here briefly summarize the preparation of this release and report results on release dynamics, habitat use and ranging patterns of released individuals based on analysis of GPS store-on-board data retrieved from the tracking collars. Finally, to evaluate the success of the CCC’s release, we compare our results with that from wild chimpanzees and other chimpanzee release projects and provide recommendations for future releases.
Subjects and Methods
The Chimpanzee Conservation Center
The CCC sanctuary is located in Somoria, 81 km from Faranah in the northwestern part of the Mafou core area of the park (Fig. 1). As of September 2010, this center cares for 38 orphaned chimpanzees. The primary aim of the CCC is to provide care to all rescued chimpanzees and to rehabilitate them in groups to prepare those apt to survive for release back to the wild. All individuals at the sanctuary are fed 4 times a day and receive routine veterinary care. Strict quarantine procedures are implemented for all new arrivals. Despite the sanctuary’s no-breeding-in-captivity policy, meaning that all cycling females receive birth control, a few females have given birth at the sanctuary. While all chimpanzees >10 yr old are maintained as a single social unit with access to an ca. 4-ha enclosure, all chimpanzees <9 yr old spend ca. 6 h/d in their social group on bush outings accompanied by a local member of staff and an expatriate volunteer. These outings constitute the core of the rehabilitation process. During this time, the young chimpanzees refine their locomotory and nest-building skills, acquire the ability to find and process a diversity of food items and avoid others, to locate and exploit natural sources of water, to recognize dangers and predators and respond appropriately, and the necessary skills to maintain and manage life in a social group. Human-initiated contact with the chimpanzees is kept to the minimum necessary. Contact varies therefore with the age of the individuals (younger chimpanzees need more contact with caretakers than older ones) and the emotional, psychological, and veterinary care needs of each individual.
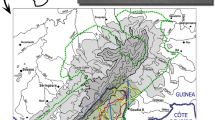
Map of the location of the Haut Niger National Park in Guinea, West Africa, highlighting the Mafou core area of the park and the relative locations of the sanctuary (Somoria) and the release site. (Adapted from Brugiere et al. 2005.).
The Haut Niger National Park
The Haut Niger National Park, located in the prefecture of Faranah and Kouroussa in the center of the Republic of Guinea, was created in 1995 and extends over ca. 10,000 km2 (Brugiere et al. 2005; Ziegler et al. 2002) (Fig. 1). It is one of only 2 national parks in Guinea (Brugiere and Kormos 2009). The park consists of 2 strictly protected core areas, where human settlement or activity are strictly prohibited, and 2 buffer zones, varying in the degree to which local people are permitted to engage in harvesting and utilization of resources. The 2 strictly protected areas include the Mafou Forest (554 km2) and the Kouya Forest (675 km2), separated by 60 km of buffer zones. The park is one of the last remaining important formations of dry forest-savanna mosaics in West Africa. The HNNP has also been flagged as a priority site for the conservation of the Western subspecies of chimpanzee (Pan troglodytes verus) (Kormos et al. 2003), harboring a viable population potentially exceeding 500 individuals (Fleury-Brugiere & Brugiere 2002; Humle et al. in review). The HNNP also harbors large carnivores including leopards (Panthera pardus) and a small remnant population of lions (P. leo) (Brugiere and Magassouba 2003; Ziegler et al. 2002).
Annual rainfall in the park ranges from 1400 to 1700 mm, with a rainy season extending between June and October. Mean monthly temperature is 17–25°C, with a maximum of 35°C at the end of the dry season in April and May (Montfort and Jansen 1993). The hydraulic network is characterized by 2 permanent water courses: the Niger River, the northern limit of the Mafou core area, and the Mafou River, the southeastern limit of the Mafou core area. Other than these 2 large rivers, a network of seasonal watercourses and streams extend throughout the park, but these typically dry up during the dry season. The road infrastructure within the park is very limited. Road access to the Mafou core area of the park consists of a single track leading to Somoria.
Release Candidates
Although lessons learned from previous releases in areas harboring wild chimpanzees highlight the risk of attack toward males by wild conspecifics (Goossens et al. 2005), the CCC decided to release 6 males (8–20 yr old) alongside 6 females (9–19 yr old) at the same time as a single unit group. These 12 individuals were rehabilitated together as a group at the CCC. They each had been rehabilitated at the CCC for 7–11 yr (Table I) and all had known each other for ≥7 yr. All had knowledge of a diversity of wild plant foods — ≥49 species, including 56 plant parts (Colin 2001; Humle unpubl. data) and edible social insects, including both Macrotermes and Pseudocanthotermes termites— good nest-building skills and species-typical social skills and cognitive abilities.
The health and genetic status of released individuals were all evaluated during the preparation stages of the release program (sensu Beck et al. 2007; Tutin et al. 2001). Health screening included examination of fecal samples for endoparasites; tuberculosis tests; as well as hematological and serotological analyses for retroviruses, filoviruses, hepatitis A, B, and C viruses, and blood parasites. Blood samples were used for the genetic analyses. All 12 release candidates proved negative for tested diseases and were all confirmed to belong to the Pan troglodytes verus ssp.
Radio- and Satellite Tracking for Postrelease Monitoring
After having worn fake collars for >12 mo, in June 2008, we fitted 4 of the older males with VHF/GPS store-on-board/ARGOS radiocollars (Model TGW-4580) and 5 of the females with simpler VHF/GPS store-on-board collars (Model TGW-4500) (Humle et al. in prep. b). We did not equip all the individuals with the sophisticated ARGOS system because of the financial costs of these collars and of the platform satellite data collection (Collecte Localisation Satellites [CLS]) services. The ARGOS system allowed us to monitor the location of the males online on a daily basis via satellite transmission. We chose males because we expected them to range initially further than females, and in the case we could no longer track them using the VHF system (Humle et al. in prep. b). One female removed her collar the day she was released and, to avoid any undue stress, we never refitted her collar. Finally, we retrieved the GPS-store-on-board data from 3 males and 4 females between May and July 2009 when they were recaptured to replace their collars with VHF-collars (we did not retrieve Zira’s collar during this period) (Table I). The data considered for analysis encompassed the period between June 2008 and May 2009.
Release Procedure
The initial plan was to move all 12 chimpanzees to an enclosure (1.5 ha) with an annex cage (25 m2) at the release site to give them time to acclimate themselves to their new surroundings. Unfortunately uncontrollable bush fires burnt down the release enclosure in January 2008 while sparing the annex cage. In September 2007, after a severe storm that disrupted the electrical wiring of the enclosures at the sanctuary, some of the chimpanzees learned to escape by placing branches on the fence wires. After repeated escape–recapture events, the decision was taken in March 2008 to move 4 of the adult males (Albert, Robert, John, Orlando) to the release annex cage at the release site.
We transferred the fifth adult male (Rappa) to the release cage in early June 2008. His reintegration with the other males was unproblematic. We transported the other 7 release candidates (6 females and 1 young male) to the release site the same day as all 12 chimpanzees were due to be released, as the release cage alone had not initially been designed to sustain all 12 chimpanzees day-in and day-out. We released all 12 chimpanzees at the same time on June 27, 2008. We opened the door of the release cage containing the 5 adult males and released the others from their individual transport cages ca. 100 m away.
Data Analysis
We used spot-image satellite maps (courtesy of Planet Action, cell resolution 2.5 × 2.5 m) of the whole Mafou core area, including peripheral zones, to generate a vegetation map. Considering that the HNNP is a mosaic of differing vegetation types (Table II), we pooled vegetation types into 4 habitat classes, i.e., river, forest, wooded savanna, and open savanna, to minimize the probability of area misclassification (Table II). Between 2007 and 2009, we gathered a large sample of ground vegetation GPS locations in situ (river: N = 47; forest: N = 660; wooded savanna: N = 968; open savanna: N = 212). We corrected the GPS points for GPS inaccuracy using ground knowledge and satellite imagery. In combination with ground knowledge, these points served to produce training sample polygons for each habitat class. We then used these polygons to generate a supervised classification of habitat type using ArcGIS multivariate tools (©ESRI). Based on a generated error matrix, we estimated overall classification accuracy at 78.5% and average production accuracy across habitat at 84.8 ± 11.8% and user accuracy across habitats at 83.7 ± 5.5% (sensu Lachowski 1996). We utilized this map to extrapolate individual habitat use patterns based on the downloaded store-on-board GPS data points.
Because the store-on-board system yielded ≤7 GPS point locations per day, the added sum of distance traveled every 2 h served to generate a minimum estimate of day range. The latter was estimated only for days for which seven data points were available. We used these data to compare daily mean minimum day range across individuals and sex, as well as season. In addition, we evaluated variation in distance traveled during the day between 06:00 h and 18:00 h, as well as maximum distance traveled and mean distance maintained from the release site (the GPS location of the release cage was used as the reference point). Finally, we estimated individual core area (>80 and 50% usage) size using the fixed kernel density estimation method with Hawths Tools in ArcGIS with the single parameter smoothing factor (h) set at 650.
Statistical Analysis
We systematically checked the data for normality using a normality probability plot and a Kolmogorov–Smirnov test. Because none of our data sets deviated significantly from normality, we used parametric statistics. All tests were 2-tailed, and significance level was set at p < 0.05. For all independent samples t-tests, we employed the Levene’s test to test for equality of variances and adjusted the degrees of freedom if equality of variances could not be assumed. Wherever appropriate, we used paired t-tests to compare means. We estimated the percentage availability of each habitat class based on the vegetation map generated in ArcGIS. We used these data to calculate percentage expected usage of each habitat class. We analyzed habitat usage with respect to availability via a χ2 test. We then used the standardized residuals to reveal patterns of habitat avoidance and preference.
Results
Initial Responses to 27 Mo Postrelease
Shortly after the release, the group split (Table III). Although the released chimpanzees were initially provisioned daily, they rarely visited the provisioning site, because the release took place at the height of the fruiting season and many succulent wild fruits were available to them. However, provisioning was thereafter maintained once a week to encourage the chimpanzees located at the release site to remain in the area and to facilitate visual monitoring of their condition and health.
Among the 12 released chimpanzees, 9 remain free-living. The youngest female (Laurence), which was born at the CCC, voluntarily returned to the sanctuary in early August 2008. An adult male (John) that had drifted away from the release site to the southwest of the Mafou core area died after failing to recover from anesthesia during a recovery mission. This mission was aimed at reuniting him with the other chimpanzees still in the release zone. Another adult male (Rappa) was brought back to the CCC after incurring injuries while in the release cage when his group was recaptured for the purpose of recovering and replacing their collars (Table III).
Between August 2008 and May 2009, 7 missions were conducted to retrieve and reunite release individuals (Table III). At 27 mo postrelease, 5 chimpanzees (2 males and 3 females) remain together at the release site. They have settled down at the release site since they were reunited in May 2009. We continue to monitor them daily. Two of the females of this core group gave birth to healthy offspring on November 11, 2009 and March 6, 2010, respectively. Zira, another female, successfully immigrated into a wild chimpanzee community. We last sighted the other 3 collarless released chimpanzees in July 2009 in the area where we last saw Zira with wild chimpanzees. We will require further sightings of these individuals to confirm their current status and dynamics. However, when last sighted, all released individuals were in good health condition and, with the exception of Rappa, had suffered minimal weight loss at least based on visual assessments. Only 1 male (Albert) incurred minor injuries to his genitals and face while alone in the southern area of the Mafou core area. However, this was the only evidence we ever recorded of aggression presumably from wild conspecifics.
Day Range and Travel Patterns
Overall mean minimum released chimpanzee day range (MDR) (±SD) was 1.4 ± 0.1 km. There was no significant sex difference in MDR (females: N = 3; mean = 1.4 ± 0.1 km; range: 0.1–5.6 km; males: N = 4; mean = 1.2 ± 0.2 km; range: 0.04–5.8 km; t 5 = 0.121; p = 0.906). There was no difference either in individual mean MDR between the dry and wet seasons (N = 6; paired t 5 = 0.270; p = 0.796). However, it must be noted that our sample size was small and differences may have emerged had we had data on more females and males. Based on the maximum distance traveled by each individual from the release site, the 4 adult males traveled significantly further than the 3 adult females (females: N = 3; mean = 8.9 ± 1.7 km; range: 6.2–12.0 km; males: N = 4; mean = 15.6 ± 0.3 km; range: 14.7–16.1 km; t 5 = −4.530; p = 0.006). Mean distance maintained from the release site was also significantly larger for the males than for the females (females: N = 3; mean = 2.5 ± 1.6 km; range: 0.7–5.6 km; males: N = 4; mean = 12.0 ± 1.3 km; range: 8.4–13.8 km; t 5 = −4.773; p = 0.005). Travel patterns during the day did not vary significantly between the sexes (paired t 6 = −0.921; p = 0.393). However, some males traveled further between 18:00 h and 06:00 h than the females, suggesting that they were active longer during the day than the females or that the females settled down earlier for the night (Fig. 2).
Habitat Use and Preference
The released chimpanzees differed significantly in their habitat usage relative to habitat availability (χ 2(3) = 24.539; p < 0.001; Fig. 3). Based on the standardized residuals, they used river in accordance to its availability. They significantly avoided open savanna areas, and used wooded savanna areas slightly less than expected, while they significantly preferred forest areas (Fig. 3).
Ranging Patterns
Males spent 80% of their time, i.e., 80% core area (Wrangham and Smuts 1980), across more areas than females (females: N = 3; mean = 1.3 ± 0.3; males: N = 4; mean = 6.0 ± 1.1; t 3.5 = −3.883; p = 0.023; Fig. 4). The mean surface area of the males’ summed 80% core area network was significantly greater than that of the females (females: N = 3; mean = 12.0 ± 1.7 km2; males: N = 4; mean = 33.8 ± 4.5 km2; t 5 = −3.975; p = 0.011; Fig. 4). In terms of 50% core area, there was no significant sex difference in area size or number of core areas (area size: females: N = 3; mean = 4.6 ± 0.9 km2; males: N = 4; mean = 7.4 ± 1.8 km2; t 5 = −1.295; p = 0.252; number of 50% core areas: females: N = 3; mean = 1.3 ± 0.3; males: N = 4; mean = 2.7 ± 0.7; t 5 = −1.523; p = 0.188). Overall, the females consistently ranged primarily only in 1 or maximum 2 areas, whereas the males ranged further afield across multiple core areas (Fig. 4). On average they ranged in 3 areas 50% of the time and twice as many 80% of time.
Discussion
This project indicates so far that rehabilitated chimpanzees can cope well with free-living in a forest-savanna landscape also harboring wild chimpanzees, as well as large predators including leopards and lions. All released chimpanzees had known each other for many years and had experienced a long rehabilitation process. Among the 12 released chimpanzees, the youngest female (Laurence), which was one of 2 born at the sanctuary, voluntarily returned to the center. The fate of the youngest male (Andrew, also captive-born) remains unconfirmed since July 2009; although when last seen he was accompanied by 2 other collarless individuals: an adult female (Nana) and an adult male (Orlando). Rappa, the second youngest male, survived several months on his own; however, he did not fare as well as the others and had lost a large amount of weight. All of the other older wild-born released adults successfully adapted to their new freedom. Age and birth status may therefore potentially influence success on release. Indeed, these results suggest that adult wild-born individuals (>14 yr old) that have benefited from a lengthy rehabilitation in a group setting in a similar environment to that provided by their future release site may have a greater chance of success on release.
Although minimal weekly provisioning is ongoing at the release site, the release chimpanzees are all nutritionally independent. Provisioning, the rate of which will gradually decrease in frequency during 2010–2011, has served the sole purpose of encouraging individuals to settle in the release zone and of facilitating visual monitoring of the chimpanzees’ health and condition. The integration of a young adult female with wild chimpanzees and the 2 births of healthy infants, presumably fathered by release males, indicate clearly that they have adjusted well to their new ecological and social surroundings.
The initial issues that arose with the group splitting shortly upon release may have been due to 1) the prerelease separation of 4 of the adult males from the remainder of the group after years together, 2) the ensuing excitement of their reunion, and 3) the unfamiliarity of the environment for the 7 individuals that were transported from the sanctuary the same day as they were all released. However, we cannot be certain that group cohesion would have been enhanced upon release had all the chimpanzees benefitted from an acclimatization period together at the release site weeks before release, as initially planned. This initial state of affairs led to 7 retrieval missions aimed at reuniting individuals, which resulted in the establishment of a core release group of 5 adult members. An acclimatization period is still a strategy that we would recommend for future releases, even though our own experience so far cannot substantiate its merit.
Habitat Use, Day Range, and Core Area
The released chimpanzees showed a significant preference for forested areas and avoidance of open savanna habitats. This pattern of habitat usage is similar to that recorded among wild chimpanzees in the HNNP, where feeding remains, nests, as well as direct sightings of wild chimpanzees, were all significantly preferentially recorded in forest habitats (Fleury-Brugiere and Brugiere 2010; Humle et al. in review).
The release chimpanzees’ day range was obviously underestimated because it was based on the sum of straight-line distances between 7 GPS locations per day. It is therefore difficult to compare our data with that from wild chimpanzees. However, day range fell within the range recorded at Taï, Côte d’Ivoire (mean: 2.4 km; range: 0.07–8.1 km; Doran 1997). We found no sex differences in minimum day range, although wild chimpanzee females tend to have shorter day ranges than males, e.g., Taï, Côte d’Ivoire: Doran (1997) and Budongo, Uganda: Bates and Byrne (2009). We also found no seasonal differences in minimum day range. However, day range variation among wild chimpanzees is not necessarily linked with food abundance and distribution, which are expected to vary seasonally (Boesch and Boesch-Achermann 2000). Similarly to wild chimpanzees (Bates and Byrne 2009), the released males tended to have longer days than the females, as some traveled further between 18:00 h and 06:00 h than their female counterparts.
The released males initially rapidly dispersed and readily shifted their ranges over time. Their core areas were less stable than the females’. The released females ranged closer to the release site and in only 1 or 2 overlapping core areas; whereas the males ranged consecutively over time over several areas. However, since the establishment of the core group in the release zone in May 2009, males have settled down, although, based on VHF recording, they still tend to travel further than the females. The released males ranged 80% and 50% of the time over areas respectively 2.8 and 1.6 times larger than the released females did. These results parallel findings on wild chimpanzees indicating that males typically range over areas 1.5–2 times larger than females do (Chapman and Wrangham 1993; Williams et al. 2002; Wrangham and Smuts 1980). Because data on individual core area size are lacking from chimpanzees in savanna-dominated areas, it is difficult to evaluate and compare the size of the core areas of the released individuals with that of wild conspecifics. However, because chimpanzees inhabiting drier and patchier habitats have significantly larger home ranges (ca. 4–12 times larger) than their forest-dwelling counterparts (Baldwin et al. 1982; Boesch and Boesch 1989; Brewer 1978; Hashimoto 1995; Kano 1972; Moore 1992; Ogawa et al. 2007), it might be expected that their core areas (80% and 50% usage) would also be significantly larger. Indeed, the released males ranged 80% of the time in areas 20 times larger than males in the Budongo Forest, Uganda (mean = 1.48 ± 0.02), where community home range was estimated at 7 km2 (Newton-Fisher 2002).
Factors Influencing Release Success
Although in the initial stages of the release, individual dispersion and travel movement were greater than initially expected, more than a year postrelease the situation has stabilized as a result of a series of retrieval missions. We believe that the success so far at least in terms of the released chimpanzees’ ability to thrive under free-living conditions and to breed can be attributed to 1) the CCC’s long rehabilitation process and 2) the heterogeneous habitat of the HNNP. The lengthy and effective rehabilitation process that the chimpanzees experienced as a group in the HNNP before their release likely favored their rapid autonomy and familiarity with the potential dangers posed by wild conspecifics or large carnivores such as lions or leopards. All released chimpanzees had previously on occasion encountered, heard, or come across signs of wild chimpanzees or large predators during the course of their rehabilitation. Although they were thus fully aware of the potential risks, this did not hinder some release individuals of both sexes from successfully thriving alongside wild chimpanzees or integrating wild communities.
The savanna-forest mosaic of the HNNP may also have facilitated the released chimpanzees’ ability to avoid conflict and aggression with wild conspecifics. In contrast to forest-dwelling chimpanzees, wild chimpanzees in savanna-dominated habitats typically tend to have lower population densities and larger home ranges (Ogawa et al. 2007). In addition, the extensive patchwork of vegetation types in the HNNP favors an even distribution of fruiting trees throughout the Mafou core area, which also potentially helps minimize competition and encounter risk. Finally, the demographics of the wild chimpanzee communities in the Mafou may also potentially explain why to date only a single case of aggression by wild conspecifics has been recorded. Both group size and the number of males affect the nature of intergroup interactions and aggressions among wild chimpanzees (Boesch et al. 2008). Further investigation aimed at precisely determining which of those factors best accounts for the low rate of aggression between release and wild chimpanzees is needed, especially because this has been a concern for other release projects (Carter 2003; Goossens et al. 2005).
Further Implications of the Release and Conclusion
Finally, releasing rehabilitated wild-born orphan chimpanzees can make a positive contribution to the conservation of the species and the release habitat. The CCC release project has provided important benefits to the HNNP. There has been a marked increase in environmental education and awareness-raising programs in and around the park. Law enforcement initiatives by local and national authorities thanks to their close collaboration with the CCC have stalled illegal logging activities in the HNNP (Humle et al. in prep. c). In addition to the dissuasive measures put in place by the local and national government, such as road blocks and patrols by park and military authorities, the presence of CCC staff at the release site is also effectively helping reduce illegal hunting activities in the core area of the Mafou (Humle et al. in prep. c). Further evaluation and analysis of the conservation impact of the release, as well as the continued adaptation of the released chimpanzees to their new free-living environment are ongoing. The lessons learnt and the experience gained so far are likely to benefit other sanctuaries that are also considering the option of releasing suitable candidates in the future. In this context, chimpanzee reintroductions are just one strategy in the grander scheme of chimpanzee conservation. We still have much to learn about how rehabilitation, pre- and postrelease procedures, and monitoring protocols impact release success. We can only hope that increased collaboration among academics, conservationists, and sanctuaries will help bridge these gaps.
References
Baldwin, P. J., McGrew, W. C., & Tutin, C. E. G. (1982). Wide ranging chimpanzees at Mt. Assirik, Senegal. International Journal of Primatology, 3(4), 367–385.
Bates, L. A., & Byrne, R. W. (2009). Sex differences in the movement patterns of free-ranging chimpanzees (Pan troglodytes schweinfurthii): Foraging and border checking. Behavioral Ecology and Sociobiology, 64(2), 247–255.
Beck, B. (2010). Chimpanzee orphans: Sanctuaries, reintroduction and cognition. In E. V. R. Lonsdorf, S. & T. Matsuzawa, T. (Eds.), Understanding chimpanzees: The mind of the chimpanzee. Chicago: Chicago University Press.
Beck, B., Walkup, K., Rodrigues, M., Unwin, S., Travis, D., & Stoinski, T. (2007). Best practice guidelines for the reintroduction of Great Apes. Gland: SSC Primate Specialist Group of the World Conservation Union.
Boesch, C., & Boesch, H. (1989). Hunting behavior of wild chimpanzees in the Taï National Park. American Journal of Physical Anthropology, 78, 547–573.
Boesch, C., & Boesch-Achermann, H. (2000). The chimpanzees of the Taï Forest. New York: Oxford University Press.
Boesch, C., Crockford, C., Herbinger, I., Wittig, R., Moebius, Y., & Normand, E. (2008). Intergroup conflicts among chimpanzees in Tai National Park: Lethal violence and the female perspective. American Journal of Primatology, 70(6), 519–532.
Borner, M. (1985). The rehabilitated chimpanzees of Rubondo Island. Oryx, 19, 151–154.
Brewer, S. M. (1978). The chimpanzees of Mt Assirik. New York: Alfred A. Knopf.
Brugiere, D., & Magassouba, B. (2003). Mammalian diversity in the National Park of Upper Niger, Republic of Guinea-an update. Oryx, 37, 19–19.
Brugiere, D., & Kormos, R. (2009). Review of the protected area network in Guinea, West Africa, and recommendations for new sites for biodiversity conservation. Biodiversity and Conservation, 18(4), 847–868.
Brugiere, D., Dia, M., Diakite, S., Gbansara, M., Mamy, M., Saliou, B., et al. (2005). Large- and medium-sized ungulates in the Haut Niger National Park, Republic of Guinea: population changes 1997–2002. Oryx, 39(1), 50–55.
Butynski, T. M. (2001). Africa’s great apes. In B. Beck, T. S. Stoinski, M. Hutchins, T. L. Maple, B. Norton, A. Rowan, E. F. Stevens, & A. Arluke (Eds.), Great apes and humans: The ethics of coexistence (pp. 3–56). Washington: Smithsonian Institution Press.
Campbell, G., Kuehl, H., N’Goran Kouame, P., & Boesch, C. (2008). Alarming decline of West African chimpanzees in Cote d’Ivoire. Current Biology, 18(19), R903–R904.
Carter, J. (2003). Orphan chimpanzees in West Africa: Experiences and prospects for viability in chimpanzee rehabilitation. In R. Kormos, C. Boesch, M. I. Bakarr, & T. Butynski (Eds.), West African chimpanzees. Status survey and conservation action plan (pp. 157–168). Gland: IUCN/SSC Primate Specialist Group. IUCN.
Chapman, C. A., & Wrangham, R. W. (1993). Range use of the forest chimpanzees of Kibale—implications for the understanding of chimpanzee social-organization. American Journal of Primatology, 31(4), 263–273.
Colin, C. (2001). Etude d’un projet de conservation d’une sous-espèce de chimpanzés (Pan troglodytes verus), menacée d’extinction, en République de Guinée. Lyon: Ecole Veterinaire.
Doran, D. (1997). Influence of seasonality on activity patterns, feeding behavior, ranging, and grouping patterns in Tai chimpanzees. International Journal of Primatology, 18(2), 183–206.
Emery-Thompson, M., Jones, J. H., Pusey, A. E., Brewer-Marsden, S., Goodall, J., Marsden, D., et al. (2007). Aging and fertility patterns in wild chimpanzees provide insights into the evolution of menopause. Current Biology, 17(24), 2150–2156.
Farmer, K. H. (2002). Pan-African sanctuary alliance: status and range of activities for great ape conservation. American Journal of Primatology, 58(3), 117–132.
Farmer, K. H., Buchanan-Smith, H. M., & Jamart, A. (2006). Behavioral adaptation of Pan troglodytes troglodytes. International Journal of Primatology, 27(3), 747–765.
Faust, L., Beck, B., & Cress, D. (2007). Estimating future capacity needs for PASA sanctuary ape populations. Paper presented at the Annual Managers Meeting of the Pan African Sanctuary Alliance, Kigali, Rwanda.
Fleury-Brugiere, M.-C., & Brugiere, D. (2002). Estimation de la population et analyse du comportement nidificateur des chimpanzés dans la zone intégralement protégée Mafou du Parc National du Haut-Niger. Faranah: Report on the Parc National du Haut-Niger/AGIR project.
Fleury-Brugiere, M. C., & Brugiere, D. (2010). High population density of Pan troglodytes verus in the Haut Niger National Park, Republic of Guinea: Implications for local and regional conservation. International Journal of Primatology, 31, 383–392.
Ghobrial, L., Lankester, F., Kiyang, J. A., Akih, A. E., de Vries, S., Fotso, R., et al. (2010). Tracing the origins of rescued chimpanzees reveals widespread chimpanzee hunting in Cameroon. BMC Ecology. Retrieved from http://www.biomedcentral.com/1472–6785/10/2.
Goodall, J. (1986). The chimpanzees of Gombe. Cambridge: Belknap Press.
Goossens, B., Setchell, J. M., Tchidongo, E., Dilambaka, E., Vidal, C., Ancrenaz, A., et al. (2005). Survival, interactions with conspecifics and reproduction in 37 chimpanzees released into the wild. Biological Conservation, 123(4), 461–475.
Hashimoto, C. (1995). Population census of the chimpanzees in the Kalinzu Forest, Uganda: Comparison between methods with nest counts. Primates, 436(4), 477–488.
Humle, T., Deniau, C., Lapeyre, V., Colin, C., & T., R. (in review). Diurnal primates and large- and medium-sized ungulates in the Haut Niger National Park, Republic of Guinea: Update on status, abundance and threats. Oryx.
IUCN. (2008). IUCN red list of threatened species. In IUCN (Ed.). Switzerland: Gland.
Kano, T. (1972). Distribution and adaptation of the chimpanzee on the eastern shore of Lake Tanganyika. Kyoto University African Studies, 7, 37–129.
Kormos, R., Humle, T., Brugière, D., Fleury-Brugière, M.-C., Matsuzawa, T., Sugiyama, Y., et al. (2003). Status surveys and recommendations: Country reports: The Republic of Guinea. In R. Kormos, C. Boesch, B. M.I. & T. M. Butynski (Eds.), Status survey and conservation action plan: West African chimpanzees (pp. 63–76). Gland, Switzerland and Cambridge, UK: IUCN/SSC Primate Specialist Group.
Lachowski, H. (1996). Guidelines for the use of digital imagery for vegetation mapping. Darby: Diane Publishing Co.
Le Hellaye, Y., Goossens, B., Jamart, A., & Curtis, D. J. (2010). Acquisition of fission-fusion social organization in a chimpanzee (Pan troglodytes troglodytes) community released into the wild. Behavioral Ecology and Sociobiology, 64(3), 349–360.
Lucas, D. C. (2004). Chimpanzee habitat survey and threat assessment in and around the Kafama and N'Dama forests, Guinea, West Africa. Study sponsored by USAID and commissioned by Project Primate Incorporated (PPI) and the Chimpanzee Conservation Center (CCC) in association with the Pan African Sanctuary Alliance (PASA).
Montfort, A., & Jansen, V. (1993). Projet de Gestion des Ressources Naturelles des Forets Classees de la Mafou et de l’Amana. Rome, Italy: Projet Faranah 1 et Kouroussa 1. Agriconsulting-Agroprogress Int.o. Document Number)
Moore, J. (1992). "Savanna" chimpanzees. In T. Nishida, W. C. McGrew, P. Marler, et al. (Eds.), Topics in primatology, Vol. 1: Human origins (pp. 99–118). Tokyo: University of Tokyo Press.
Moscovice, L. R., Issa, M. H., Petrzelkova, K. J., Keuler, N. S., Snowdon, C. T., & Huffman, M. A. (2007). Fruit availability, chimpanzee diet, and grouping patterns on Rubondo Island, Tanzania. American Journal of Primatology, 69(5), 487–502.
Newton-Fisher, N. E. (2002). Ranging patterns of male chimpanzees in the Budongo Forest, Uganda: Range structure and individual differences. In C. S. Harcourt & B. Sherwood (Eds.), New perspectives in primate evolution and behaviour (pp. 287–308). Otley: Westbury Academic.
Ogawa, H., Idani, G., Moore, J., Pintea, L., & Hernandez-Aguilar, A. (2007). Sleeping parties and nest distribution of chimpanzees in the savanna woodland, Ugalla, Tanzania. International Journal of Primatology, 28(6), 1397–1412.
Raballand, E. (2004). Proposal for the release of chimpanzees into the Parc National du Haut Niger, Guinea. Chimpanzee Conservation Center.
Seddon, P. J., Armstrong, D. P., & Maloney, R. F. (2007). Developing the science of reintroduction biology. Conservation Biology, 21(2), 303–312.
Teleki, G. (1989). Population status of wild chimpanzees (Pan troglodytes) and threats to survival. In P. G. Heltne & L. A. Marquardt (Eds.), Understanding chimpanzees (pp. 312–353). Cambridge: Harvard University Press.
Tutin, C. E. G., Ancrenaz, M., Paredes, J., Vacher-Vallas, M., Vidal, C., Goossens, B., et al. (2001). Conservation biologgy framework for the release of wild-born orphaned chimpanzees into the Conkouati Reserve, Congo. Conservation Biology, 15(5), 1247–1257.
Williams, J. M., Pusey, A. E., Carlis, J. V., Farm, B. P., & Goodall, J. (2002). Female competition and male territorial behaviour influence female chimpanzees’ ranging patterns. Animal Behaviour, 63, 347–360.
Wrangham, R. W., & Smuts, B. B. (1980). Sex differences in the behavioural ecology of chimpanzees in the Gombe National Park, Tanzania. Journal of Reproduction and Fertility, Supplement, 28(Supplement), 13–31.
Ziegler, S., Nikolaus, G., & Hutterer, R. (2002). High mammalian diversity in the newly established National Park of Upper Niger, Republic of Guinea. Oryx, 36(1), 73–80.
Acknowledgments
This study was conducted through the Chimpanzee Conservation Centre (CCC) with the assistance of CCC expatriate and local staff, as well as volunteers from Projet Primates France (PPF) and Project Primate Inc. (PPI), with financial support from the U.S. Fish and Wildlife Services (USFW) and Fauna and Flora International (FFI). The CCC also thanks Dr. Andrew Rowan and Dr. Geza Teleki for their advice and the Arcus Foundation, the Edith J. Goode Trust fund, the Fondation Brigitte Bardot, Fondation Le Pal Nature, IPPL-UK, IPPL-US, and the Tusk foundation for their financial support. We also thank Dr. Henk Niphuis at the Biomedical Primate Research Centre, Rijswijk, the Netherlands, for all the testing of serological samples; Dr. Wendi Bailey at the Liverpool School of Tropical Medicine, UK, for her parasitological expertise; Planet Action for donating high-resolution satellite maps of HNNP and ArcGIS; Telonics for providing us with extra necessary software; and CLS (Collecte Localisation Satellites, Toulouse, France) and Dr. Scott Wilson from Chester Zoo, UK, for their logistical support. We also thank the 2 anonymous reviewers who have provided us with helpful comments and suggestions for improving this manuscript. Estelle Raballand is also deeply grateful to PASA for advice and help during the entire release process and the workshop hosted at Apenheul, the Netherlands, which led to the creation of a CCC-release working group (thank you to Dr. Marc Ancrenaz, Dr. Benoit Goossens, Mike Jordan, Frands Carlsen, Norm Rosen, and Dr. Benjamin Beck). We also thank Christine Sagno, director of the Direction Nationale des Eaux et Forêts, Mr. Aboubacar Oulare, director of the Direction Nationale de la Diversité Biologique et des Aires Protégées and the late Aliomou Diallo, Director of the HNNP. Dr. Tatyana Humle, as scientific advisor to the CCC, also thanks Dr. Mamby Keita, national director of the Direction Nationale de la Recherche Scientifique et Technologique and Mr. Aboubacar Oulare, national director of the Direction de la Diversité Biologique et des Aires Protégées, for granting permission to conduct research in the Haut Niger National Park (HNNP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Humle, T., Colin, C., Laurans, M. et al. Group Release of Sanctuary Chimpanzees (Pan troglodytes) in the Haut Niger National Park, Guinea, West Africa: Ranging Patterns and Lessons So Far. Int J Primatol 32, 456–473 (2011). https://doi.org/10.1007/s10764-010-9482-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-010-9482-7