Abstract
The Nimba Mountains are a West African Natural World Heritage site located in the range of the Guineo–equatorial evergreen rainforest, renowned for its rich biodiversity with a high level of endemism. In 1976, Yukimaru Sugiyama from Kyoto University initiated the long-term study of chimpanzees at Bossou, a Guinean village situated 5 km from the northern foothills of Nimba. This Japanese initiative has provided key discoveries and insights on our closest living evolutionary relatives over the 40 past years, and has grown to become an international collaboration with a research focus extended to adjacent chimpanzee communities. The present paper describes a mid-term behavioral and ecological study on wild chimpanzees populating the southern slope of the Nimba Mountains, conducted in the framework of this collaborative project. It aimed to assess the status and ecological requirements of chimpanzees in order to formulate purpose-built actions for their conservation. We estimated a density of 0.46 chimpanzee per km2 using nest count methods from line transects. We used logistic and Poisson regressions to investigate basic ecological characteristics of chimpanzees in relation to habitat composition and structure, topography and seasonality. We performed an in-depth analysis of their nesting and feeding behaviors, and identified important components of their diet; we also recorded their year-round ranging patterns. Our findings highlight the importance of old secondary forest and high-altitude habitats for these chimpanzees. We discuss the results in the light of other studies from the perspective of the conservation of the species and its natural habitat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the heart of the biodiversity hotspot of the Guinean forests of West Africa stands the World Heritage site of the Nimba Mountains (Myers et al. 2000). This 40-km long relief, emerging sharply more than 1000 m above a large plain, stretches out as a landmark at the tri-national border between Guinea, Côte d’Ivoire and Liberia. The massif’s crest peaks at 1752 m, covered by altitude grasslands that maintain labile relationships with a lush evergreen rainforest covering its slopes (Lamotte 1998). This complex and dynamic biotope is home to a rich biodiversity including chimpanzee populations estimated to be in the hundreds (Matsuzawa et al. 2011).
Northerly from the Nimba Mountains, the Guinean village of Bossou is surrounded by small hills that are inhabited by a group of chimpanzees. Bossou provides a rare example of a site where wild chimpanzees have been living alongside humans for generations, sharing parts of their habitats and resources (Yamakoshi 2011). This unique coexistence stems from the belief of the founding family of Bossou who have traditionally worshiped and protected chimpanzees as sacred animals that are reincarnations of their ancestors (Yamakoshi 2006). As a consequence, Bossou chimpanzees were not afraid of humans and could easily be observed in 1976, when Yukimaru Sugiyama from the Primate Research Institute of Kyoto University (KUPRI, Japan) initiated their long-term study. In 1986, Tetsuro Matsuzawa joined Sugiyama, followed by other Japanese researchers, and from 1995 by non-Japanese researchers. As the research team became more international, collaboration with the Guinean government tightened, and the project progressively gained in strength. Throughout its 40 years of existence, this scientific enterprise has devoted increasing time and endeavor to promote environmental conservation and local development in the region of Bossou, and has strengthened efforts with regard to chimpanzees populating surrounding areas, like the Nimba Mountains. On the occasion of a seminar held in September 2002 regrouping chimpanzee researchers and conservationists working in West Africa, I first met with Sugiyama and Matsuzawa. An outcome of this seminar was the establishment of the Nimba Mountains as one of five exceptionally important areas for the conservation of West African chimpanzee (Kormos et al. 2003). A second outcome was my incorporation into the KUPRI–International team.
Chimpanzees are humans’ closest living evolutionary relatives (Kortland 1974; Prüfer et al. 2012). They are efficient seed dispersers, playing a key role in forest ecology and renewal, recognized as a good flagship, umbrella and environmental indicator species (Junker et al. 2012). These apes sleep in nests (also called beds or sleeping platforms) that are most of the time built in trees and not re-used (Plumptre and Reynolds 1996). Chimpanzees are omnivorous, but strongly depend on ripe fruits as preferred foods (Hladik 1977). Fruits are of high nutritional value and easy to process, but their availability fluctuates greatly across seasons and from year to year (Wrangham et al. 1991). Chimpanzees have developed various strategies to cope with periods of fruit scarcity, like splitting into smaller subgroups and traveling further to exploit more productive areas, or shifting their diet to lower quality but more abundant fallback foods such as leaves, stems, piths and terrestrial herbaceous vegetation (THV; Wrangham et al. 1991; Doran 1997). Fallback foods are higher in fiber, lower in energy and harder to process than ripe fleshy fruits, but more uniformly distributed in the habitat and available year-round (Yamakoshi 1998). Marshall and Wrangham (2007) distinguished staple fallback resources—available year-round they tend to be eaten throughout the year and can constitute up to 100 % of the diet—from filler fallback resources that never constitute 100 % of the diet, and may be completely avoided for weeks at a time.
Although chimpanzees exhibit a wide range of socio-ecological adaptations and cultural variations between communities, their survival is put at risk by increased global deterioration of their living conditions, mainly the loss, degradation and fragmentation of their natural habitats (Oates et al. 2008). In order to efficiently protect chimpanzees and to concurrently favor the sustainability of naturally co-occurring wildlife species, it is crucial to assess their populations’ status and to understand their specific ecological requirements where they still occur (Lambert 2011). In this context, I developed a mid-term ecological and behavioral study of chimpanzees on the southern slope of the Nimba Mountains with perspectives on the sustainable conservation of natural habitats. This consisted of investigating basic ecological characteristics of the chimpanzees, including their nesting, feeding and ranging behaviors in relation to habitat structure and composition, topography, seasonality and other species presence. The primary aim was to understand why chimpanzees appeared to mainly exploit high-altitude habitats colonized by secondary vegetation and with high ground declivity. We tested the hypotheses that these chimpanzees showed a year-round preference for old secondary habitats because they constantly harbored food resources while providing sufficiently sturdy trees in which to build nests, and offering a relatively low human frequentation.
Methods
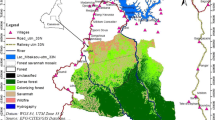
Based on results of preliminary surveys conducted in the eastern part of the Nimba Biosphere Reserve between 2006 and 2008, I established a 60-km2 study area in the middle of the massif’s southern slope, where chimpanzees were thought to permanently dwell (Granier 2011). This study area encompassed the entire Ivorian Nimba Mountains plus the bordering Guinean Nimba Mountains (Fig. 1). With the cooperation of local field assistants, we systematically set up 80 km of survey itineraries consisting of three kinds: linear transects, contour surveys and loop reconnaissance surveys (Fig. 1). Three linear transects were drawn from where the three main rivers outflow from the reserve, following the north azimuth and continuing as high as allowed by topography (mean length = 4.15 ± SD 0.27 km; altitude range = 441–1031 m asl). Two contour reconnaissance surveys stretched between the Guinean and Liberian borders following the 450 and 750 contour lines (mean length = 17 ± SD 0.71 km). Finally, three loop reconnaissance surveys started from where the three main secondary rivers outflow from the reserve, extending as high as allowed by topography and going back down in a large “u turn” forming a loop (mean length = 10.90 ± SD 2.27 km; altitude range = 415–1081 m asl).
The Nimba Mountains and surroundings. The study area appears on the southern slope of the massif with survey itineraries (photo from Lamotte 1998)
We carefully described the structure and composition of vegetation and topography along these survey itineraries. Vegetation structure was described according to nine variables characterizing the different vegetation strata: forest type, forest disturbance level, canopy closing, plus six features describing understory (Schnell 1951; Marchesi et al. 1995; Table 1). The composition of woody species was assessed on a 10-m wide strip along the three transects, identifying all trees of more than 10 cm in diameter at breast height. We then walked these survey itineraries each month during 19 months between 2009 and 2011, monitoring the fruit phenology and recording all signs of chimpanzee presence (nests, feeding remains and ant-dipping sites, feces, footprints and trails, hairs, vocalizations and direct sightings) together with signs of other large mammals (footprints, feces, vocalizations, direct sightings) and human activity (trails, cartridges, trash, gunshots, fishing materials, tree cuttings, direct sightings).
Results
Habitat and fruit phenology
Along the survey itineraries, we identified a total tree diversity of 437 species belonging to 64 families (Granier 2014). Mountain forest was slightly more represented than both lowland and gallery forests (Table 2). About two-thirds of forested habitats were of old-growth type; the remaining third corresponded to different stages of secondary forests. Marantaceae plants were recorded in the understory of 52.4 % of sampled habitats, whereas Zingiberaceae flora was present in 15 %.
Half of the total fruiting trees, shrubs and lianas (N = 11,898) were recorded during the late dry season (January–April), whereas fruiting species were consistently scarcer during the other months (Fig. 2). Mann–Whitney U tests revealed that general fruit availability was markedly seasonal in the Nimba forest (U obs = 0, P < 0.01, N = 11). However, the abundance of 45 principal fruit food species—that were concurrently the most frequently recorded as feeding signs or through macroscopic analysis of feces and the most represented along the three transects—showed aseasonal fluctuations (U obs = 13, P < 0.05, N = 11; Granier 2014).
Year-round patterns of fructification. Seasonal variation of fruit availability between the dry (dark gray) and rainy (light gray) seasons appears in red with the monthly numbers of overall fruiting plants recorded on all itineraries (number total fruits). Green dotted line shows the aseasonal variation of principal species fruit abundance index (abundance principal species fruits). Principal species were concurrently the most eaten by chimpanzees and the most represented along the three transects (color figure online)
Feeding behavior
We identified 87 vegetal foods as part of the Nimba chimpanzees’ diet through direct and indirect observations. Most of these foods consisted of fruits, but chimpanzees also consumed terrestrial herbaceous vegetation (THV) such as piths of six Marantaceae species and piths and fruits of seven Zingiberaceae species. Modeling signs of chimpanzee presence as a function of monthly fruit abundance and Marantaceae/Zingiberaceae availability using logistic regression revealed important fluctuations in the species and abundance of fruits and THV that composed the diet. During the rainy months (May–November), the overall number of fruiting plants was low but principal fruit food species were periodically abundant (Fig. 2), and chimpanzee presence was linked to Zingiberaceae and seasonal fleshy fruits. During the dry season (January–April), the overall number of fruiting plant species peaked, but principal fruit food species were scarce; chimpanzees were drawn to Zingiberaceae and Marantaceae. Based on Marshall and Wrangham (2007), we identified Marantaceae plants as filler fallback resources with wide spatiotemporal distribution, which attracted chimpanzees only during periods of general fruit food scarcity. Zingiberaceae plants, however, constituted staple fallback foods: although less represented in the study area than Marantaceae, they attracted apes across seasons and were eaten every month. We also identified the fleshy fruits of two species (Nauclea diderrichii and Grewia barombiensis) as preferred foods that were actively sought by Nimba chimpanzees (Granier 2014).
Nesting behavior
We recorded 764 nests in 338 groups consisting of 1–11 nests (mean group size = 2.23 ± SD 1.57 nests). Nests were found mainly in steep locations (mean ground declivity = 15.54 ± SD 10.81 %). Nimba chimpanzees used 114 vegetal species for nesting (108 woody, 3 Marantaceae and 3 Zingiberaceae), but 10 tree species accounted for 52 % of all nests (Granier et al. 2014). A G test for goodness of fit revealed a high selectivity in chimpanzees’ choice of nesting-tree species that was independent of overall tree availability (G = 577.49, df = 41, P < 0.00001; Granier et al. 2014). Calculation of Pearson’s normalized residuals for each nesting-tree species pinpointed 21 preferred nesting-tree species (that were used significantly more than expected from general availability) and 15 avoided species (used less than expected).
Abundance
We used Distance 6.0 software to estimate chimpanzee density from the standing crop (SCNC) and marked nest count methods (MNC), based on both nest groups and individual nests (Plumptre and Reynolds 1996). Population density ranged between 0.14 and 0.65 chimpanzee per km2, which corresponds to a population of 8–39 chimpanzees in the study area. Based on lower Akaike information criterion, the MNC method applied to nest groups provided the most reliable estimate, which is 0.46 chimpanzee/km2 (Granier et al. 2014).
Habitat use and ranging
We used Poisson regression to investigate the influences of habitat structure and human and other mammal presence on chimpanzee sign counts (Granier 2014). Chimpanzees overwhelmingly used more gallery and mountain forests than lowland forest. They selected old-growth forest slightly more than secondary forest for nesting, whereas they showed a marked preference for the old secondary forest for feeding, moving or resting. Considering the range of altitudes sampled (415–1081 m asl), chimpanzees used more upper-altitude than lower-altitude habitats, with the maximum number of chimpanzee sign observations at 975 m. They avoided habitats containing signs of human presence, and clearly exploited the same habitats as the seven other primate species observed in Nimba forests (n = 387 signs related to the presence of Cercopithecus campbelli campbelli, C. diana diana, C. nictitans martini, C. petaurista buettikoferi, Colobus polykomos, Cercocebus atys atys and Galagoides demidovii).
Plotting all evidence of chimpanzee presence reveals two distinct clusters separated by a gap (Fig. 3). When combined with nest group characteristics and low population density estimates, this pattern suggests that the study area straddles the territories of two distinct communities (Granier et al. 2014). The spacing between observations of chimpanzee signs in Gouéla II, and their seasonality, suggest that this area is a peripheral part of one community’s territory. In contrast, considering the high concentration and the permanence of signs observed in the Nuon area, we propose that this cluster shows part of the core zone of a second community’s territory.
Spatial distribution of chimpanzee signs and nest groups. Chimpanzee signs (red) and nest groups (orange) were observed in the upper part of the study area in two distinct clusters. The Nuon cluster on the left contained N = 430 densely distributed observations (including n = 227 nest groups), and the Gouéla II cluster on the right N = 363 dispersed observations, more widely distributed across the altitudinal gradient (including n = 111 nest groups) (color figure online)
Discussion
Chimpanzee research
When Sugiyama first visited Bossou in 1976, Guinea was governed by A. Sékou Touré, the first president in the post-colonization era, who remained in power from 1958 to 1984. At that time, the country was rather closed and it was not easy to conduct in situ research. Thanks to Sugiyama’s tenacity and to the hospitality of Bossou villagers, after 10 years of activity, the research project was well established. Through the efforts of Professor Matsuzawa, since 1986, the research team has grown and become increasingly international. At least 50 persons from nine nations and four continents have participated as core researchers in the Bossou–Nimba project (Matsuzawa et al. 2011). This international collaboration has been initiated, continuously driven and mainly financed by Japan during the 40 years. Still ongoing, it has become a landmark project that has provided key discoveries and central insights into the life history of Bossou chimpanzees, while promoting their conservation and the protection of natural habitats. Surveys conducted in different zones of the Nimba Mountains established the presence of chimpanzees (Sugiyama 1995; Matsuzawa and Yamakoshi 1996; Shimada 2000). Two areas with permanent presence emerged as particularly valuable for further behavioral and ecological studies: Seringbara on the northern slope, and Yealé on the southern slope (Fig. 1; Humle and Matsuzawa 2001). A permanent research site was established in Seringbara in 2003, but the war that erupted in Côte d’Ivoire in 2002 had interrupted research in Yealé until my first visit in 2007 (Koops 2011; Granier 2014). The present study has provided tangible information on the ecology and population status of the chimpanzee population on the southern slope of the Nimba Mountains. Almost all the information obtained so far is based on indirect evidence (nests and other signs of presence), recorded with care taken to avoid any habituation effect on these wild chimpanzees, and aiming to have minimal impact on their habitats. We estimated chimpanzee abundance, described their year-round ranging and feeding behavior, identified some of their preferred and fallback foods and documented their nesting behavior. Combining all the knowledge achieved to date, we conclude that two distinct communities of chimpanzees inhabit the study area, and we have proposed ranging patterns for each (Granier et al. 2014).
Conservation
Our analyses additionally shed light on the importance of secondary vegetation and high-altitude habitats for these chimpanzee populations, and allowed us to pinpoint woody species of particular importance in their daily life. Throughout the year, 53 species of trees and lianas were preferentially selected for building nests or as sources of fruits (Granier 2014). Three of these species of special value in chimpanzees’ life history emerged as critically important in terms of conservation, namely Parinari excelsa—an orophilous species producing fleshy fruits—and Pouteria altissima—listed as ‘conservation dependent’ in the IUCN red list of threatened species—both used for feeding and nesting; and Nauclea diderrichii—a preferred food listed as ‘vulnerable’ in the IUCN red list.
Our findings validate the working hypothesis that Nimba chimpanzees frequently select high-altitude habitats and particularly old secondary forest because this biotope continuously provides them with food, while offering a low probability of encountering humans. Though secondary vegetation is not at first sight of great conservation value, numerous studies have revealed the central role of regrowth habitat in a primate's diet, and its importance for the conservation/management of wildlife (Fimbel 1994). However, in a context of growing human demographics and concomitantly increasing pressures on wildlife, with the unavoidable menace of iron ore mining in the Guinean Nimba, it is extremely important to maintain and encourage tracts of old-growth forest. These habitats support a greater diversity and biomass of primates per unit area than secondary forest, and they can also act as refuges and re-population centers for surrounding degraded habitats (Chapman et al. 2010). In addition to this general measure of compensation, further studies are needed to assess chimpanzee adaptations to habitats heavily modified by humans, and specifically by extractive activities. Our experience showed that chimpanzees often approached and sometimes entered the mining concession. We need to achieve better understanding of this behavior in order to prevent increasing proximity with humans and increased risks of accidents, inter-species conflict and transmission of infectious diseases (Hockings and Humle 2009).
A conservation “shortcut” proposed by Fleishman et al. (2000) consists of selecting particular taxa as representative of a given ecological community, and focusing all efforts on these ‘umbrella’ taxa. Moreover, Lambert (2011) referred to Cercopithecus and Pan as good umbrella taxa of the Kibale Forest in Uganda. Nimba chimpanzees clearly exploit the same habitats as the other primates inhabiting the mountain, and we sometimes observed polyspecific associations between Pan and Cercopithecus species (personal observations). We build on these statements to propose a holistic approach toward research and conservation targeting the Cercopithecus monkeys and the chimpanzee, as an effective way forward for preserving a wide range of species, thereby ensuring the sustainability of Nimba forested ecosystems.
References
Chapman CA, Struhsaker TT, Skorupa JP, Snaith TV, Rothman JM (2010) Understanding long-term primate community dynamics: implications of forest change. Ecol Appl 20:179–191
Doran D (1997) Influence of seasonality on activity patterns, feeding behavior, ranging and grouping patterns in Taï chimpanzees. Int J Primatol 18:183–206
Fimbel C (1994) The relative use of abandoned farm clearings and old forest habitats by primates and a forest antelope at Tiwai, Sierra Leone. Biol Conserv 70:277–286
Fleishman E, Murphy DD, Brussard PF (2000) A new method for selection of umbrella species for conservation planning. Ecol Appl 10:569–579
Granier N (2011) Chimpanzees in the eastern part of the Nimba Mountain Biosphere Reserve: Gouéla II and Déré Forest. In: Matsuzawa T, Humle T, Sugiyam Y (eds) The chimpanzees of Bossou and Nimba. Springer-verlag, Tokyo, pp 289–299
Granier N (2014) Ecology and conservation of wild chimpanzees Pan troglodytes verus in the Nimba Mountain. Dissertation, Liège University, Belgium
Granier N, Hambuckers A, Matsuzawa T, Huynen MC (2014) Density estimates and nesting-site selection in chimpanzees of the Nimba Mountains, Côte d’Ivoire and Guinea. Am J Primatol. doi:10.1002/ajp.22278
Hladik CM (1977) Chimpanzees of Gabon and Gombe: comparative data on the diet. In: Clutton-Brock TH (ed) Primate ecology. Academic Press, New York, pp 481–501
Hockings K, Humle T (2009) Best practice guidelines for the prevention and mitigation of conflict between humans and great apes. Gland, Switzerland: IUCN/SSC Primate Specialist Group (PSG)
Humle T, Matsuzawa T (2001) Behavioural diversity among the wild chimpanzee populations of Bossou and neighboring areas, Guinea and Côte d’Ivoire, West Africa. Folia Primatol 72:57–68
Junker J, Blake S, Boesch C, Campbell G et al (2012) Recent decline in suitable environmental conditions for African great apes. Divers Distrib 18:1077–1091
Koops K (2011) Chimpanzees in the Seringbara region of the Nimba Mountains. In: Matsuzawa T, Humle T, Sugiyama Y (eds) The chimpanzees of Bossou and Nimba. Springer-verlag, Tokyo
Kormos R, Boesch C, Bakarr M, Butynski TM (2003) West African chimpanzees: status survey and conservation action plan. IUCN/SSC Primate Specialist Group, Gland, Switzerland and Cambridge, UK
Kortland A (1974) New perspectives on ape and human evolution. Curr Anthropol 15:427–448
Lambert JE (2011) Primate seed dispersers as umbrella species: a case study from Kibale National Park, Uganda, with implications for Afrotropical forest conservation. Am J Primatol 73:9–24
Lamotte M (1998) Le Mont Nimba, réserve de Biosphère et site du patrimoine mondial—initiation à la géomorphologie et biogéographie. UNESCO, Paris
Marchesi P, Marchesi N, Fruth B, Boesch C (1995) Census and distribution of chimpanzees in Côte d’Ivoire. Primates 36:591–607
Marshall AJ, Wrangham RW (2007) Evolutionary consequences of fallback foods. Int J Primatol 28:1219–1235
Matsuzawa T, Yamakoshi G (1996) Comparison of chimpanzee material culture between Bossou and Nimba, West Africa. In: Russon AE, Bard K, Parker ST (eds) Reaching into thought. Cambridge University Press, Cambridge, pp 211–232
Matsuzawa T, Humle T, Sugiyama Y (2011) The Chimpanzees of Bossou and Nimba. Springer-verlag, Tokyo
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Oates JF, Tutin CEG, Humle T, Wilson ML et al. (2008) Pan troglodytes. The IUCN red list of threatened species 2008. Downloaded on 31 August 2015
Plumptre AJ, Reynolds V (1996) Censusing chimpanzees in the Budongo forest, Uganda. Int J Primatol 17:85–99
Prüfer K, Munch K, Hellmann I, Akagi K et al (2012) The bonobo genome compared with the chimpanzee and human genomes. Nature 486:527–531
Schnell R (1951) Végétation et flore des Monts Nimba (Afrique Occidentale Française). Vegetation 3:350–406
Shimada MK (2000) A survey of the Nimba Mountains, West Africa from three routes: confirmed new habitat and ant-catching wands use of chimpanzees. Pan Africa News 7:7–10
Sugiyama Y (1995) Tool-use for catching ants by chimpanzees at Bossou and Monts Nimba, West Africa. Primates 36:193–205
Wrangham RW, Conklin NL, Chapman CA, Hunt KD (1991) The significance of fibrous foods for Kibale forest chimpanzees. Philos Trans R Soc B 334:171–178
Yamakoshi G (1998) Dietary responses to fruit scarcity of wild chimpanzees at Bossou, Guinea: possible implications for ecological importance of tool-use. Am J Phys Anthropol 106:283–295
Yamakoshi G (2006) An indigenous concept of landscape management for chimpanzee conservation at Bossou, Guinea. In: Maruyama J, Wang L, Fujikura T, Ito M (eds) Proceedings of Kyoto Symposium 2006, Crossing disciplinary boundaries and re-visioning area studies: perspectives from Asia and Africa. Kyoto University, pp 3–10
Yamakoshi G (2011) The “prehistory” before 1976: looking back on 3 decades of research on Bossou chimpanzees. In: Matsuzawa T, Humle T, Sugiyama Y (eds) The chimpanzees of Bossou and Nimba. Springer-verlag, Tokyo, pp 35–44
Acknowledgments
I am grateful to Professor Matsuzawa for his support and confidence. I am indebted to the eight local field assistants for their dedication and essential help. Thanks are due to the ‘Office Ivoirien des Parcs et Réserves’ in Côte d’Ivoire and the ‘Direction Nationale de la Recherche Scientifique et de l’Innovation Technologique’ in Guinea for research authorizations. Research was financially supported by the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT #16002001, #20002001, #24000001), the Japanese Society for the Promotion of Science—International Training Program—Primate Origins of Human Evolution grants addressed to T. Matsuzawa, and by a scholarship from the Japanese Society for the Promotion of Science to N. Granier.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Granier, N. Chimpanzee research and conservation in Bossou and the Nimba Mountains: a long-term international collaborative effort in West Africa. Primates 57, 349–357 (2016). https://doi.org/10.1007/s10329-016-0519-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-016-0519-1