Abstract
Infanticide by males is widespread across mammals and especially prevalent among primates. Considerable research has examined how fitness benefits can explain the occurrence of this behavior; less is known, however, about intrapopulation variation in its occurrence. We evaluated 10 infanticides by males in wild blue monkeys according to the sexual selection hypothesis. To explore intrapopulation variation in occurrence of infanticide, we compared these cases to 38 cases that were contextually similar but in which infanticide did not occur. We examined male reproductive benefit, infant age, maternal parity, postconception estrus, group defense, available mating partners, and context of takeover. We based comparisons on daily or near daily records of male presence in the study groups, infant birth dates, and male-female sexual interactions. Infanticides followed predictions of the sexual selection hypothesis: males were unlikely to kill their own offspring, the period for the mother’s return to conception was reduced by half, and males increased their chance of siring her next offspring. Difference in male reproductive benefit, costs, and motivation did not fully explain the observed variation in infanticide occurrence. Infants were more likely to be spared if they were older when a male first arrived, or if their mother had mated with the male in the second month after conception. The most important determinant of infant fate, however, was male identity, a finding consistent with 2 scenarios: 1) an infanticidal tendency may be influenced by a genetic polymorphism that is not fixed in this population or 2) infanticidal behavior may be a conditional male strategy. Further research on intrapopulation variation in infanticidal behavior should focus especially on characteristics of males.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infanticide by males occurs in many mammalian species and is especially prevalent among primates (Ebensperger 1998; van Schaik 2000a). Hrdy (1979) articulated alternative adaptive hypotheses for infanticidal behavior in males, i.e., that they may reduce food competition for themselves or their offspring or both, gain a nutritional advantage when infanticide is associated with cannibalism, or increase their likelihood of siring offspring while reducing the relative genetic contribution of other males. A nonadaptive explanation suggests that infanticide is a byproduct of male aggression toward females that is associated with changing male membership (Bartlett et al. 1993).
The position that male infanticide is adaptive has been challenged, mainly on perceived deficiencies in supporting data (Sussman et al. 1995). However, observed cases from wild primate populations (van Schaik 2000b) continue to accumulate (Agoramoorthy and Hsu 2004; Enstam et al. 2002; Gibson et al. 2008; Harris and Monfort 2003; Knopff et al. 2004; Lewis et al. 2003; Manson et al. 2004; Mori et al. 2003; Murray et al. 2007; Onderdonk 2000; Ramirez-Llorens et al. 2008; Rasoloharijaona et al. 2000; Sherrow and Amsler 2007; Singh et al. 2006; Teichroeb and Sicotte 2008; Watts et al. 2002; Xiang and Grueter 2007; Yamada and Nakamichi 2006) and for primates, as well as other taxa, there is considerable support for the sexual selection hypothesis. Most empirical data suggest 1) that infanticide by a male typically occurs shortly after he enters a group or ascends to high rank, when his likelihood of paternity is low, and 2) that females’ return to ovulation is accelerated afterwards (Pradhan and van Schaik 2008; van Schaik 2000b).
Although there is strong evidence that infanticide by males is adaptive, there can be considerable variation in its occurrence both between and within populations. Interpopulation variation seems generally related to differences in benefits and opportunity (Janson and van Schaik 2000). In brown bears (Ursus arctos), such differences were attributed to variable competitive pressure among males that derived from demographic differences (Bellemain et al. 2006). Butynski (1990) contrasted 2 subpopulations of blue monkeys (Cercopithecus mitis stuhlmanni) and found higher rates of infanticide where the density of adult males relative to adult females and to number of groups was higher. Palombit (2003) found that variation in infanticide rates among multimale baboon populations related to males’ ability to monopolize matings (and presumably increase certainty of paternity) and access to fertile females (a function of reproductive rates and male tenure length).
Intrapopulation variation in infanticide is much more difficult to interpret. Certainly not all males exhibit infanticidal behavior and not all infants are attacked or killed by infanticidal males (Butynski 1982a; Palombit et al. 2000; Teichroeb and Sicotte 2008; Yamagiwa and Kahekwa 2004). For example, in Sommer’s (1994) examination of 18 yr of data pooled from 1 langur population, approximately half the infants present during 22 male residence changes were not harmed. Feh and Munkhtuya (2008), studying Przewalski’s horses over 10 yr, similarly observed that only 5 of 39 foals were attacked by newly arrived stallions. These results stand in contrast to reports from other species, such as lions (Packer and Pusey 1984) and mice (Wolff and Cicirello 1991), in which newly arrived males consistently kill most or all infants.
The degree of observed variation, particularly within populations, raises the question of what male decision rules guide infanticidal attacks, as well as what factors influence the success of attacks once launched. Given the support for the sexual selection hypothesis, a male’s assessment of an infant’s paternity, particularly his own likelihood of being the father, should be one critical component (Pradhan and van Schaik 2008; van Schaik et al. 2004). This assessment may derive from his mating history with an infant’s mother, including the timing of mating relative to the mother’s fertility or peak attractiveness (Borries et al. 1999b; Gibson et al. 2008; Murray et al. 2007; Soltis 2002; van Schaik 2000b). A second component may be assessment of potential reproductive benefits in given circumstances (Soltis et al. 2000; van Schaik 2000b), though how males gauge these is uncertain; infant’s age, revealed in some species by a natal coat (Treves 1997), may be an important clue to the mother’s expected return to ovulation. A third component may be an assessment of the costs of infanticidal attack (van Schaik 2000b). Although it is recognized that males intent on killing infants usually eventually succeed (van Schaik 2000b), anecdotal evidence suggests that success of attacks may relate to defensive actions by the mother or other group members (Treves 2000; van Schaik 2000b).
In a long-term study of social behavior in wild blue monkeys, we documented 10 cases of infanticide or highly probable infanticide by 6 males over a 12-yr period. During this same period, we also observed 8 takeovers by new males in which no infanticide occurred. We predicted that, consistent with an adaptive hypothesis for infanticide, this observed variation in occurrence would relate to differences in potential reproductive benefit to males. We therefore compared cases of killed vs. unharmed infants according to Hrdy’s (1979) criteria for the sexual selection hypothesis, i.e., that 1) the infant is unlikely to be the offspring of the infanticidal male, 2) the mother of the infant returns to ovulation more quickly than she would have had the infant lived, and 3) the infanticidal male is likely to sire her next offspring.
We also examined other factors related to male cost and motivation, as well as female counterstrategies such as direct defense or postconception estrus (Hrdy 1979), that might explain an infant’s fate. Specifically, we predicted that older infants or those whose mothers were more experienced, and might therefore provide better protection, would be less likely to be killed. We also expected that infants would more likely be spared if other group members defended them or if males had mated with the pregnant mother. We predicted that males with more mating partners during their first months in a new group would be less motivated to gain additional partners through infanticide. Lastly, we predicted that males arriving as part of a breeding season influx would be less likely than males arriving on their own to kill infants because of the potential continued presence of an infant’s protective father or a lower probability of siring the next offspring (Borries and Koenig 2000).
Methods
Study Site and Subjects
The Isecheno study site is located in the Kakamega Forest, a moist semideciduous forest in western Kenya (0°14′N, 34°52′E, elevation 1580 m, annual rainfall averaging 2.2 m; Fashing and Gathua 2004; Cords unpubl data). This area supports a relatively dense population of blue monkeys (Cercopithecus mitis stuhlmanni), ca. 170–220 individuals/km2 (Fashing and Cords 2000).
Female blue monkeys at Kakamega are strictly philopatric, whereas males leave their natal groups at puberty (Ekernas and Cords 2007); as adults, males may live within or outside a heterosexual group. Groups typically include only 1 resident adult male, but multiple males may regularly be present during the 3–4 mo breeding season (Cords 2000). Unusually, one of our study groups included multiple males (usually 3–6/d) continuously over 61 mo. Males that become sole residents may enter a group during a multimale influx, or they may take over a group from a single resident. Breeding season influxes do not necessarily lead to the ouster of a long-term resident, which may regain his sole resident status after the breeding season (Cords 2002a). Blue monkeys at Kakamega are territorial; females actively defend territory boundaries and intergroup relations are predictable according to their location relative to these boundaries (Cords 2002b, 2007).
We report data recorded as part of a long-term study of blue monkey social behavior that started in 1979. The study population included 2 groups in 1996, with each fissioning subsequently (1999, 2005), so that there were 4 groups by 2008. All group members were well habituated and individually recognized. We followed study groups on a daily or near daily basis from June 16 to December 15, 1996 and from June 8, 1997 to August 31, 2009. On each 9–10 h observation day, 1–3 observers took roll, allowing us to specify when births, disappearances (or deaths), and male entry into and departure from groups occurred. In addition, we recorded all observed occurrences of sexual activity (copulations, nonejaculatory mounts, and proceptive presenting and puckering; Tsingalia and Rowell 1984), as well as aggressive behavior (threats, chases, and attacks with physical contact) involving males. Recording sexual behavior had the highest priority through June 2006, and observers focused attention on breeding adults, i.e., adult males and sexually active (henceforth estrous) females. Given visibility conditions in the forest and the fact that copulations were infrequent, we judged such focused group follows to provide a more complete daily record of sexual partners than rotating, briefer focal follows of adult females.
Criteria for Including Cases in the Data Set
Cases included as infanticides (killed infants)
In 1 of the 10 infanticides, we observed the male continuously as he attacked a female, seized her infant, and killed and ate it. In 3 other cases, we observed the male eating (n = 2) or carrying a dead infant in his mouth and simultaneously verified that the mother had lost her infant. In another 3 cases, the male severely attacked a mother-infant pair, but it took us up to 24 hr to resight the female that no longer had her infant. In the remaining 3 cases of presumed infanticide, the male repeatedly attacked or stalked a female with a live infant for a few days (n = 2) or weeks (n = 1) and ceased this aggression abruptly after the infant disappeared. We refer to all the above cases as infanticides (see Bartlett et al. 1993; Borries et al. 1999a; Palombit 2003).
Cases included as noninfanticides (unharmed infants)
We included as noninfanticides infants from groups in which a new male entered and eventually achieved sole resident status during the same 12-yr period. In documented blue monkey infanticides, the oldest infant killed was ca. 6 mo old (Butynski 1982a), and the longest known interval between male arrival and infanticide is nine months (Table I, Case 5). We therefore included only infants that were ≤6 mo old within the first 9 mo of a male’s arrival. Although the sexual selection hypothesis suggests males would benefit substantially by killing older infants as well, there may be reasons they do not (such as higher costs or lowered motivation; Sommer 1994); we preferred to identify vulnerable infants conservatively, and thus used empirically derived limits.
Comparisons
We compared infanticides with noninfanticides, using data on the circumstances and sequelae of infanticides and analogous demographic data from noninfanticides. We inferred a male’s likelihood of being an infant’s father by examining his mating history with the mother relative to the infant’s conception date. Lacking hormonal data, we inferred a conception window for each infant using the estimate of gestation length of Pazol et al. (2002: 176 d, 95% confidence interval: 162–190 d) derived for blue monkeys from this population; we estimated the conception period for each infant by counting back 162–190 d from its birth. If a male had not yet entered the infant’s group at the time of this 28-d conception window and we had observed no social interaction between him and the group, we judged his probability of paternity as very low. Given the extent of daily observations, but allowing for the possibility of missed interactions, if a male had joined a group but we observed no sexual interactions between him and the mother during the conception window, we judged probability of paternity as moderately low. Conversely, if we observed sexual behavior between a male and mother during the conception period, we judged his chance of paternity to be higher than in either of the previous scenarios.
We considered sexual behavior by a female as being postconception if it occurred ≤161 d before giving birth. We likewise inferred a female’s return to ovulation by counting back 162–190 d from her next birth. It is possible that females began to ovulate sooner without becoming pregnant or that some pregnancies went unnoticed if they were prematurely terminated; our measure of return to ovulation and interbirth interval is thus conservative with respect to the predictions of the sexual selection hypothesis.
We also compared killed vs. unharmed infants with regard to the parity of their mothers. For all killed infants and 29 of the 38 unharmed infants, we knew the mother’s parity exactly. For the mothers of the remaining 9 unharmed infants, we did not know if our record of the female’s first observed birth was her actual first birth: however, we estimated her parity at the time of the infanticide as if this were so. None of these 9 infants represented the mother’s first observed birth.
We compared the number of females with which each male mated during the 2 mo after his arrival in the group or, for infanticidal males, before he killed his first infant (whichever was shorter). Because some infanticidal males did not kill their first victim for >2 mo, we repeated the analysis comparing all preinfanticide residence time for these males to the first 2 mo of residence of the noninfanticidal males. In one case, we were uncertain which of 2 males (Osca or SS) committed an infanticide; we therefore used the average number of sexual partners for these 2 males as 1 record in the analysis (as neither of these males interacted with the mother during the killed infant’s conception or postconception period, this uncertainty did not affect other analyses). We excluded one infanticidal male (Tiny) from the analysis because his arrival in the group predated daily monitoring.
Finally, we compared infanticidal vs. noninfanticidal males in terms of the circumstances of their arrival in the group; specifically we compared whether the new male joined the group as part of a multimale influx (Cords 2000) or took over a group on his own.
Results
Infanticides
From 1996 to 2008, 6 males in the study population killed 10 infants (Table I). In 9 of these cases, a newly arrived resident male killed infants in his own group. In the tenth infanticide, a resident male attacked a female from a neighboring, non-study group and killed and ate her infant.
Infanticide accounted for 17% of infant mortality in the study groups (9 out of 53 infants that died before reaching the age of 6 mo) between 1996 and 2008. The median age of infants at the time of infanticide was 16 d (range: 1–147 days, n = 9); the age of the infant killed in the intergroup infanticide was unknown.
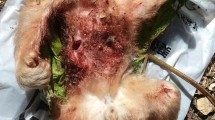
In the best observed attacks, patterns of aggression and killing were similar to those described in other blue monkey infanticides (Butynski 1982a, Fairgrieve 1995). The male specifically targeted the mother-infant pair for aggressive stalking, appearing to pay little or no attention to other monkeys in the vicinity. In the closely observed killing, the infant received a fatal bite to the back of the neck, consistent with the classic craniocervical killing bite used by predators on innocuous prey (Steklis and King 1978).
In 3 cases, we observed the male eating portions of the carcass, but he did not consume it entirely. Other individuals showed extreme interest, continuously observing the male as he ate, repeatedly approaching and withdrawing, and sniffing the carcass and patches of blood left on branches; no monkeys other than the infanticidal male consumed any part of the carcass. When the male eventually dropped the body, neither he nor any other monkey attempted to retrieve it.
Testing the Sexual Selection Hypothesis
We review the 9 cases of intragroup infanticide according to Hrdy’s (1979) criteria. The one case of intergroup infanticide is considerably different and we therefore address it separately.
-
1.
Probability of paternity. In 6 of the 9 intragroup infanticides, killed infants were conceived before the infanticidal male entered the group. None of these males was known to be a temporary visitor or to have social or sexual contact with the group before joining. The likelihood that these males sired the infants they killed is therefore very low.
The 3 remaining infants were killed by the same male (Putn) and were all conceived after he first joined the group. During their conception periods (which ran continuously from July 18 to September 22, 2001), Putn was in the group on 45% of days; he was not a consistent daily resident until November 5, 2001. During the 3 infants’ conception periods, we did not see Putn mate with their mothers; they mated only with other males at these times. Thus, despite his being partially present in the group during the conception periods of his victims, copulation records suggest the likelihood that this male sired the infants he killed is moderately low.
-
2.
Return to ovulation. The median interbirth interval for this population, derived from measurements made during continuous monitoring over 11 yr and excluding the 9 infanticide cases, is 24.7 mo (range: 7.2–62, n = 148). Postinfanticide interbirth intervals were less than half this length (median: 11.2, range: 10.7–12.9, n = 9), a significant reduction (Mann-Whitney U Test, Z = –4.22, 2-tailed p < 0.0001). The median interval between infanticide and conception of the next born infant was 160 d (65–385, n = 9).
-
3.
Probability of siring next offspring. All females whose infants were killed copulated with the infanticidal male 6–177 d (median: 46 d, n = 9) after losing their infant. In all but one case, however, these first postinfanticide copulations either did not lead to pregnancy or pregnancies were terminated prior to detection. In 3 of 9 cases we had no data regarding sexual activity of the mother during the conception period of the new infant. In all 6 cases when we did observe mating during the subsequent conception period, mothers mated with the infanticidal male. Three of these females also mated with ≥1 other male at this time.
Intergroup Infanticide
The single case of intergroup infanticide differed considerably from the other 9 cases: there was no change of resident males in the infant’s group and the infant was killed by the resident male of a neighboring group. The infanticidal male (PH) had been the sole resident of his group (GN) for 4.7 yr at the time of the infanticide. He had a history of infanticide in his own group, having killed 2 infants there shortly after taking over (Table I). The infant’s social group (F) was not a study group but had been observed intermittently over 25 yr. Its composition was unknown, though group size appeared comparable to that of GN. F group’s resident male was 12 yr old and had been sole resident male in F for 2.2 yr. F’s territory bordered GN’s, and intergroup encounters between these groups were common; the infanticide took place along their territorial border, which had been stable for >10 yr.
There was no observed sexual activity between PH and any female in F group before the infanticide and therefore, while we have occasionally seen copulations across groups in this population, PH’s likelihood of having sired the infant he killed was judged to be very low. We have no information about the mother of the killed infant regarding her return to ovulation or next pregnancy. We did not see her interact sexually with PH after the infanticide.
Noninfanticides
During the period in which the 10 infanticides occurred, 38 infants survived the arrival of 11 new males that eventually achieved sole resident status in their respective groups (Table II). Three of these infants were spared by three males that killed other infants; the remaining 35 infants were left unharmed by 8 males that did not kill any infants when they joined a group. We first review these 38 cases of noninfanticide in the context of the sexual selection hypothesis criteria and then compare them with the victims of infanticide.
Testing the Sexual Selection Hypothesis Criteria
-
1)
Probability of paternity. In 35 of 38 cases, the male had not joined the group nor was there any recorded interaction between him and the mother at the time the infant was conceived. In 2 cases, the male’s arrival coincided with the infant’s conception period but we observed no mating between the new male and the infant’s mother. In only 1 case did we observe the infant’s mother mate with the new male during the infant’s conception period, though she did not mate exclusively with him. The likelihood that the males sired these infants is low or very low in all but this one case. These results do not differ from the infanticide cases (Fisher Exact Test, p = 1.00).
-
2)
Return to ovulation. Seven of the 38 mothers did not have a subsequent offspring, either because they died (n = 5) or had not given birth again (n = 2) at the time of writing. For those mothers that gave birth again, the median interbirth interval was 26.8 mo (range: 10.5–62 mo, n = 31). These intervals did not differ from the set of all population values from which the infanticide cases and these 31 cases had been excluded (median: 24.2, range: 7.2–59.8, n = 117; Mann-Whitney U test, Z = 1.62, 2-tailed p = 0.105). They were, however, significantly longer than those of the infanticide victims (Mann-Whitney U test, Z = 4.24, 2-tailed p < 0.0001).
-
3)
Probability of siring next offspring. Of 31 mothers who gave birth again, 13 mated during their next conception period with the noninfanticidal male. In 5 additional cases, the noninfanticidal male was present during the next conception period, but all observed mating was with other males. In another 5 cases, we observed no copulations during the conception period of the subsequent infant. In the remaining 8 cases, the noninfanticidal male was no longer resident in nor visiting the group when the mother next conceived, so was unavailable as a partner. Thus, in 13 of the 18 (72%) subsequent conception periods during which the noninfanticidal male was present and for which mating behavior was observed, females mated with the noninfanticidal male. This proportion is lower than but not statistically different from the infanticide cases (Fisher Exact Test, 2-tailed p = 0.545).
Overall, in regard to potential reproductive benefit to the male as delineated in the criteria of the sexual selection hypothesis, we found no difference between the infanticides and noninfanticides.
Comparisons of Infanticides and Noninfanticides
We evaluated other variables that might explain the difference between the 2 groups of infants, including: 1) sex and 2) age of infants, 3) mother’s parity, 4) group defense of mother-infant pair, 5) postconception estrus by mother with new male, 6) number of mating partners available to new male, and 7) circumstances of male’s arrival (Table III).
Sex of Infants
We found no significant difference in the sexes of killed vs. unharmed infants, but note that the sample size for killed infants was very small (Table III). The sex ratio of unharmed infants matched the 1:1 sex ratio for births in this population (Cords and Chowdhury, in press).
Age of Infants
Two of the 9 infants killed were born before the infanticidal male entered their group and 7 were born afterwards. Twenty-two of the 38 unharmed infants were born before the new male entered their group and 16 were born afterwards. Unharmed infants were born earlier relative to the new male’s arrival than infanticide victims (Fig. 1, Table III). The main difference between the 2 age distributions is the relatively large number of unharmed infants born 5–6 mo before males entered their groups. These infants were relatively old, approaching the oldest known age for infanticide in this species, when the male first arrived. Only infants aged <3 mo on a male’s entry, as well as those born after he entered, were sometimes infanticide victims. Indeed, if one excludes from consideration infants born 4–6 mo before the male’s arrival, then the ages of infants that were killed are indistinguishable from those of infants that were unharmed (Mann-Whitney U test, Z = 1.07, 2-tailed p = 0.285).
Mother’s Parity
Of the 9 mothers of infants killed by their own resident males, only 1 was primiparous; the rest were parous females, with the killed infant representing their 2nd to 9th birth. Of the 38 unharmed infants, 5 were firstborn offspring and 33 were born to parous mothers, representing their 3 rd to 6th births. Maternal parity was not significantly different for infants that were killed vs. those that were not (Table III).
Defense by Other Group Members
During all fatal and nonfatal attacks by infanticidal males, other group members intervened to defend the mother-infant pair. Defensive tactics typically included aggressive and alarm vocalizations (growls, chirps) directed at the attacking male, and sometimes chases by ≥1 females. On one occasion, females appeared to try to pacify or distract a male (Tiny) as he attacked a female (Wag) in the days before her infant’s disappearance; this involved five adult females repeatedly presenting and puckering to and grooming the male over 3 h while he stalked and chased the mother-infant pair.
Adult females participated in all observed cases of defense; juveniles as young as 1–2 yr also participated occasionally. We never observed adult males protecting females, even though other adult males (in 3 cases, males that had mated with the mother) were present in the group on all days when attacks occurred. In the one case of intergroup infanticide, the resident male of the killed infant’s group was not present during the actual attack; however, he appeared soon afterwards and participated in an aggressive intergroup encounter involving several females from both groups as well as the infanticidal male. Although no physical contact occurred, the 2 males oriented toward each other within 25 m for >15 min and vocalized frequently.
In 2 of the 38 noninfanticide cases, males (PH, Putn) threatened and chased the mothers of unharmed infants at least once. Other group members rose to the defense and the males did not persist in stalking or harassing the mother-infant pair. Both these males were more persistent in attacks on other infants and succeeded in killing 2 (PH) or 3 (Putn), despite similar defense by group members. In the remaining 36 cases, we never observed intense, persistent aggression by the male against the mother-infant pair. We therefore cannot compare occurrence of group defense between killed and unharmed infants as no such defense arose in most of the noninfanticide cases.
Postconception Estrus
For 8 infants that were killed, we monitored the mother’s pregnancy continuously before the infant’s birth; we observed postconception estrus in all 8 cases. In 7 of them, sexual behavior included copulation, whereas in 1 case (Gigi08) we observed only female proceptive behavior. In 6 cases, including Gigi08, postconception sexual partners included the infanticidal male, whereas in 2 cases females mated only with other males. The largest number of mothers mated with the infanticidal male in the 4th and 5th mo after conception, although differences across months were small (Fig. 2).
Number of mothers of killed (K) and unharmed (NK) infants that engaged in post-conception mating (black bars) with the newly arrived male in each post-conception month. No such matings were observed in the first month. The number of females who had the opportunity to mate with the male in each month is indicated by the white bars; this number varied according to which males were present in each month of pregnancy. Six mothers of killed infants and 8 mothers of unharmed infants were known to mate with the male at some time after conception. A given female may have been counted in more than one month.
Mothers of 16 unharmed infants had an opportunity to interact sexually with the newly arrived male after conception but before the birth of their infant (in the other 22 cases, the infant was already born when the male joined the group). In 8 of these 16 cases, the mother showed postconception mating with the male, whereas in 8 she did not. The likelihood that infants’ mothers engaged in postconception sexual behavior with the newly arrived male was no different for killed vs. unharmed infants (Table III).
The timing of postconception sexual interactions with the newly arrived male, however, differed for the mothers of killed vs. unharmed infants (Fig. 2). Specifically, mothers of unharmed infants interacted sexually with newly arrived males earlier in pregnancy. The greatest difference occurred in the 2nd postconception month, when none of the mothers of killed infants mated with the infanticidal male, whereas 5 of 8 mothers of unharmed infants did so (Fisher Exact Test, 1-tailed p = 0.024).
Number of Mating Partners
Infanticidal and noninfanticidal males did not have different numbers of mates, whether we considered only the first 2 mo of their tenure or their entire tenure prior to the first infanticide (Table III).
Circumstances of Male Arrival
Of the 14 males in this study, 9 joined a new group as part of a multimale influx (5 infanticidal and 4 noninfanticidal males), whereas 5 others took over a group on their own, 1–7 d after the previous resident male disappeared (1 infanticidal and 4 noninfanticidal males). The way males joined the group, i.e., as part of an influx or as a single-male takeover, did not differ significantly between infanticidal and noninfanticidal males (Table III).
Males that Killed Some Infants and Left Others Unharmed
Three males each killed 1–3 infants after joining a group but left another unharmed; these particular cases provided an opportunity for a comparative analysis in which male identity was controlled. We predicted that differences associated with infant’s fate between infants killed and spared might be more easily detected in these cases; accordingly, we reevaluated the killed and not killed infants associated with only these 3 males, with particular attention to the coresidence and copulation histories of their mothers with these males (Table IV).
Patterns of residence and observed copulation did not predict which infants were killed and which were not killed by these 3 males. Two were not resident when infants of either category (killed or not) were conceived, whereas the 3rd was present during conception periods of both killed and unharmed infants. None of the infants’ mothers copulated with these males during conception periods. Postconception sexual activity occurred with the mothers of all 6 killed infants (most during midpregnancy), but with only 1 of 3 mothers of unharmed infants (Table IV).
As noted previously, 2 of these 3 males threatened and chased the mothers of unharmed infants at least once, but neither persisted in stalking the mother in the face of typical group defense. Defense by group members characterized successful attacks by these males as well, and we were unable to detect any difference in the type of defense given to mothers of killed vs. unharmed infants. In fact, we could discern no obvious reason why the 2 males that each ceased to harass a mother-infant pair did not persist.
Discussion
Sexual Selection and Blue Monkey Infanticide
Each of the 9 intragroup infanticides meets the criteria of the sexual selection hypothesis very well. Males were unlikely to have fathered the infants they killed, either because they were not present in the group or did not mate with the mother when the infant was conceived. Based on the 6-mo gestation and an interbirth interval exceeding 25 mo, we infer the duration of lactational amenorrhea at roughly 18 mo. Given a lactation period about triple the gestation length, we conclude that infanticide would be advantageous for males of this species (van Schaik 2000a). This inference is supported by the fact that infant death reduces the interbirth interval by >50%. Finally, in every case for which we had data, infanticidal males did mate with the mothers of infanticide victims at the conception period of the subsequent infant. Although some males were not her exclusive sexual partner at this time, each infanticidal male had a high probability of siring the next offspring, and certainly an increased chance relative to the previous infant. Although genetic paternity data would allow even more confidence in this conclusion (Borries et al. 1999a), these behavioral patterns are clearly consistent with the sexual selection hypothesis.
Alternative hypotheses do not fit our observations as well. In 3–4 of the 7 observed attacks, males ate part of the infant’s body, as Fairgrieve (1995) and Macleod (2000) similarly reported for other wild populations of Cercopithecus mitis. Although nutritional gain could be an adaptive benefit of infanticidal behavior, several observations suggest that it is not the primary explanatory factor. First, cannibalism is not frequent, either in absolute terms or in relation to infanticide events. Second, if there were a significant benefit, males might be expected to target juveniles at least occasionally; however, all attacks we witnessed, and all those reported for the species, involve infants <6 mo old, with the majority of infants killed within the first 2 mo. Third, if meat were highly prized, one would expect more complete consumption of the carcass and participation by others; the 2 carcasses we saw well, however, were only half-consumed by the male and neither he nor others retrieved the remains when they fell to the ground. One report of several adult female Cercopithecus mitis erythrarchus killing an unfamiliar female (Payne et al. 2003) also did not include cannibalism. Fourth, if infanticide provided a nutritional benefit, one might expect females to kill and eat infants, at least occasionally. Female blue monkeys do kill and eat mice (Wahome et al. 1988), bats, squirrels (Cords, unpubl. data), and bushbabies (Butynski 1982b). Some of these prey are substantially larger than a conspecific infant, suggesting that it is not a physical limitation that inhibits females from hunting conspecific infants. These considerations lead us to conclude that cannibalism in blue monkeys is best considered a byproduct of infanticide that is committed for other reasons.
The hypothesis that infanticide reduces competition makes different predictions about the sex of targeted infants depending on the resource in question (Crockett 2003). To reduce competition for mates, males should selectively kill male infants, whereas they should selectively kill female infants to reduce food competition for their offspring or the mothers of their offspring. We did not sex enough killed infants to distinguish either pattern. As with cannibalism, however, this hypothesis predicts juveniles would occasionally be targets and that females (as their offspring would also benefit) would at least attempt to kill infants; in blue monkeys, however, infanticide appears to be practiced exclusively by males against the very young.
The nonadaptive hypothesis that infants are killed as a result of generalized aggression by males (Bartlett et al. 1993) also does not fit our observations. All infanticidal males appeared to target only mothers of young infants, and did not stalk other females or other group members. Some new males showed other forms of aggression —mainly threats and lunges— toward adult females without infants, but this was almost invariably toward females who persistently solicited the males for copulations. Thus both the form and context of male aggression toward nonmothers were completely different.
Intergroup Infanticide
The 1 case of intergroup infanticide recorded at Kakamega is a rare event among rare events and is difficult to assess in regard to any adaptive hypothesis (see also Palombit et al. 2000). In accordance with the sexual selection hypothesis, it is likely that the mother returned to ovulation sooner than she would have had the infant lived. It is far more difficult, however, to infer the paternity of the killed infant, or the infanticidal male’s chances of siring the next infant. Although it is conceivable that a male could increase his chance of siring infants in a neighboring group via infanticide, especially given that nonresident males may sire as many as half the infants in a group (Hatcher et al., in prep.), we have too little information in this case to make confident inferences.
Some of the other adaptive explanations offered for intergroup infanticide do not seem to apply. In Thomas langurs (Presbytis thomasi), for example, infanticide may influence female transfer and mate choice (Sterck 1997), potentially to the infanticidal male’s advantage. Female blue monkeys at Kakamega are strictly philopatric, however, making this explanation inappropriate. Van Schaik (2000b) proposed that infanticide increases a male’s likelihood of being able to transfer into or take over a group. As the male in our case was the sole resident male in a stable group, takeover advantage is also not a plausible explanation. Reduction of resource competition, as suggested for intercommunity infanticide in carnivores (Packer and Pusey 1984), guerezas (Harris and Monfort 2003), and chimpanzees (Sherrow and Amsler 2007; Watts et al. 2002), is potentially relevant in this case, as the perpetrator and his offspring could benefit from the removal of a competitor. The rarity of intergroup infanticide and the lack of participation by females, however, undermine this argument.
Unlike other reports of intergroup infanticide (Shopland 1982; Singh et al. 2006), this attack did not occur during an aggressive intergroup encounter (cf. Yamada and Nakamichi 2006). Perhaps males simply target infants of unfamiliar females, and this male acted on a rule of thumb at an opportune moment; assessing his paternity likelihood as low, he exercised a low-cost behavior despite a potentially low probability of benefit.
As observed for most other primates (van Schaik 2000b), our observations suggest that infanticidal attacks are not costly for blue monkey males. Although 1 male reopened a small wound during his attack, most males suffered at most a vigorous chase by females and juveniles. Even when present, other males, certainly capable of inflicting serious wounds, never defended the mother-infant pair. In the 1 case of intergroup infanticide, the aftermath of the event included a prolonged aggressive intergroup encounter that involved the infanticidal male, the resident male of the killed infant’s group, and several females from both groups. This encounter did not escalate to contact aggression, however, and no changes in territorial patterns were observed in the weeks that followed. The willingness of the infanticidal male to kill an infant outside his own group, to no apparent (or at least a very uncertain) reproductive advantage, also argues for the low cost of this behavior.
Variation in Infanticidal Behavior Between and Within Males
We hypothesized that infanticide and noninfanticide cases would differ either in relation to male reproductive benefit, i.e., sexual selection hypothesis criteria, or in a trait particular to the infant (e.g., age), its mother (e.g., parity, sexual interaction with male), or the circumstances (e.g., defense, available mates, presence of other males). Our analysis of killed vs. unharmed infants, however, revealed very few differences. As in infanticide cases, the new males were unlikely to have fathered the unharmed infants, and it is probable, based on the data from infanticide cases, that the subsequent birth intervals would have been significantly shortened had males killed those infants. These males would also have increased their likelihood of impregnating the mother. In fact, males that did not harm infants were slightly less likely than those that did kill infants to mate with the mother at the next conception period, further suggesting that infanticide would have benefited them. Overall, potential reproductive benefits to males from killing infants appear equivalent and thus do not explain why some infants are killed and others are not.
Even if reproductive benefits to the male of killing an infant are the same in both groups, differential costs could, in principle, explain the difference. Others have suggested that defenses mounted by group members may explain why some presumably vulnerable infants are not killed (Butynski 1982a; Crockett 2003; Enstam et al. 2002; Palombit et al. 2000; Teichroeb and Sicotte 2008; van Schaik 2000b). In our study, such an explanation may apply to 2 cases in which mother-infant pairs were attacked but ultimately spared after defense by group-mates. It cannot, however, explain why another 36 infants were unharmed as we observed no attacks (and hence no such defense). Variation in defense by others, therefore, does not generally explain the fate of the infants in our study.
Some researchers have suggested that variable opportunity may influence the occurrence of infanticide (Crockett 2003; Crockett and Janson 2000). It is difficult, however, to identify and measure opportunity directly. The male blue monkeys that did not kill infants seemed to have ample opportunity to do so: young infants were present over periods of months when these males were newly arrived in their groups, and each infant may be vulnerable for at least half a year. Further, we agree with van Schaik (2000b) that males intent on killing an infant seem able to do so. The observation that defense by the mother or by other group members is typically ineffective agrees with such a proposal.
It is reasonable to assume that more experienced mothers may have strategies to evade or thwart infanticidal males more effectively, or that males may otherwise assess them as less suitable targets. Maternal parity and infant fate, however, were not related, again, perhaps, because males intent on killing infants usually succeed (van Schaik 2000b).
Males with fewer mating opportunities might be more motivated to kill infants and thus increase their pool of potential mates. We found, however, that the number of females with whom a male mated shortly after arriving in a group did not distinguish infanticidal from noninfanticidal males. Newly arrived males are generally attractive to females, which appear to be sexually stimulated by novel partners (Cords 2000; Cords et al. 1986; Pazol 2003). Similarly, the presence of other males should increase male reproductive motivation, though it could also act as deterrent against infanticide. Occurrence of infanticide is typically lower in multimale groups than in unimale groups, a pattern potentially explained by higher degrees of paternity confusion in multimale groups, the presence of fathers, and lower reproductive benefits due to increased mating competition (Borries and Koenig 2000). We examined the context of male takeovers, i.e., single male or multimale influx, in regard to likelihood of infanticide, and found that presence of other males in the group when a male arrived did not relate systematically to infanticidal behavior.
Occurrence of postconception mating by an infant’s mother did not distinguish killed and unharmed infants but the timing of such mating did. Mothers of killed infants were less likely to have mated with the newly arrived male in the 2nd month after conception than were mothers of unharmed infants. Thus in contrast to the findings of Soltis et al. (2000) for Japanese macaques and Murray et al.’s (2007) suggestion for chimpanzees that males are inhibited by their memory of “a general tendency to mate,” our results suggest that postconception mating later in pregnancy is not associated with the likelihood that an infant will be killed. Our findings also contrast with Fairgrieve’s (1995) report on blue monkeys, which implicated mating up to the 5th postconception month as an effective deterrent of infanticide in blue monkeys: however, Fairgrieve compared only 1 killed and 1 unharmed infant. We further note that although early postconception matings may inhibit infanticide, they do not seem to fully explain its absence. Of 16 mothers of unharmed infants that had opportunities for postconception mating with a newly arrived male, we observed only 8 to engage in such mating. An additional 22 mothers of unharmed infants had no opportunity for postconception mating, and yet their infants also were spared.
We found only 1 significant difference between killed and unharmed infants: killed infants were born somewhat later, relative to a male’s entry into the group, i.e., spared infants were older when the new male arrived. There are several possible reasons this difference might predict the fate of infants, though we find none especially compelling in these cases. Infants get bigger as they get older and therefore might present a greater challenge to a male; even at 6 mo (oldest victim), however, a blue monkey infant is much smaller than an adult male, making this an unlikely explanation. In terms of shortening interbirth interval, the relative benefit to a male decreases as an infant approaches the age of weaning; one might argue that the fitness gain is lower for killing older infants and thus males would be less likely to target them. However, the life history of blue monkeys, particularly the >2 yr interbirth interval, would make it advantageous for males to kill infants even older than the unharmed infants we observed. Further, for a decline in benefit to be influential, we would expect to perceive some cost to the male that we have not detected. For these reasons, we doubt that observed differences in age at time of male arrival are sufficient to explain the infants’ fates.
While both postconception estrus early in pregnancy and infant age may influence an infant’s chance of survival, we found the most striking difference between killed and unharmed infants was the identity of the male. Our results suggest that male identity accounts for most of the variation over the sample as a whole. Over 12 yr, 8 newly arrived males never killed infants whereas 3 killed the only infant available and 3 killed all but 1. The fact that 3 of 6 infanticidal males each spared an infant may appear to weaken the suggestion that male identity is the strongest predictor of an infant’s fate. However, we did observe severe attacks, consistent with infanticide cases, by 2 of these males on the spared infant-mother pairs; this targeted aggression, never observed in any noninfanticidal males, strengthens our conclusion that infanticide is more strongly linked to male identity than to some characteristic of the infant, mother, or group.
Others have similarly described how infanticidal behavior occurs in only some adult males in a population. For example, Butynski (1982a), also studying blue monkeys, found that 3 of 6 males neither chased nor killed young infants after arriving in a group. Palombit et al. (2000) found infanticidal behavior limited to 3 of 8 male chacma baboons, and Feh and Munkhtuya (2008) found it limited to 2 of 9 Przewalski stallions. Butynski had little contextual information to evaluate; in the baboons and horses, however, differences in contextual factors that related to benefit and opportunity for the males may explain the observed patterns (Feh and Munkhtuya 2008; Palombit et al. 2000). These reports thus differ from ours in that the variation among males remains plausibly related to circumstances, instead of to characteristics of the male himself. All these cases, including our observations, contrast with reports from other taxa in which infanticide is much more invariant. In wild deermice, all males are infanticidal, if they have not lived together with the mothers of neonates (Wolff and Cicirello 1991). Similarly, Packer and Pusey (1984) reported that every coalition of male lions that took over a pride killed infants, except for 1 case in which a male was related to the females.
Observations like ours, in which infanticide is limited to only some males in a population, are consistent with 2 scenarios. First, there may be a real genetic polymorphism that influences infanticidal behavior. Along these lines, Perrigo et al. (1993) reported strain differences in male infanticidal tendency in house mice (Mus domesticus) and, based on experimental testcrosses, demonstrated that infanticidal behavior is a heritable trait that exhibits dominant-recessive inheritance patterns in males. In the case of blue monkeys and perhaps other species, the degree to which this trait provides fitness advantage may not be pronounced enough for it to have become fixed in the population, or it may be in the process of becoming fixed. The second scenario is that infanticidal behavior is a conditional behavior of males, and only some males at any one time meet the conditions for its expression. Our analysis appears to rule out conditions related to the adaptiveness of the behavior, but other features of the males themselves, such as age or body size, may be important. We have little information about most of the males before their entering our focal groups, precluding systematic comparison. We note, however, that 2 of the 8 noninfanticidal males were known to be young at the time they entered the group (7.8 and 11 yr old, respectively), and a third was relatively small-bodied, and thus possibly young as well. In contrast, infanticidal Milo was in his 2nd stint as a resident male when he entered Gs group (his first residency was in a nonfocal group), and Osca, 1 of 2 males that attacked a mother-infant pair in Tw group, was definitely an older male based on the wear of his canines. Overall, our findings reject aspects of the social or demographic circumstances as important determinants of infanticidal behavior. We therefore propose that further research on intrapopulation variation in infanticidal behavior focus especially on characteristics of different males.
References
Agoramoorthy, G., & Hsu, M. J. (2004). Occurrence of infanticide among wild proboscis monkeys (Nasalis larvatus) in Sabah, northern Borneo. Folia Primatologica, 76, 177–179.
Bartlett, T. Q., Sussman, R. W., & Cheverud, J. M. (1993). Infant killing in primates: A review of observed cases with specific reference to the sexual selection hypothesis. American Anthropologist, 95, 958–990.
Bellemain, E., Swenson, J. E., & Taberlet, P. (2006). Mating strategies in relation to sexually selected infanticide in a non-social carnivore: The brown bear. Ethology, 112, 238–246.
Borries, C., & Koenig, A. (2000). Infanticide in hanuman langurs: Social organization, male migration, and weaning age. In C. P. van Schaik & C. H. Janson (Eds.), Infanticide by males and its implications (pp. 99–122). Cambridge, UK: Cambridge University Press.
Borries, C., Launhardt, K., Epplen, C., Epplen, J. T., & Winkler, P. (1999a). DNA analyses support the hypothesis that infanticide is adaptive in langur monkeys. Proceedings of the Royal Society of London. Series B. Biological Sciences, 266, 901–904.
Borries, C., Launhardt, K., Epplen, C., Epplen, J. T., & Winkler, P. (1999b). Males as infant protectors in Hanuman langurs (Presbytis entellus) living in multimale groups – defence pattern, paternity and sexual behaviour. Behavioral Ecology and Sociobiology, 46, 350–356.
Butynski, T. M. (1982a). Harem male replacement and infanticide in the blue monkey (Cercopithecus mitis stuhlmanni) in the Kibale Forest, Uganda. American Journal of Primatology, 3, 1–22.
Butynski, T. M. (1982b). Blue monkey (Cercopithecus mitis stuhlmanni) predation on galagos. Primates, 23, 563–566.
Butynski, T. M. (1990). Comparative ecology of blue monkeys (Cercopithecus mitis) in high and low density subpopulations. Ecological Monographs, 60, 1–26.
Cords, M. (2000). The number of males in guenon groups. In P. M. Kappeler (Ed.), Primate males (pp. 84–96). Cambridge, UK: Cambridge University Press.
Cords, M. (2002a). When are there influxes in blue monkey groups? In M. E. Glenn & M. Cords (Eds.), The guenons: diversity and adaptation in African monkeys (pp. 189–201). New York: Kluwer Academic/Plenum Press.
Cords, M. (2002b). Friendship among adult female blue monkeys (Cercopithecus mitis). Behaviour, 139, 291–314.
Cords, M. (2007). Variable participation in the defense of communal feeding territories by blue monkeys in the Kakamega Forest, Kenya. Behaviour, 144, 1537–1550.
Cords, M., & Chowdhury, S. C. (in press). Life history of blue monkeys (Cercopithecus mitis stuhlmanni) in the Kakamega Forest, Kenya. International Journal of Primatology.
Cords, M., Mitchell, B. J., Tsingalia, H. M., & Rowell, T. E. (1986). Promiscuous mating among blue monkeys in the Kakamega Forest, Kenya. Ethology, 72, 214–226.
Crockett, C. M. (2003). Re-evaluating the sexual selection hypothesis for infanticide by Alouatta males. In C. B. Jones (Ed.), Sexual selection and reproductive competition in primates: new perspectives and directions (pp. 327–365). Norman, OK: American Society of Primatologists.
Crockett, C. M., & Janson, C. H. (2000). Infanticide in red howlers: Female group size, male membership, and a possible link to folivory. In C. P. van Schaik & C. H. Janson (Eds.), Infanticide by males and its implications (pp. 75–98). Cambridge, UK: Cambridge University Press.
Ebensperger, L. A. (1998). Strategies and counterstrategies to infanticide in mammals. Biological Reviews, 73, 321–346.
Ekernas, L. S., & Cords, M. (2007). Social and environmental factors influencing natal dispersal in blue monkeys (Cercopithecus mitis stuhlmanni). Animal Behaviour, 73, 1009–1020.
Enstam, K. L., Isbell, L. A., & DeMaar, T. W. (2002). Male demography, female mating behavior, and infanticide in wild patas monkeys (Erythrocebus patas). International Journal of Primatology, 23, 85–104.
Fairgrieve, C. (1995). Infanticide and infant eating in the blue monkey (Cercopithecus mitis stuhlmanni) in the Budongo Forest Reserve, Uganda. Folia Primatologica, 64, 69–72.
Fashing, P. J., & Cords, M. (2000). Diurnal primate densities and biomass in the Kakamega Forest: An evaluation of census methods and a comparison with other forests. American Journal of Primatology, 50, 139–152.
Fashing, P. J., & Gathua, J. M. (2004). Spatial variability in the vegetation structure and composition of an East African rain forest. African Journal of Ecology, 42, 189–197.
Feh, C., & Munkhtuya, B. (2008). Male infanticide and paternity analyses in a socially natural herd of Przewalski’s horses: Sexual selection? Behavioral Processes, 78, 335–339.
Gibson, K. N., Vick, L. G., Palma, A. C., et al. (2008). Intra-community infanticide and forced copulation in spider monkeys: A multi-site comparison between Cocha Cashu, Peru and Punta Laguna, Mexico. American Journal of Primatology, 70, 485–489.
Harris, T. R., & Monfort, S. L. (2003). Behavioral and endocrine dynamics associated with infanticide in a black and white colobus monkey (Colobus guereza). American Journal of Primatology, 61, 135–142.
Hrdy, S. B. (1979). Infanticide among animals: a review, classification, and examination of the implications for the reproductive strategies of females. Ethology and Sociobiology, 1, 13–40.
Janson, C. H., & van Schaik, C. P. (2000). The behavioral ecology of infanticide by males. In C. P. van Schaik & C. H. Janson (Eds.), Infanticide by males and its implications (pp. 469–494). Cambridge, UK: Cambridge University Press.
Knopff, K. H., Knopff, A. R. A., & Pavelka, M. S. M. (2004). Observed case of infanticide committed by a resident male Central American black howler monkey (Alouatta pigra). American Journal of Primatology, 63, 239–244.
Lewis, R. J., Razafindrasamba, S. M., & Tolojanahary, J. P. (2003). Observed infanticide in a seasonal breeding prosimian (Propithecus verreauxi verreauxi) in Kirindy Forest, Madagascar. Folia Primatologica, 74, 101–103.
Macleod, M. K. (2000). The reproductive strategies of samango monkeys (Cercopithecus mitis erythrarchus). PhD dissertation: School of Life Sciences, University of Surrey Roehampton.
Manson, J. H., Gros-Louis, J., & Perry, S. (2004). Three apparent cases of infanticide by males in wild white-faced capuchins (Cebus capucinus). Folia Primatologica, 75, 104–106.
Mori, A., Belay, G., & Iwamoto, T. (2003). Changes in unit structures and infanticide in Arsi geladas. Primates, 44, 217–223.
Murray, C. M., Wroblewski, E., & Pusey, A. E. (2007). New case of intragroup infanticide in the chimpanzees of Gombe National Park. International Journal of Primatology, 28, 23–37.
Onderdonk, D. (2000). Infanticide of a newborn black-and-white colobus monkey (Colobus guereza) in Kibale National Park, Uganda. Primates, 41, 209–212.
Packer, C., & Pusey, A. E. (1984). Infanticide in carnivores. In G. Hausfater & S. B. Hrdy (Eds.), Infanticide: comparative and evolutionary perspectives (pp. 31–42). New York: Aldine.
Palombit, R. A. (2003). Male infanticide in savanna baboons: Adaptive significance and intraspecific variation. In C. B. Jones (Ed.), Sexual selection and reproductive competition in primates: New perspectives and directions (pp. 367–412). Norman, OK: American Society of Primatologists.
Palombit, R. A., Cheney, D. L., Fischer, J., et al. (2000). Male infanticide and defense of infants in chacma baboons. In C. P. van Schaik & C. H. Janson (Eds.), Infanticide by males and its implications (pp. 123–152). Cambridge: Cambridge University Press.
Payne, H. F. P., Lawes, M. J., & Henzi, S. P. (2003). Fatal attack on an adult female Cercopithecus mitis erythrarchus: Implications for female dispersal in female-bonded societies. International Journal of Primatology, 24, 1245–1250.
Pazol, K. (2003). Mating in the Kakamega Forest blue monkeys (Cercopithecus mitis): Does female sexual behavior function to manipulate paternity assessment? Behaviour, 140, 473–499.
Pazol, K., Carlson, A. A., & Ziegler, T. E. (2002). Female reproductive endocrinology in wild blue monkeys: A preliminary assessment and discussion of adaptive functions. In M. E. Glenn & M. Cords (Eds.), The guenons: Diversity and adaptation in African monkeys (pp. 217–232). New York: Plenum Press.
Perrigo, G., Belvin, L., Quindry, P., Kadir, T., Becker, J., van Look, C., et al. (1993). Genetic mediation of infanticide and parental behavior in male and female domestic and wild stock house mice. Behavior Genetics, 23, 525–531.
Pradhan, G. R., & van Schaik, C. P. (2008). Infanticide-driven intersexual conflict over matings in primates and its effect on social organization. Behaviour, 145, 251–275.
Ramirez-Llorens, P., Di Bitteti, M. S., Baldovino, M. C., & Janson, C. H. (2008). Infanticide in black capuchin monkeys (Cebus apella nigritus) in Iguazú National Park, Argentina. American Journal of Primatology, 70, 473–484.
Rasoloharijaona, S., Rakotosamimanana, B., & Zimmerman, E. (2000). Infanticide by a male Milne-Edwards’ sportive lemur (Lepilemur edwardsi) in Ampijoroa, NW-Madagascar. International Journal of Primatology, 21, 41–45.
Sherrow, H. M., & Amsler, S. J. (2007). New intercommunity infanticides by the chimpanzees of Ngogo, Kibale National Park, Uganda. International Journal of Primatology, 28, 9–22.
Shopland, J. (1982). An intergroup encounter with fatal consequences in yellow baboons (Papio cynocephalus). American Journal of Primatology, 3, 263–266.
Singh, M., Kumara, H. N., Kumar, A., Singh, M., & Cooper, M. (2006). Male influx, infanticide, and female transfer in Macaca radiata radiata. International Journal of Primatology, 27, 515–528.
Soltis, J. (2002). Do primate females gain non-procreative benefits by mating with multiple males? Theoretical and empirical considerations. Evolutionary Anthropology, 11, 187–197.
Soltis, J., Thomsen, R., Matsubayashi, K., & Takenaka, O. (2000). Infanticide by resident males and female counter-strategies in wild Japanese macaques (Macaca fuscata). Behavioral Ecology and Sociobiology, 48, 195–202.
Sommer, V. (1994). Infanticide among the langurs of Jodhpur: Testing the sexual selection hypothesis with a long-term record. In S. Parmigiani, & F. S. vom Saal (Eds.), Infanticide and parental care (pp 155–198). Chur, Switzerland: Harwood Academic Publishers.
Steklis, H. D., & King, G. E. (1978). The craniocervical killing bite: Toward an ethology of primate predatory behavior. Journal of Human Evolution, 7, 567–581.
Sterck, E. H. M. (1997). Determinants of female dispersal in Thomas langurs. American Journal of Primatology, 42, 179–198.
Sussman, R. W., Cheverud, J. M., & Bartlett, T. Q. (1995). Infant killing as an evolutionary strategy: Reality or myth? Evolutionary Anthropology, 3, 149–151.
Teichroeb, J. A., & Sicotte, P. (2008). Infanticide in ursine colobus monkeys (Colobus verllerosus) in Ghana: New cases and a test of existing hypotheses. Behaviour, 145, 727–755.
Treves, A. (1997). Primate natal coats: A preliminary analysis of distribution and function. American Journal of Physical Anthropology, 104, 47–70.
Treves, A. (2000). Prevention of infanticide: the perspective of infant primates. In C. P. van Schaik & C. H. Janson (Eds.), Infanticide by males and its implications (pp. 223–238). Cambridge, UK: Cambridge University Press.
Tsingalia, H. M., & Rowell, T. E. (1984). The behavior of adult male blue monkeys. Zeitschrift für Tierpsychologie, 64, 253–268.
van Schaik, C. P. (2000a). Vulnerability to infanticide by males: Patterns among mammals. In C. P. van Schaik & C. H. Janson (Eds.), Infanticide by males and its implications (pp. 61–71). Cambridge, UK: Cambridge University Press.
van Schaik, C. P. (2000b). Infanticide by male primates: The sexual selection hypothesis revisited. In C. P. van Schaik & C. H. Janson (Eds.), Infanticide by males and its implications (pp. 27–60). Cambridge, UK: Cambridge University Press.
van Schaik, C. P., Pradhan, G. R., & van Noordwijk, M. A. (2004). Mating conflict in primates: infanticide, sexual harassment and female sexuality. In P. M. Kappeler & C. P. van Schaik (Eds.), Sexual selection in primates: new and comparative perspectives (pp. 131–150). Cambridge, UK: Cambridge University Press.
Wahome, J. M., Cords, M., & Rowell, T. E. (1988). Blue monkeys eat mice. Folia Primatologica, 51, 158–160.
Watts, D. P., Mitani, J. C., & Sherrow, H. M. (2002). New cases of inter-community infanticide by male chimpanzees at Ngogo, Kibale National Park, Uganda. Primates, 43, 263–270.
Wolff, J. O., & Cicirello, D. (1991). Comparative paternal and infanticidal behavior of sympatric white-footed mice (Peromyscus leucopus novaeboracensis) and deermice (P. maniculatus nubiterrae). Behavioral Ecology, 2, 38–45.
Xiang, Z., & Grueter, C. (2007). First direct evidence of infanticide and cannibalism in wild snub-nosed monkeys (Rhinopithecus bieti). American Journal of Primatology, 69, 249–254.
Yamada, K., & Nakamichi, M. (2006). A fatal attack on an unweaned infant by a non-resident male in a free-ranging group of Japanese macaques (Macaca fuscata) at Katsuyama. Primates, 47, 165–169.
Yamagiwa, J., & Kahekwa, J. (2004). First observations of infanticides by a silverback in Kahuzi-Biega. Gorilla Journal, 29, 6–9.
Acknowledgments
We thank the Ministry of Science, Education and Technology, Government of Kenya, for permission to work in the Kakamega Forest, the University of Nairobi Zoology Department, Institute for Primate Research (National Museum of Kenya), Moi University Department of Wildlife Management and Masinde Muliro University Department of Biological Sciences for local sponsorship, and local Forest Department (now Forest Service) staff for cooperation at the field site. Long-term funding for this study was provided by Columbia University, the L. S. B. Leakey Foundation, the Wenner-Gren Foundation, AAAS, and the National Science Foundation (especially BCS 9808273, BCS 0554747). Many Kenyan and American field assistants contributed to collection of longitudinal data; we especially thank P. Akelo, M. Atamba, S. Brace, N. Cohen, S. Förster, A. Fulghum, J. Kirika, S. Maisonneuve, C. Makalasia, K. McLean, C. Mitchell, N. Mitchell, S. Mugatha, C. Oduor, C. Okoyo, J. Omondi, B. Pav, A. Piel, S. Roberts, M. Sheehan, E. Shikanga, B. Shimenga, and E. Widava. We thank the editor, 2 anonymous reviewers, and S. Förster for comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cords, M., Fuller, J.L. Infanticide in Cercopithecus mitis stuhlmanni in the Kakamega Forest, Kenya: Variation in the Occurrence of an Adaptive Behavior. Int J Primatol 31, 409–431 (2010). https://doi.org/10.1007/s10764-010-9400-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-010-9400-z