Abstract
Forest fragmentation alters plant species diversity and composition, and causes diverse affects on the feeding behavior of wild primates. We investigated the feeding behavior and diet of two groups of western hoolock gibbon (Hoolock hoolock) inhabiting a small isolated forest patch (21 km2) in Hollongapar Gibbon Wildlife Sanctuary, Assam, Northeast India, over a year using focal animal sampling. H. hoolock adults spent, on average, 35.2% of their total annual activity budget on feeding, and fed on young leaves, mature leaves, flowers, fruits, petioles, buds and also on animal matter. There was marked seasonal variation in the proportions of the dietary items consumed. Fruits accounted for an average of 51% (range 34–71% per month) of feeding time over the year. This highly frugivorous diet may limit the ability of the species to survive in small and disturbed forest fragments. A total of 54 plant species (32 families) were consumed by the focal groups during the study period, but there were variations between months in the selection of these plant species. Non-tree species such as lianas were among the most highly selected species in the diet. Moraceae, comprising ten species, was the most dominant family among the food plants, accounting for 36% of annual feeding time. The present study presents quantitative and qualitative data on dietary composition, preference and selection of food plants of H. hoolock in a fragmented habitat, which can contribute to the restoration and manipulation of degraded habitats of H. hoolock.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fragmentation of large, contiguous and undisturbed forests into small patches is one of the most serious threats to biodiversity. Primates are susceptible to deforestation and habitat fragmentation, and are increasingly forced to inhabit isolated and small forest patches surrounded by an anthropogenic matrix (Estrada and Coates-Estrada 1996; Arroyo-Rodríguez and Dias 2010). Several characteristics of primates have been identified that may influence their ability to live in forest fragments (Onderdonk and Chapman 2000). For example, a highly frugivorous diet may limit the ability of the species to live in a fragmented habitat (Lovejoy et al. 1986; Estrada and Coates-Estrada 1996) because fruit is usually patchily distributed, both spatially and temporally. Moreover, when the size of the fragment decreases, the overall plant diversity decreases and the vegetation structure becomes degraded (Arroyo-Rodríguez et al. 2007), which may lead to lower food availability for species that inhabit the fragments (Zanette et al. 2000; Fahrig 2003). The effect of fragmentation on primate populations can also be seen at different temporal scales (Irwin 2008). Altered habitat characteristics in fragmented areas can have major effects on primate populations due to the changes in the availability of food resources. However, primates presumably can adjust to the altered conditions via ecological and behavioral shifts, within limits. Moreover, the diet of some species differs between intact forest and fragments, suggesting a degree of flexibility (Chiarello 1993; Galetti et al. 1994). Notably, fragmentation and isolation of the tropical forest patches affect certain specialized characteristics of primates such as frugivory, arboreality, territoriality, monogamy, etc. [in the western hoolock gibbon Hoolock hoolock (Kakati et al. 2009)]. Thus, the fragmentation of habitat has the potential to affect the feeding ecology of a species due to changes in habitat quality by affecting the presence, abundance or phenology of food plant resources.
Hoolock hoolock is widely distributed throughout the northeastern states of India with the exception of Sikkim. Globally, H. hoolock is restricted to monsoon evergreen and semi-evergreen forests of Northeast India, Bangladesh, south and east of the Brahmaputra River (Anderson 1878; Mukherjee 1982; Choudhury 1987; Das et al. 2003a, b), Northwest Myanmar and west of the Chindwin River (Tickell 1864; Brockelman et al. 2008). The Brahmaputra River Valley in the state of Assam covers most of the remaining lowland tropical forests of Northeast India, and these are the abodes of H. hoolock. However, nearly three-quarters of the gibbons’ habitats in this region has already been cleared or degraded (Rawat et al. 2001). Habitat loss, fragmentation, and hunting have led to declines in the populations of other gibbon species including Nomascus concolor, Hoolock leuconedys (Ni et al. 2014; Sarma et al. 2014). Due to the continued destruction of forest areas inhabited by gibbons, for commercial logging, agriculture and horticultural crops, permanent settlement, expansion of road networks, etc., combined with traditional bushmeat hunting, most populations of H. hoolock in Northeast India have become highly fragmented. They occupy isolated forest patches, most of which are degraded forest (Choudhury 1990; Das et al. 2003a; Walker 2005; Dam 2006; Walker et al. 2007). A rapid decline in at least 90% of the population of H. hoolock has been reported by Walker (2005) over the last three to four decades, and H. hoolock has been categorized as Endangered in India under International Union for Conservation of Nature (IUCN) Threat Criteria (Brockelman et al. 2008).
Several studies have been carried out on the dietary diversity of H. hoolock in Northeast India (Tilson 1979; Das 2002; Gupta et al. 2004; Chetry et al. 2007; Sarma et al. 2013; Sarma 2015) and in Bangladesh (Feeroz and Islam 1992; Islam and Feeroz 1992; Das et al. 2003b; Hasan et al. 2007). However, studies on the diet, its seasonal variation, and food preferences of H. hoolock in India are limited, although a few studies on diet and food preference of primate species in fragmented habitats have been reported for howler monkeys (Estrada and Coates-Estrada 1996), black crested gibbon (Ni et al. 2014), brown howler monkey (Chiarello 1994), primates in tropical deciduous forests in Bolivia (Lennart et al. 2010), and primates in the Amazon (Gilbert 2003). Kakati (2004) reported changes in feeding behavior of H. hoolock in eastern Assam: leaf content in the diet increased as forest fragment size became smaller. Das (2002) found that fruit consumption was high in gibbons in undisturbed forests in comparison with those in disturbed forest in Northeast India. Kakati et al. (2009) and Pachuau (2011) reported that changes in food plant species diversity and density due to forest clearance had impacts on the dietary pattern of H. hoolock.
The possible effects of forest fragmentation on the composition of the diet of primates are an important issue because these can impact the ecology, behavior and health (and ultimately the viability) of the population (Milton 1996; Chapman et al. 2005; Irwin 2008). We undertook the present study to examine the dietary diversity, preference and food selection of H. hoolock in different months of the year in a small isolated forest of Hollongapar Gibbon Wildlife Sanctuary (HGWLS), Assam, India. The sanctuary is under continuous pressure from illegal tree felling, firewood collection, cattle grazing, etc., by humans who live in fringe areas, including Adivashi (tea plantation workers) and Assamese communities. Stronger selectivity would be expected when fruit is abundant and the animal is able to exercise choice (McConkey et al. 2002). The aim of our study was to understand how the gibbons cope with the fragmented habitat condition and how they select and determine their diet, being a truly arboreal and frugivorous ape species.
Methods
Study site
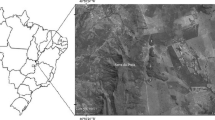
The present study was conducted in HGWLS, which is located on the southern bank of the Brahmaputra River system in the Mariani area of Jorhat District, Assam, India (Fig. 1). The sanctuary is a totally isolated forest patch covering about 21 km2 and situated between 26°40′ and 26°45′N and between 94°20 and 94°25′E at an altitude of 100–120 m a.s.l. It is surrounded by tea gardens and human settlements. The sanctuary receives 1777 mm of rainfall annually, and the monthly mean temperature ranges from 9.2° to 31.8 °C, and humidity from 40 to 95%. We divided the study period into four seasons: winter (December–February), premonsoon (March–May), monsoon (June–September) and post-monsoon (October–November), which correspond to the general seasonal pattern of Assam (Borah et al. 2014). The forest type of HGWLS is classified as Eastern Alluvial Secondary Semi-Evergreen Forest (1/2/2B/2S2) under the category Moist Tropical Forest of India (Champion and Seth 1968). This is the only sanctuary in Northeast India that harbors seven species of primate together in one small forest patch: H. hoolock, Trachypithecus pileatus, Macaca assamensis, Macaca arctoides, Macaca leonina, Macaca mulatta and Nycticebus bengalensis (Chetry et al. 2007). Twenty-five groups of western hoolock gibbon comprising 101 individuals in total (mean group size = 4.4 ± SE 1.1 individuals) were reported for HGWLS by Sharma (2008).
Study groups and methods
We studied feeding behavior and diet preference in two groups of H. hoolock. These two groups were habituated for 3 months prior to collection of behavioral data for 1 year, from January to December 2011. Group 1 consisted of two individuals (one adult male, one adult female) and group 2 comprised four individuals (one adult male, one adult female, one sub-adult male and one infant). The selected focal groups were followed from 0600 to 1600 hours for 12–15 days in every month for 1 year to record feeding behavior using focal animal sampling of the adult males and females (Altmann 1974; Bartlett 1999). Each focal animal was sampled for 1 h continuously. The total observation time was 1440 h in the 1-year study period; of these, 660-h were used for data analysis in males and 660 h for data analysis in female, after discarding very incomplete daily samples.
We recorded the time spent by the focal animals’ consumption of each food plant species and other food items. The food items were divided into seven categories: young leaves, mature leaves, flowers, fruits, petioles, buds and animal matter (e.g., insects, birds’ eggs). In general, leaves that appeared fresh and light green were considered young leaves while dark green leaves with developed texture were considered mature leaves. All trees, shrubs, lianas, climbers, epiphytes and climbing epiphytes eaten by H. hoolock in each month were recorded and herbarium specimens of each plant species were prepared and submitted to the Ecology and Biodiversity Laboratory, Department of Environmental Science, Tezpur University, Assam. Plant species were identified using Kanjilal and Bor (2005) and after consultation with plant taxonomists. We calculated the percentage of daily feeding time on different food categories in relation to total feeding time for each month (Gupta and Kumar 1994):
where T a = percentage of time spent on food item a, N a = number of records of food a, and n = total number of feeding records per day.
A plant survey was conducted by randomly placing 50 quadrats of 10 m × 10 m in size (total area sampled = 0.5 ha) for trees (girth measured at breast height, or 1.3 m), lianas and climbers (girth/collar measured at base) following Muller-Dombois and Ellenberg (1974), inside compartment no. 2 of the sanctuary containing the study groups.
Quantitative community parameters like density (stems/0.5 ha) and basal area (m2/0.5 ha) were calculated for each food plant species (Cottam and Curtis 1956). The species were categorized as “rare” species (those with less than 10 stem/ha on average), “uncommon” (<10 stems/ha), “common” (<25 stems/ha), “dominant” (<50 stems/ha) and “predominant” (>50 stems/ha) on the basis of the density of the plant species (Kadavul and Parthasarathy 1999).
The selection ratio for plant species, which tells us whether gibbons select a particular food or feed on it in proportion to its availability, was calculated using the formula given below (modified from Sarkar 2000). The ratio of feeding frequency, based on feeding observations, to food availability in terms of either relative dominance or relative density of the plant species gives the selection ratio. Relative dominance was used for tree species and relative density for lianas, climbers and epiphytes.
Here,
Values of SR < 1 signify low priority regards selection of a food species, those ≈1 denote species eaten in proportion to their density, and values >1 indicate species that are actively selected and apparently preferred. We used SPSS 16.0 software for statistical analysis. ANOVA was used in order to compare total feeding time across months and preference for plant species across months.
Results
Feeding behavior
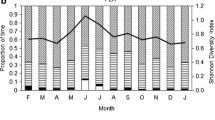
Hoolock hoolock spent 35.2% of total (annual) activity time on feeding during the study period. Variation in feeding time across months in a year was highly significant (F = 3.8, df = 11, P < 0.05). Monthly time spent on feeding varied from 30.5 ± SE 9.0% (December) to 40.0 ± SE 6.4% (September).
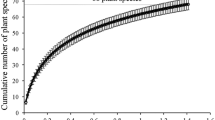
During the study period, the focal animals consumed food from 54 plant species belonging to 32 families, accounting for 90.3% of feeding time. The rest of the time was spent eating animal matter. Among the food plant species, 51.8% were trees (n = 28) followed by climbers (16.7%, n = 9), epiphytes (16.7%, n = 9), lianas (7.4%, n = 4), shrubs (3.7%, n = 2) and climbing shrubs (3.7%, n = 2) (Table 1).
Fruits comprised 51.1 ± SE 3.2% of the diet of H. hoolock and were highly preferred food items over the year (Fig. 2), ranging between 34.0 and 71.4% in different months (Fig. 3a). Other major food categories such as young leaves comprised 19.1 ± SE 3.0%, followed by mature leaves (15.7 ± SE 3.9%) and animal matter (9.7 ± SE 2.6%). Less than 1% each of flowers, flower buds and petioles were eaten (Fig. 2). However, the total leaf consumption (young leaves + mature leaves) was much higher than the amount of fruit consumed in some months of the year during the study period, namely, January (leaves = 63%, fruits = 37%), February (leaves = 54%, fruits = 40%) and May (leaves = 51%, fruits = 34%).
Monthly (a–g) and seasonal (h) variation in feeding time (%) on different food items in seven categories consumed by H. hoolock in HGWLS. YL Young leaves; ML mature leaves; FL flowers; FR fruits; P petioles; B buds; AM animal matter (insects, eggs, etc.). Asterisk Statistically significant at P < 0.01
Monthly and seasonal variation in feeding time devoted to different types of food
We observed significant variation among months in the proportions of different food categories in the diet. Variation in feeding time on fruits was significant across months (F = 7.3, df = 11, P < 0.05). In May and January, feeding time on fruits was relatively low (34.0 and 37.1%, respectively) (Fig. 3a). Feeding time on young leaves was also significantly different among months (F = 11.9, df = 11, P < 0.05) with a maximum recorded in May (40.9%) and minimum in July (5.6%) (Fig. 3b). Mature leaves constituted a significant fraction of the diet, which was at a minimum in August (0%) and a maximum in January (38.4%) (F = 13.9, df = 11, P < 0.05) (Fig. 3c). Feeding on flowers and buds was observed only for 4 and 3 months, respectively, and the variation among months was significant (F = 3.6, df = 11, P < 0.05; F = 6.9, df = 11, P < 0.05, respectively) (Fig. 3d, f). Feeding on petioles was observed for 9 months during February to November, with the exception of May, but the variation was not statistically significant (F = 0.8, df = 11, P > 0.05) (Fig. 3e).
A significant seasonal change in the diet of H. hoolock in HGWLS was observed during the study period. Fruits comprised the highest percentage in every season, among the various food items eaten, and the proportion varied significantly among the four seasons. Young leaves, mature leaves, buds and animal matter also differed significantly across seasons. The proportion of young leaves consumed was highest in the premonsoon (27.8%), whereas, proportion of mature leaves was highest during winter (32.5%). Flowers comprised a small portion of the diet of H. hoolock during winter (1%) and the premonsoon period (4.5%), while consumption of buds was observed only in the premonsoon. Gibbons were observed feeding on petioles in all four seasons in small amounts. Consumption of animal matter was recorded in every season with highest consumption in the rainy hot monsoon period (Fig. 3h).
Species’ usage and preference
Family Moraceae constituted the highest number of food plants, with ten species out of the 32 families of food plants that H. hoolock consumed during the study period (Table 1). The gibbons spent 36% of total annual feeding time on the ten species of Moraceae, followed by one species of Apocynaceae (13%), three species of Rubiaceae (6%), two species of Euphorbiaceae (4%), four species of Papilionaceae (2%), with the remainder of the 34 species, belonging to 27 families, representing 29% of annual average feeding time. Ichnocarpus frutescens, Ficus lepidosa, Artocarpus chaplasha, Ficus ramentacea, Anthocephalus chinensis and Balakata baccata were the top six species on each of which H. hoolock spent more than 4% of annual feeding time. Two distinctive species, F. lepidosa and F. ramentacea, out of the 54 food plant species, were eaten throughout the year followed by I. frutescens for 11 months, Trichosanthes truncata for 9 months and Pothos hookerii, Hoya parasitica, Abrus pulchellus and Ficus laevis for 8 months (Table 1).
The number of food plant species eaten in a full day varied from two to 11 (mean 7.7 ± SD 1.9) and the variation among days was highly significant (F = 3.6, df = 11, P < 0.05). However, the number of plant species eaten in each month ranged from ten in July to 23 in March with an average 16.4 ± SD 4.1 per month (Fig. 4). The variation in the number of plant species eaten in each month was also significant (F = 6.7, df = 11, P < 0.05).
The pattern of food selection revealed that Ailanthus grandis, I. frutescens, T. truncata and F. ramentacea were the most selected plant species among trees, lianas, climbers and epiphytes, respectively, ranking at the top of the selection list. The highest percentage of feeding frequency was found for F. lepidosa among trees, I. frutescens among lianas, T. truncata among the climbers and F. ramentacea among the epiphytic food plants (Table 2). Nine species out of the top 15 selected tree species were recorded as “rare” in the habitat, while one liana, four climbers and one epiphyte were also found to be rare (Table 2).
The top three “preferred” (most highly selected) food plants differed between months, but certain species appeared on the list in more than 1 month (Table 3). Of the 54 food plant species recorded in the diet of H. hoolock over the year, 17 (31%) were listed in the top three selected species in more than 1 month. Consumption of the top three food plants comprised an average of 57.0% of total feeding time per month, and more than 50% in 10 months. Consumption of the first, second and third preferences comprised on average 32.8, 15.5 and 8.6% of feeding time, respectively. I. frutescens occurred among the top three eight times, and twice in first place (in May and October), and F. lepidosa occurred among the top three five times, twice in first place (January and February). Usually a fruit species was the top selection, exceptions being in May and October when leaves of I. frutescens were most highly selected.
Discussion
The annual average percentage feeding time (35%) in the present study is generally consistent with the study of Alfred and Sati (1994), which reported 25–45% feeding time for H. hoolock in the Garo Hills, Meghalaya, India. Moreover, variation in terms of the time spent on feeding in response to monthly changes shows that food availability plays a major role in temporal feeding variation. Relatively low feeding time recorded during July (31%) and August (33%) may be due to the easy availability of juicy ripe fruits of A. chaplasha and other preferred species, which are relatively large, rich and heavy. However, other factors such as the short day length may also have caused the low feeding activity in the winter. Lower feeding time during short days was also reported by Whitten (1982) for Kloss’s gibbons.
Fruit consumption (51% in the present study) was found to be lower than the 67% recorded by Tilson (1979) in the same study site, 60% recorded in the Garo Hills of Meghalaya (Alfred and Sati 1994) and 62% recorded in Namdapha National Park, Arunachal Pradesh (Das 2002). Kakati (2004) reported that gibbon groups that live in medium-size fragments and large forest areas consume between 56 and 62% of fruit and figs. However, a similarly low fruit contribution was recorded by Feeroz and Islam (1992) and Ahsan (2001) in Bangladesh, and by Mukherjee (1986), Kakati (1997) and Das (2002) in fragmented and degraded habitats of Northeast India (Table 4). Relatively low fruit consumption compared to leaf consumption in some months indicates an altered diet of the gibbons in the study area, which has been reported in other study sites by Kakati (2004). This reduction of fruit content in the diet of H. hoolock may be attributed to the decreased availability of preferred fruits due to fragmentation or due to the impact of a changing climate on the phenology of fruit production (Poulsen et al. 2001).
No primate is known to be wholly frugivorous and some leaf material and animal matter seem to be necessary components of the frugivorous diet (Hladik 1978). H. hoolock also spent a considerable fraction of feeding time (9.7%) ingesting animal matter, which was higher than the time spent on minor plant items (buds, flowers, petioles; 4.3%). Animal matter was consumed mostly during June–August, probably because of the higher availability of insects during these hot and rainy months, or due to the presence of quality and nutritious animal items during this period. Some common insect species consumed by H. hoolock included Microcentrum sp., Cyclosia papilionaries, Oecophylla smaragdina, Antheraea assamensis and Odontotermes assamensis. High insect feeding in July was also recorded in Hanuman langur (Presbytis entellus) by Srivastava (1991).
Fruits, leaves and flowers have been the main parts of the diet of forest-dwelling primates for most of their evolutionary history (Milton 1986, 1987, 1993 ). The present study confirms that H. hoolock at HGWLS are highly frugivorous, as has been supported by several studies (Tilson 1979; Feeroz and Islam 1992; Alfred and Sati1994; Ahsan 2001; Das 2002). We also found that in addition to fruits, young and mature leaves of some species were preferred in some months depending on their availability. Variation in the proportion of fruits in the diet of H. hoolock could be caused by fragmentation and degradation of habitats of HGWLS and non-availability of fruiting trees throughout the year. Lower fruit consumption has been reported in fragmented forests than in contiguous habitat of H. hoolock (Kakati 2004). Fruits provide necessary nutrients, fibers, antioxidants and water for the body (Milton and Jenness 1987; Milton 1999). It was found that, on a monthly percentage basis, young leaves of I. frutescens were selected over fruits of F. lepidosa and F. laevis in May, and in October mature leaves of I. frutescens were selected over fruits of Elaeocarpus ganitrus, which may have been due to the limited availability of these fruits in the home range. Seasonal changes in the proportions of different plant parts in the diet are attributed to phenological changes in availability. Increase in the proportion of mature leaves in the diet during winter is due to the decreased availability of young leaves on the food plants as the dry season progresses (Oates et al. 1980), whereas, an increase in young leaves during the premonsoon is attributed to the increased availability of young leaves during this season.
The genus Hoolock has been reported to rely upon 460 plant species belonging to 84 families in its entire distribution range in Northeast India (Chetry et al. 2007). Kakati (2004) reported a maximum of 21 food plant species belonging to 13 families and as few as eight species (four families) consumed by H. hoolock among five disturbed forests of Assam, which comprised 75% of the diet. The present study found 19 species belonging to 15 families of food plants contributing 75% of the diet, which is comparable with the study of Kakati (2004). The highest number of food plants were members of the family Moraceae, with ten species in the study area, whereas 29 species of food plants from the Moraceae were reported in eastern Assam (Das et al. 2005). H. hoolock is highly selective in its food choice, which is evident in its ranging pattern (Das 2002). They maximize their food intake by a goal-directed search of food, and thus are able to use locally abundant or patchily distributed food sources efficiently (Davies 1978). In our study, the 54 species of foods used were not equally selected. The highest consumption (13%) as well as the highest selection ratio (5.4) among all food plants was recorded for the liana I. frutescens, which is known to have medicinal properties (Singh et al. 2012). Consumption of I. frutescens by H. hoolock was also recorded in Kakojan Reserve Forest, Assam (Kakati 2004). Chemically, I. frutescens is composed of phenylpropanoids, phenolic acids, coumarines, flavonoids, sterols and pentacyclic triterpenoids (Verma et al. 1987). Among trees, the food selection ratio was high for A. grandis, and gibbons consumed non-plant items such as insects, caterpillars, etc. along with resins and young and mature leaves from this species. The selection ratio was also high for the climber T. truncata and the epiphytic fig F. ramentacea, due partly to the very low dominance of these species in the habitat. Kibaja (2014) suggested that monkeys selected certain food plants based on accessibility, abundance and nutritional content. Some plant species selected by monkeys were not abundant and some that were abundant had low selection ratios despite having high feeding frequencies. Mturi (1991) regarded the less-eaten plant species to be “unpreferred” when their selection ratios were less than 1.0; however, even unselected species may contribute significant portions of the diet and may actually be preferred in a general sense. Several studies have reported that the plant species that are highly selected despite their low abundance in the habitat have rich protein content and are poor in secondary compounds (McKey and Gartlan 1981; Mturi 1991; Chapman and Chapman 2002; Fashing et al. 2007). The presence of nine “rare” species out of the 15 top selected tree species in the diet also indicates a potential future resource bottleneck for populations of H. hoolock in the study area. Low densities of the preferred plant species may have a significant impact on the gibbons’ diet by lowering feeding percentages and may alter the choice of food plants in the future. Hence, the dietary pattern of H. hoolock is altered by the temporal changes in the structural composition of food plant species and their density due to various ecological factors.
The loss of H. hoolock from the HGWLS, as well as other isolated, fragmented and degraded forest ecosystems of Northeast India, may have harmful consequences for forest regeneration, which is already severely compromised. Our study suggests that a suitable conservation plan needs to be introduced in the fragmented and degraded habitats of H. hoolock as well as isolated forest areas, which may be unable to provide quality food throughout the year. Planting the most preferred or selected native food plants of H. hoolock may improve their habitat. Species of figs (Moraceae), climbers, lianas and epiphytes are among the most important components in the diet of gibbons and maintenance or restoration of their presence in degraded habitats poses a challenge for conservation which needs to be addressed. Increasing liana and climber density will also provide support in traveling and foraging opportunities for gibbons. In addition, plantations of native species are also recommended in open areas to fill in the gaps inside the sanctuary, which will help to provide canopy cover as well as canopy links enabling gibbons to explore more food resources. Thus, our findings provide useful information which may aid habitat restoration and manipulation efforts in order to help maintain healthy populations of H. hoolock in the fragmented forests of Northeastern India.
References
Ahsan MF (2001) Socio-ecology of the hoolock gibbon (Hylobates hoolock) in two forests of Bangladesh. Brookfield Zoo, compiler. The apes: challenges for the 21st century: conference proceedings. Brookfield Zoo, Brookfield, pp 286–299
Alfred JRB (1992) The hoolock gibbon Hylobates hoolock. Primate Rep 34:65–69
Alfred JRB, Sati JP (1986) The gibbons with special reference to Hylobates hoolock. In: Majupuria TC (ed) Wildlife wealth of India. Resources and management. Tec Press Service, Bangkok, pp 384–390
Alfred JRB, Sati JP (1994) Diet and feeding in the hoolock gibbon of Garo Hills in Northeast India. Ann For 2:109–122
Altmann J (1974) Observational study of behaviour: sampling methods. Behaviour 49(3):227–267
Anderson J (1878) Anatomical and zoological researches: comprising an account of the zoological results of the two expeditions to western Yunnan in 1868 and 1875; and a monograph of the two cetacean genera, Platanista and Orcella. Quaritch, London
Arroyo-Rodríguez V, Dias PAD (2010) Effects of habitat fragmentation and disturbance on howler monkeys: a review. Am J Primatol 72:1–16
Arroyo-Rodríguez V, Mandujano S, BenítezMalvido J, CuendeFanton C (2007) The influence of large tree density on howler monkey (Alouatta palliate mexicana) presence in very small rain forest fragments. Biotropica 39:760–766
Bartlett TQ (1999) Feeding and ranging behaviour of the white-headed gibbon (Hylobates lar) in Khai Yai National Park, Thailand. Ph.D. thesis, Washington University
Borah M, Devi A, Kumar A (2014) Feeding on non-plant food items by western hoolock gibbon (Hoolock hoolock). Curr Sci 107:1657–1660
Brockelman W, Molur S, Geissmann T (2008) Hoolock hoolock. In: IUCN 2013. IUCN Red List of Threatened Species version 2013.2 http://www.iucnredlist.org. Downloaded on 23 February 2016
Champion HG, Seth SK (1968) A revised survey of the forest types of India. Natraj, Dehra Dun
Chapman CA, Chapman LJ (2002) Foraging challenges of red colobus monkeys: influence of nutrients and secondary compounds. Comp Biochem Physiol Part A 133:861–875
Chapman CA, Gillespie TR, Goldberg TL (2005) Primates and the ecology of their infectious diseases: how will anthropogenic change affect host-parasite interactions? Evol Anthr 14:134–144
Chetry D, Chetry R, Bhattacharjee PC (2007) Hoolock: the ape of India. Gibbon Conservation Centre, Assam, pp 23–26
Chiarello AG (1993) Activity pattern of the brown howler monkey Alouatta fusca, Geoffroy 1812, in a forest fragment of Southern Brazil. Primates 34:289–293
Chiarello AG (1994) Diet of the brown howler monkey Alouatta fusca in a semideciduous forest fragment of southern Brazil. Primates 35:25–34
Choudhury A (1987) Notes on the distribution and conservation of Phayre’s leaf monkey and hoolock gibbon in India. Tiger Paper 14:2–6
Choudhury A (1990) Population dynamics of hoolock gibbons (Hylobates hoolock) in Assam, India. Am J Primatol 20:37–41
Cottam G, Curtis JT (1956) The use of distance measurement in phytosociological sampling. Ecol 37:451–460
Dam SN (2006) A short study on wild hoolock gibbons (Hoolock hoolock) in Assam and Bangladesh. Gibbon J 2:40–47
Das J (2002) Socio-ecology of hoolock gibbon Hylobates hoolock hoolock (Harlan, 1834) in response to habitat change. Ph.D. thesis, Gauhati University, Guwahati, India
Das J, Biswas J, Medhi R, Bose J, Chetry D, Bujorborua P, Begum F (2003a) Distributional status of hoolock gibbon (Bunopithecus hoolock) and their conservation in southern Assam, India. Tiger Paper 30(4):26–29
Das J, Feeroz MM, Islam MA, Biswas J, Bujorborua P, Chetry D, Medhi R, Bose J (2003b) Distribution of hoolock gibbon (Bunopithecus hoolock hoolock) in India and Bangladesh. Zoos Print J 18:969–976
Das J, Bhattacherjee PC, Biswas J, Chetry D (2005) Western hoolock gibbon: socioecology, threats and conservation action plan. Department of Zoology, Gauhati University, and Primate Research Centre, Northeast Centre, Guwahati, India
Davies NB (1978) Ecological questions about territorial behaviour. In: Krebs JR, Davies NB (eds) Behavioural ecology: an evolutionary approach. Sinauer, Sunderland
Estrada A, Coates-Estrada R (1996) Tropical rain forest fragmentation and wild populations of primates at Los Tuxtlas. Int J Primatol 5:759–783
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515
Fashing PJ, Dierenfeld ES, Mowry CB (2007) Influence of plant and soil chemistry on food selection, ranging patterns, and biomass of Colobus guereza in Kakamega Forest, Kenya. Int J Primatol 28:673–703
Feeroz MM, Islam MA (1992) Ecology and behaviour of hoolock gibbon of Bangladesh. MARK (Multidisciplinary Action Research Centre), Dhaka, Bangladesh
Galetti M, Pedroni F, Morellato LPC (1994) Diet of the brown howler monkey (Alouatta fusca) in a forest fragment in Southern Brazil. Mammalia 58:111–118
Gilbert KA (2003) Primates and fragmentation of the Amazon forest. In: Marsh LK (ed) Primates in fragments ecology and conservation. Kluwer Academic, New York, pp 145–157
Gupta AK, Kumar A (1994) Feeding ecology and conservation of the Phayre’s leaf monkey Presbytis phayrei in Northeast India. Biol Conserv 69:301–306
Gupta AK, Sharma N, Dasgupta S, Chakraboty D, Hazarika H (2004) Conservation of hoolock gibbon (Bunopithecus hoolock) in Northeast India. ENVIS Bull: Wildl Prot Area 8:1–26
Hasan MK, Feeroz MM, Islam MA, Kabir MM, Begum S (2007) Substrate used by western Hoolock gibbon (Hoolock hoolock) in a semi-evergreen forest of Bangladesh. Zoos Print J 22:2702–2705
Hladik A (1978) Phenology of leaf production in rain forest of Gabon: distribution and composition of food for folivores. In: Montgomery GG (ed) The ecology of arboreal folivores. Smithsonian Institute Press, Washington, pp 51–71
Irwin MT (2008) Feeding ecology of Propithecus diadema in forest fragments and continuous forest. Int J Primatol 29:95–115
Islam MA, Feeroz MM (1992) Ecology of hoolock gibbon in Bangladesh. Primates 33:451–464
Kadavul K, Parthasarathy N (1999) Structure and composition of woody species in tropical semi-evergreen forest of Kalrayan hills, Eastern Ghats, India. Trop Ecol 40:77–90
Kakati K (1997) Food selection and ranging in the hoolock gibbon (Hylobates hoolock Harlan 1834) in Borajan reserve forest, Assam. M.Sc. dissertation. Wildlife Institute of India, Dehra Dun, India
Kakati K (2004) Impact of forest fragmentation on the hoolock gibbon (Hylobates hoolock Harlan 1834) in Assam, India. Ph.D. thesis, University of Cambridge, Cambridge, UK
Kakati K, Raghavan R, Chellam R, Qureshi Q, Chivers DJ (2009) Status of western hoolock gibbon (Hoolock hoolock) population in fragmented forests of Eastern Assam. Primate Conserv 24:127–137
Kanjilal UN, Bor NL (2005) Flora of Assam. Omsons, New Delhi
Kibaja M (2014) Diet of the ashy red colobus (Piliocolobus tephrosceles) and crop-raiding in a forest-farm mosaic, Mbuzi, Rukwa Region, Tanzania. Primate Conserv 28:109–116
Lennart WP, Büntge ABS, Herzog SK, Michael K (2010) Effects of habitat structure and fragmentation on diversity and abundance of primates in tropical deciduous forests in Bolivia. Int J Primatol 31(5):796–812
Lovejoy TE, Bieregaard RO Jr, Rylands AB, Malcolm JR, Quintela CE, Harper LH, Brown KS Jr, Powell AH, Powell GVN, Schubart HOR, Mays MB (1986) Edge and other effects of isolation on Amazon forest fragments. In: Soule ME (ed) Conservation biology: the science of scarcity and diversity. Sinauer, Sunderland, pp 257–285
McConkey KR, Aldy F, Ario A, Chivers DJ (2002) Selection of fruits by gibbons (Hylobates mulleri × agilis) in the rain forests of central Borneo. Int J Primatol 23(1):123–145
McKey DB, Gartlan JS (1981) Food selection by black colobus monkeys (Colobus satanas) in relation to plant chemistry. Biol J Linn Soc 16:115–146
Milton K (1986) Features of digestive physiology in primates. News Physiol Sci 1:76–79
Milton K (1987) Primate diets and gut morphology: implications for human evolution. In: Harris M, Ross EB (eds) Food and evolution: toward a theory of human food habits. Temple University Press, Philadelphia, pp 93–116
Milton K (1993) Diet and primate evolution. Sci Am 269:86
Milton K (1996) Interactions between a host-specific bot fly, Alouatta myiabaeri, and a free-ranging howler monkey (Alouatta palliata) population in Panama. J Zool 239:39–63
Milton K (1999) Nutritional characteristics of wild primate foods: do the diets of our closest living relatives have lessons for us? Nutrition 15:488–498
Milton K, Jenness R (1987) Ascorbate content of Neotropical plant parts available to monkeys and bats. Experientia 43:339
Mturi A (1991) The feeding ecology and behaviour of the red colobus (Colobus badius kirkii). Ph.D. thesis, University of Dar es Salaam, Dar es Salaam
Mukherjee RP (1982) Survey of non-human primates of Tripura, India. J Zool Soc India 34:70–81
Mukherjee RP (1986) The ecology of hoolock gibbon (Hylobates hoolock) in Tripura, India. In: Else JG, Lee PC (eds) Primate ecology and conservation. Cambridge University Press, UK, pp 115–123
Muller-Dombois D, Ellenberg H (1974) Aims and methods of vegetation ecology. Wiley, New York
Ni Q-Y, Huang B, Liang Z-L, Wang X-W, Jiang X-L (2014) Dietary variability in the western black crested gibbon (Nomascus concolor) inhabiting an isolated and disturbed forest fragment in Southern Yunnan, China. Am J Primatol 76:217–229
Oates JF, Waterman PG, Choo GM (1980) Food selection by the South Indian leaf monkey Presbytis johnii, in relation of leaf chemistry. Oecologia 45:45–56
Onderdonk DA, Chapman CA (2000) Coping with forest fragmentation: the primates of Kibale National Park, Uganda. Int J Primatol 21:587–611
Pachuau SV (2011) Response of western Hoolock Gibbon (Hoolock hoolock) in terms of population abundance and resource utilization across various disturbance regimes in Dampa Tiger Reserve. Mizoram. M.Sc. thesis, Saurashtra University, Gujarat, pp. 66
Poulsen JR, Clark CJ, Smith TB (2001) Seasonal variation in the feeding ecology of the gray-cheeked mangabey (Lophocebus albigena) in Cameroon. Am J Primatol 54:91–105
Rawat GS, Desai A, Somanathan H, Wikramanayake ED (2001) Brahmaputra Valley semi-evergreen forests. (IM0105) available at: https://www.worldwildlife.org/ecoregions/im0105
Sarkar P (2000) Ecology and dynamics of social relationships of Assamese macaque: Macaca assamensis (McClelland, 1839). Ph.D. thesis, Gauhati University, India
Sarma K (2015) Studies on population status, behavioural and habitat ecology of eastern hoolock gibbon (Hoolock leuconedys) in Arunachal Pradesh, India. Ph.D. thesis, Department of Forestry, North Eastern Regional Institute of Science and Technology (deemed university), Arunachal Pradesh, India
Sarma K, Kumar A, Krishna M, Tripathi OP, Gajurel PR (2013) Ground feeding observations on corn (Zea mays) by eastern hoolock gibbon (Hoolock leuconedys). Curr Sci 104(5):587–589
Sarma K, Krishna M, Kumar A (2014) Fragmented populations of the Vulnerable eastern hoolock gibbon Hoolock leuconedys in the Lower Dibang Valley district, Arunachal Pradesh, India. Oryx 49:133–139
Sharma N (2008) Evaluation of the conservation status of the diurnal primates of Gibbon Wildlife Sanctuary, Assam, northeastern India. Final report, National Institute of Advanced Studies, Bangalore, India
Singh N, Mani TT, Prakash D, Singh P (2012) A review on medicinal properties of Ichnocarpous frutescens. Indian J Novel Drug Deliv 4(1):24–27
Srivastava A (1991) Insectivory and its significance to langur diets. Primates 32:237–241
Tickell SR (1864) Notes on the gibbon of Tenasserim, Hylobates lar. J Asiat Soc Bengal 33:196–199
Tilson RL (1979) On the behaviour of hoolock gibbons (Hylobates hoolock) during different seasons in Assam, India. J Bombay Nat Hist Soc 76:1–16
Verma RK, Singh N, Gupta MM (1987) Triterpenoids of Ichnocarpus frutescens. Fitoterapia 8(4):271–272
Walker S (2005) Highlights of the population and habitat viability assessment for western hoolock gibbon (Bunopithecus hoolock hoolock) held in Bangladesh. Zoos Print J 20(4):2–3
Walker S, Molur S, Brockelman WY (2007) Western hoolock gibbon, Hoolock hoolock (Harlan, 1831). In: Mittermeier RA et al (eds) Primates in peril: the world’s 25 most endangered primates, 2006–2008, vol 22. Primate Conserv, Conservation International, Arlington, Virginia, pp 1–40
Whitten AJ (1982) Diet and feeding behavior of Kloss gibbons on Siberut Island, Indonesia. Folia Primatol 37:177–208
Zanette L, Doyle P, Tre´mont S (2000) Food shortage in small fragments: evidence from an area-sensitive passerine. Ecology 81:1654–1666
Acknowledgements
We highly acknowledge Dr. Warren Y. Brockelman for his valuable suggestions, comments and sincere effort to improve the quality of the manuscript. We extend our sincere gratitude to the Principal Chief Conservator of Forests (Wildlife), Basistha, Guwahati, Assam, for his kind permission to carry out the research work in HGWLS, Assam. We sincerely thank forest officials of the Meleng Beat Office, HGWLS, especially Mr. Daben Borah, for his assistance during the entire fieldwork. We also thank Dr. Gitamani Dutta and Dr. Rajeev Basumatary for their valuable suggestions and help, and Mr. Arup Kumar Das, Aaranyak, for help in making the geographic information system-based map of the study area. We also thank Mr. Lakshminath Rabha, Assistant Professor, D. R. College, Golaghat for language editing.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Borah, M., Devi, A. & Kumar, A. Diet and feeding ecology of the western hoolock gibbon (Hoolock hoolock) in a tropical forest fragment of Northeast India. Primates 59, 31–44 (2018). https://doi.org/10.1007/s10329-017-0627-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-017-0627-6