Abstract
Angiotensin II (Ang-II) is a widely studied hypertensive, profibrotic, and pro-inflammatory peptide. In the heart, cardiac fibroblasts (CF) express type 1 angiotensin II receptors (AT1R), Toll-like receptor-4 (TLR4), and the NLRP3 inflammasome complex, which play important roles in pro-inflammatory processes. When activated, the NLRP3 inflammasome triggers proteolytic cleavage of pro-IL-1, resulting in its activation. However, in CF the mechanism by which Ang-II assembles and activates the NLRP3 inflammasome remains not fully known. To elucidate this important point, we stimulated TLR4 receptors in CF and evaluated the signaling pathways by which Ang-II triggers the assembly and activity. In cultured rat CF, pro-IL-1β levels, NLRP3, ASC, and caspase-1 expression levels were determined by Western blot. NLRP3 inflammasome complex assembly was analyzed by immunocytochemistry, whereas by ELISA, we analyzed NLRP3 inflammasome activity and \(IL-1\beta\) release. In CF, Ang-II triggered NLRP3 inflammasome assembly and caspase-1 activity; and in LPS-pretreated CF, Ang-II also triggered \(IL-1\beta\) secretion. These effects were blocked by losartan (AT1R antagonist), U73221 (PLC inhibitor), 2-APB (IP3R antagonist), and BAPTA-AM (Ca2+ chelator) indicating that the AT1R/PLC/IP3R/Ca2+ pathway is involved. Finally, bafilomycin A1 prevented Ang-II-induced \(IL-1\beta\) secretion, indicating that a non-classical protein secretion mechanism is involved. These findings suggest that in CF, Ang-II by a Ca2+-dependent mechanism triggers NLRP3 inflammasome assembly and activation leading to \(IL-1\beta\) secretion through a non-conventional protein secretion mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Angiotensin II (Ang-II) is an octapeptide that physiologically regulates blood pressure and is a strong cardiac remodeling inductor [1]. Accumulating evidence reveals that Ang-II triggers cardiac inflammation, leading to an imbalance between pro-inflammatory and anti-inflammatory cytokine secretion [2, 3]. Ang-II-induced inflammation has been reported to increase endothelial dysfunction, vascular permeability, and the expression of adhesion molecules that ultimately promote inflammatory cell recruitment and its infiltration into tissues [4]. In addition Gan et al. [5] reported that male mice B6/129S treated with Ang-II mini-osmotic pumps after 7 days developed cardiac fibrosis dependent on activation of the NLRP3 inflammasome, which was attenuated with EMD638683 (a highly selective SGK1 inhibitor) by inhibiting the NLRP3 inflammasome/IL-1β secretion axis [5].
Several studies have shown that during cardiac damage, there is the secretion of interleukin-1beta (IL-1β), a pro-inflammatory cytokine that is synthesized by resident heart cells, including cardiac fibroblast (CF), through the NLRP3 inflammasome complex [6, 7]. At the cellular level, \(IL-1\beta\) exhibits differential growth regulation in cardiac cells, inducing cardiomyocyte hypertrophy and repressing CF proliferation [8], while preclinical and clinical findings support the key pathogenetic role of the IL-1 cytokines and the NLRP3 inflammasome in the formation, progression, and complications of atherosclerosis and in ischemic (acute myocardial infarction) and non-ischemic injury to the myocardium (myocarditis) and the progression to heart failure [9]. The ability of the NLRP3 inflammasome complex to produce pro-inflammatory cytokines (IL-1β and IL-18) in resident cardiac cells and in infiltrating cells has been recognized as a major cause of cardiac inflammation and tissue remodeling, also resulting in the progression of cardiac fibrosis [10, 11]. The NLRP3 inflammasome is a multiprotein complex involved in the innate immune system that acts as an intracellular sensor. It mediates caspase-1 activation and production of pro-inflammatory cytokines in response to pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs). The NLRP3 inflammasome is composed of NLRP3 (intracellular PRR receptor), ASC (apoptosis-associated speck-like protein, the adaptor), and the protease caspase-1. Inflammasome activation is a two-signal process—first, the priming or signal 1 and the activation or signal 2—the former is provided by PAMPs, DAMPs, or cytokine stimuli with the main purpose of upregulating the expression of NLRP3, ASC, caspase-1, and pro-IL-1β; and the latter occurs through a wide variety of PRR activators (PAMPs or DAMPS, adenosine triphosphate (ATP), cholesterol, sodium monourate (MSU), and asbestos) that activate multiple upstream signaling events, for example, an increase of cytosolic Ca2+ flux from the endoplasmic reticulum, K+ efflux, intracellular increases of ROS, lysosomal destabilization, or a decrease in intracellular levels of cyclic AMP, among others. Upon the stimuli, NLRP3 oligomerizes and then recruits ASC and the pro-caspase-1 into a complex. This promotes the transformation of pro-caspase-1 into caspase-1, which in turn cleaves the pro-forms of cytokines and the secretion of mature IL-1β and IL-18 into extracellular media [12, 13].
CF can detect external signals and trigger an inflammatory response, so they have been termed “sentinel cells” [14]. CF express type 1 Ang-II receptors (AT1R), which are coupled to PLC-IP3R signaling pathways increasing the cytosolic Ca2+ concentration and activating NADPH producing an increase in ROS [15, 16]. PAMPs or DAMPs can also activate the inflammatory response in CF [17,18,19,20], and these signals are recognized by molecular pattern recognition receptors (PRR) such as NLRP3. The activation of these receptors in CF is associated with the production of inflammatory cytokines and chemokines, which can have a direct impact on neighboring or immune cells [18, 19, 21]. Bracey et al. [22] reported the upregulation of NLRP3 protein levels in human CF after 24 h of stimulation with Ang-II; but Ang-II alone was not able to induce an increase in the IL-1β secretion [22].
Despite all this information, the mechanism by which Ang-II could act as an inductor of signal 2 to activate the NLRP3 inflammasome on CF remains not fully known. Thus, we hypothesized that Ang-II through AT1R activation and by a Ca2+ signaling-dependent pathway could trigger NLRP3 inflammasome assembly and IL-1β release in a previously primed CF.
MATERIALS AND METHODS
Materials
Sterile cell culture plastics were purchased from Costar® (NJ, USA). The following reagents were purchased from Sigma Chemical Co. (St. Louis, MO, USA): trypan blue, trypsin/EDTA, molecular mass standard, ultrapure LPS, angiotensin II, 2-APB, AC-YVAD-CMK, and bafilomycin A1. Fetal bovine serum (FBS) was purchased from GIBCO BRL (Carlsbad, CA, USA). All organic and inorganic compounds were purchased from Merck (Darmstadt, Germany). The chemiluminescence enhancement reagent (ECL) was purchased from Perkin Elmer Life Sciences, Inc. (Boston, MA, USA). The primary antibodies for NLRP3, the secondary anti-goat antibodies, and the NLRP3 blocking peptide were purchased from Santa Cruz Biotechnology (CA, USA), and the IL-1β antibody was from Merck Millipore (MA, USA). Anti-rabbit and anti-mouse secondary antibodies, in addition to the primary antibody for tubulin, were purchased from Calbiochem (Darmstadt, Germany). The ELISA kit for rat IL-1β was purchased from R & D Systems (MN, USA). ATP, losartan, U73122, and the caspase-1 activity kit (fluorometric) were purchased from ABCAM (Cambridge, UK). The LDH cytotoxicity kit Pierce™, NLRP3 Select Silencer®, and brefeldin A were requested from Thermo Fisher Scientific (MA, USA). BAPTA-AM and ac-YVAD-cmk were purchased from Cayman Chemical (MI, USA).
Cell Culture
The cells used were primary cultures of neonatal CF brought to confluence. These cell cultures were isolated from the hearts of neonatal rats using the protocol described by Vivar et al. [16]. The cells were seeded in culture plates with DMEM-F12 + FBS (10%); after 4 h, they were washed and deprived with DMEM-F-12 until the next day. The Bioethics Committee of the Faculty of Chemical and Pharmaceutical Sciences of the University of Chile with the code CBE2012-20 approved the management and care of laboratory animals.
Stimuli
The signal 1 stimulus of the inflammasome was performed with ultrapure lipopolysaccharide (LPS: 1 μg/mL) dissolved in bi-distilled water. The stimulus time was from 0 to 72 h depending on the experiment. The stimuli for signal 2 of the inflammasome corresponded to ATP (3 mM) and Ang-II (1 μM). These substances were added after 8 h of LPS stimulation, without medium washing, and samples were obtained between 1 and 16 h after the initiation of this second stimulus (Fig. 1A). Antagonists or inhibitors: losartan (10 μM), U73122 (1 μM), 2-APB (10 μM), ac-YVAD-cmk (10 μM) brefeldin A (100 nM), and bafilomycin A1 (1 nM) were used 1 h after LPS and 1 h before stimulation with Ang-II 16 h. BAPTA-AM (10 μM) was added 1 h before stimulation with Ang-II 4 h. All the stimuli, antagonists, and water-soluble inhibitors were dissolved in water, and the rest were dissolved in DMSO, reaching a maximum concentration of 1% DMSO in the cell culture media.
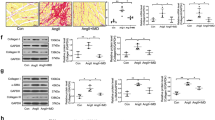
Ang-II increases NLRP3 and ASC colocalization. A Protocol of experimental design. Representative diagram of the experimental groups in the biological assays. CF were stimulated with LPS (1 μg/mL) for 24 h alone or with Ang-II (1 μM) for the last 16 h. Cells were fixed, and immunocytochemistry was performed. B Representative immunocytochemistry images of NLRP3 (green), ASC (red), and nucleus (blue). C Quantification of the percentage of cells that colocalize NLRP3 and ASC in perinuclear/nuclear zone. The values are the means ± SEM ***p < 0.001 vs control, ###p < 0.001 vs LPS 8 h. n = 4 independent experiments. D 3D reconstruction of the images obtained with stimulation of LPS 8 h + Ang-II 16 h with ASC and NLRP3 separately.
Western Blot
The western blots were performed on the cell lysates or from the precipitation of the supernatant proteins of the treated cells. The protocol used was described before by Boza et al. [17], and then the following antibodies were added: IL-1β (1: 1000 for cell lysate).
Immunocytochemistry
The immunocytochemistry images were obtained with confocal microscopy. CF were seeded on glass coverslips. At the end of the experimental period, the cells were washed with PBS and fixed for 20 min with 4% paraformaldehyde. They were then washed and permeabilized with 0.1% Triton x-100 for 10 min. Subsequently, they were washed with PBS and blocked with 3% BSA for 1 h. After blocking, they were rewashed and incubated with primary antibodies for NLRP3 (1:50) and ASC (1:50) in BSA overnight. In the next day, the cells were washed, and the Alexa Fluor® anti-goat and anti-rabbit (1: 400) secondary antibodies, in addition to Hoechst (1: 100) for nuclear labeling, were added for 2 h. Finally, the coverslips were again washed and placed on a slide with DAKO until dry. The images were captured by confocal microscopy (Zeiss, model LSM 700) in 10 × and 40 × magnification. Fourteen cross-sections were made from the base to the apex of the cell to perform the z-stack and later the 3D reconstruction.
ELISA
Rat IL-1β ELISA (R & D Systems) was performed in conditioned cell culture media. These media were centrifuged at 2000 rpm for 5 min after collection. The methodology of the kit instructions was followed, without diluting the samples. The experiments were performed with fresh culture media on the same day or collected the day before the end of each stimulus. The results were expressed in pg IL-1β/mL and normalized against the total amount of cellular proteins, expressed in pg IL-1β/μg of protein. The total protein concentration of the lysates was quantified using the Bradford method.
Caspase-1 Activity
Caspase-1 activity was determined using a commercial kit (Abcam). For this, cells were stimulated with LPS (8 h) + ATP (16 h) or LPS (8 h) + Ang II (16 h) and their respective controls. At the end of the experimental period, the analysis was performed according to the manufacturer’s instructions. Active caspase-1 (intracellular) recognizes and cleaves a specific amino acid sequence that fluoresces when it is cleaved by this active protease. The substrate corresponds to ac-YVAD-AFC, a tetrapeptide linked to a coumarin (AFC) that, when cut, emits fluorescence at 505 nm.
Cytotoxicity
Cytotoxicity assays were performed using a commercial lactate dehydrogenase (LDH) kit (Thermo Fisher). The kit used LDH released during cell lysis of CF with lysis buffer. The percentage of cytotoxicity of the compounds used was calculated from the results obtained.
Silencing of NLRP3
NLRP3 gene silencing was performed using NLRP3 Select Silencer® (Thermo Fisher). The transfection protocol was performed according to the manufacturer’s instructions. The siRNA and the scrambled control were used at a final concentration of 5 nM. The blocking peptide was co-incubated with the NLRP3 antibody at a final concentration of 1:40.
Statistical Analysis
Data were analyzed by one-way ANOVA, with Dunnett’s post-test. The plotted values correspond to the mean values ± standard error of the mean (SEM). The presented data correspond to at least three independent experiments. A statistically significant difference was considered p < 0.05.
RESULTS
Cardiac Fibroblasts Caspase-1 Activity and IL-1β Secretion
Our first aim was to determine pro-IL-1β expression levels, and to this aim, we use LPS, a recognized TLR4 agonist. In Supplementary Fig. 1A, the results show low pro-IL-1β expression levels in CF; however, LPS increased expression levels of this cytokine, achieving a maximum fivefold increase at 8 h and then decay of the signal at 24 h. Note that no changes were observed in the 24-h control.
Once it was established that CF express pro-IL-1β, it was determined whether ATP (a classic inflammasome activator) was able to activate caspase-1. In Supplementary Fig. 1B, the results show that ATP (16 h) and LPS (8 h) + ATP (16 h), giving a total time of 24 h, significantly increased caspase-1 activity compared to controls. However, LPS alone (24 h) was unable to increase caspase-1 activation relative to the control.
Once ATP was found to induce caspase-1 activity, we wanted to determine if ATP also induced the secretion of mature IL-1β. Supplementary Fig. 1C shows that neither LPS alone (24 h) nor ATP alone (16 h) induced increases in IL-1β secretion. However, when the stimuli (LPS 8 h + ATP 1–16 h) were combined, there was a time-dependent increase in IL-1β secretion that reached statistical significance after 16 h. It is important to note that although ATP alone (16 h) increased caspase-1 activity, it did not induce IL-1β secretion.
Ang-II Assembles and Activates the NLRP3 Inflammasome in Cardiac Fibroblasts
It is well known that when NLRP3 is activated, it is mobilized to the perinuclear sector where after the oligomerization, it colocalizes with ASC and caspase-1 to generate its assembly, thus constructing the active NLRP3 inflammasome.
To determine whether Ang-II induced NLRP3 and ASC protein assembly, CF were stimulated with LPS (8 h) + Ang-II for an additional 16 h (a total of 24 h). The results of the confocal microscopy (Fig. 1B–D) showed that under control conditions, the NLRP3 (green) and ASC (red) proteins were homogeneously located throughout the cell (the colocalization analysis of both proteins showed an intense yellow color in whole cell: NLRP3 + ASC). However, after stimulation with only Ang-II (16 h) or with LPS (8 h) + Ang-II (16 h), there is a redistribution and increase of both proteins in the perinuclear/nuclear sector (the colocalization analysis of both proteins showed an intense yellow color in the perinuclear/nuclear zone: NLRP3 + ASC). Nuclear colocalization was confirmed by nuclear Hoechst staining (the colocalization analysis of both proteins showed an intense white color in the nuclear area: NLRP3 + ASC + Hoechst) (Fig. 1B). Finally, the results showed that FC stimulated only with LPS (24 h) did not induce colocalization of both proteins in the perinuclear/nuclear zone, indicating that LPS alone did not induce the assembly of NLRP3 and ASC.
Furthermore, the graphical analysis showed that Ang-II (16 h) and LPS (8 h) + Ang-II (16 h) significantly increased the percentage of cells showing perinuclear/nuclear colocalization, compared to control (Fig. 1C) and also compared to LPS alone (24 h).
Finally, to corroborate that Ang-II promoted the mobilization of NLRP3 and ASC towards the same intracellular sector, a 3D reconstruction was performed using the images obtained from 14 slices (z-stack), ranging from the base of the cell to the appendix. Following the above, it was observed in the 3D reconstruction of a cell stimulated with LPS (8 h) + Ang-II (16 h) that the colocalization of NLRP3 and ASC in the sector where the nucleus is located (merge condition of NLRP3, ASC, and nucleus, Fig. 1D).
Additionally, it was demonstrated that the stimuli did not cause pyroptosis. This type of cell death is induced by the activation of the inflammasome and caspase-1. For this, the activity of lactate dehydrogenase (LDH) was quantified. The results showed that none of the stimuli used generated cytotoxicity, and consequently, the cells did not experience pyroptosis (Supplementary Fig. 2).
Ang-II Activates Caspase-1 Activity and the Secretion of IL-1β
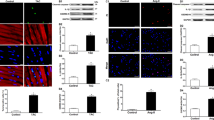
To define the participation of NLRP3 in Ang-II effects in CF, NLRP3 was silenced using a specific siRNA. To verify if the siRNA decreased NLRP3 expression in CF, the total cellular fluorescence was quantified from images by confocal microscopy. The results showed that in CF, the NLRP3 siRNA decreased NLRP3 expression in comparison to the control (untreated cells) and scrambled siRNA (Fig. 2A, B) by ~ 70%. In addition, the blocking peptide of NLRP3 was used as a positive control of the technique. This peptide, specific for the antibody used, was able to decrease the fluorescence by ~ 80% in comparison to the control. No statistical differences were observed between the use of siRNA and the NLRP3 blocking peptide (Fig. 2A, B).
In CF the NLRP3 inflammasome is activated by Ang-II. A Immunocytochemistry representing silencing of NLRP3 (green) and nucleus (blue). NLRP3 blocking peptide corresponds to the positive control of the technique. Photographs are at 40 × magnification. B Quantification of the relative total fluorescence of the images obtained by confocal microscopy. C Caspase-1 activity was analyzed in CF stimulated with LPS (1 μg/mL) for 8 h and then with Ang-II (1 μM) for 16 h. D Caspase-1 activity was analyzed in CF treated with ac-YVAD-cmk (10 μM). CF were treated with LPS 1 μg/mL for 7 h, ac-YVAD-cmk was added for 1 h, and finally, cells were stimulated with 1 μM Ang-II for 16 h. Values are mean ± SEM. For B *p < 0.05 and **p < 0.01 vs control; #p < 0.05 and ##p < 0.01 vs scramble. For C *p < 0.05 vs control and #p < 0.05 vs LPS 8 h. For D **p < 0.01 vs control; ##p < 0.01 vs YVAD and &&&p < 0.001 vs LPS 8 h + ATP 16 h. n = 5 independent experiments.
To establish whether Ang-II produced an increase in caspase-1 activity, CF were stimulated with LPS (8 h), followed by Ang-II for an additional 16 h. The results showed that both Ang-II (16 h) and LPS (8 h) + Ang II (16 h) generated a significant increase in caspase-1 activity in comparison to the control (Fig. 2C); by contrast, LPS alone (24 h) did not cause changes in caspase-1 activity. To define the participation of caspase-1 in Ang-II effects in CF, caspase-1 was irreversibly inhibited with ac-YVAD-cmk (10 μM). Thus, CF were treated with LPS (8 h) and pretreated with ac-YVAD-cmk, 1 h before stimulation with Ang-II (16 h). The results showed that ac-YVAD-cmk prevented the increase in caspase-1 activity induced by Ang-II (Fig. 2D).
To establish whether Ang-II induced IL-1β secretion, CF were stimulated with LPS (8 h) and then with Ang-II for an additional 16 h. The results showed that co-stimulation of LPS (8 h) + Ang-II (16 h) induced IL-1β secretion significantly in comparison to the control (Fig. 3A), while LPS (24 h) or Ang-II (16 h) by themselves did not induce cytokine secretion. Furthermore, once siRNA functionality was established, IL-1β secretion was quantified. The results showed that the NLRP3-silenced CF did not secrete IL-1β compared to LPS-stimulated cells (8 h) + Ang-II (16 h), and IL-1β secretion was similar to the control (Fig. 3B). Finally, when CF were treated with LPS for (8 h) and pretreated with ac-YVAD-cmk, 1 h before stimulation with Ang-II (16 h), the results showed that ac-YVAD-cmk prevented the increase in IL-1β secretion (Fig. 3C) induced by LPS (8 h) + Ang-II (16 h).
IL-1β secretion triggered by Ang-II is NRLP3-dependent. An ELISA of IL-1β secretion (expressed in pg IL-1β/μg protein) was performed on the supernatant. A CF were stimulated with LPS (1 μg/mL) for 8 h and then with Ang II (1 μM) for 16 h; B CF treated with NLRP3 siRNA and then stimulated with LPS (1 μg/mL) for 8 h and then with Ang II (1 μM) for 16 h; and C CF were treated with LPS 1 μg/mL for 7 h, ac-YVAD-cmk was added for 1 h, and finally cells were stimulated with 1 μM Ang-II for 16 h. The values are the mean ± SEM. For A *p < 0.05 vs control, #p < 0.05 vs LPS, and &p < 0.05 vs Ang-II. For B *p < 0.05 vs LPS 8 h + Ang-II. For C *p < 0.05 vs control, #p < 0.05 vs YVAD, and &p < 0.05 vs LPS + Ang-II. n = 5 independent experiments.
Ang-II Activates Caspase-1 and Triggers IL-1β Secretion by an AT1R/PLC/IP3R/Ca.2+-Dependent Mechanism
It is known that Ang-II binds to the AT1R receptor and that upon activation it can trigger the following signaling pathway: AT1R-PLC-IP3/DAG-IP3R-Ca2+. Losartan (10 μM, AT1R antagonist) was used to establish the role of AT1R activation. CF were stimulated with LPS (8 h), and without changing media, losartan was added for 1 h before the addition of Ang-II (16 h). The results showed that losartan prevented the increase in caspase-1 activity (Fig. 4A) and IL-1β secretion (Fig. 4B) induced by Ang-II. Subsequently, the participation of phospholipase C (PLC) and the IP3 receptor were evaluated using U73122 (PLC inhibitor) and 2-APB (IP3R antagonist), respectively. CF were stimulated with LPS (8 h) and then pretreated for 1 h with U73122 (1 μM) or 2-APB (10 μM), before the addition of Ang-II (16 h). The results showed that U73122 and 2-APB prevented both caspase-1 activity (Fig. 4C) and IL-1β secretion (Fig. 4D) induced by Ang-II. Finally, the BAPTA-AM chelator was used to determine if Ca2+ was the key in NLRP3 inflammasome activation. CF were incubated with BAPTA-AM (10 μM) for 1 h, and then Ang-II was added for 4 h. It should be considered that this experiment was performed in the presence of culture media with Ca2+. It is important to clarify that the methodology and stimulus time used were changed because BAPTA-AM induced cell death in culture from 6 to 8 h. The methodology was adjusted to achieve a maximum exposure time of BAPTA-AM of 5 h, so it was only performed in the caspase-1 activity assay and not IL-1β secretion because the latter requires a longer incubation time. It was observed that BAPTA-AM prevented caspase-1 activity induced by Ang-II (Fig. 4E).
Ang-II through AT1R/PLC/IP3R/Ca2+ signaling pathway activates caspase-1 and IL-1β secretion. CF were stimulated with LPS (1 μg/mL) for 8 h and preincubated with losartan (10 μM), U73122 (1 μM), or 2-APB (10 μM) for 1 h before stimulating with Ang-II (1 μM) for 16 h. A Caspase-1 activity in the presence of losartan; B ELISA for IL-1β was secretion expressed in pg IL-1β/μg protein in the presence of losartan. C Caspase-1 activity in presence of U73122 and 2-APB; D ELISA for IL-1β was secretion expressed in pg IL-1β/μg protein in the presence of U73122 and 2-APB. E Caspase-1 activity was quantified in CF preincubated with BAPTA-AM (10 μM) for 1 h and then stimulated with Ang-II (1 μM) for 4 h. The values are means ± SEM. For A and B, *p < 0.05 vs control, #p < 0.05 vs losartan, and &p < 0.05 vs LPS 8 h + Ang-II 16 h. For C and D, *p < 0.05 vs control, #p < 0.05 vs U73; &p < 0.05 vs 2-APB, and $p < 0.05 vs LPS 8 h + Ang-II 16 h. n = 5 independent experiments.
Ang-II Induces the Secretion of IL-1β by a Non-classical Vesicular Mechanism
Finally, we aimed to determine the \(IL-1\beta\) secretory mechanism in response to LPS + Ang-II, identifying the classical reticulum/Golgi secretion or an alternative non-classical mechanism. To do this, CF were stimulated with LPS (8 h), and then brefeldin A (100 nM), a molecule that interferes with the anterograde mechanism from the endoplasmic reticulum to the Golgi apparatus, was added for 1 h before cells were stimulated with Ang-II for 16 h. Brefeldin was unable to prevent IL-1β secretion induced by LPS (8 h) + Ang-II (16 h) (Fig. 5A). Subsequently, bafilomycin A1 (1 nM), an inhibitor of V-ATPase that prevents the formation of vesicles that have this H+ pump, was used. Bafilomycin A1 decreased IL-1β secretion mediated by LPS (8 h) + Ang-II (16 h) (Fig. 5B).
Ang-II induces IL-1β secretion by a non-classical protein secretion mechanism. CF were treated with LPS 1 μg/mL for 7 h, then preincubated for 1 h with brefeldin A (Bref) or bafilomycin A1 (Baf), and finally stimulated with Ang-II (1 μM) for 16 h. ELISA for IL-1β was secretion expressed in pg IL-1β/μg protein. A Cells treated with brefeldin A (100 nM). B Cells treated with bafilomycin A1 (1 nM). Values are mean ± SEM. For A **p < 0.01 vs control, #p < 0.05 vs brefeldin A. For B **p < 0.01 vs control; #p < 0.05 vs LPS 8 h + Ang-II 16 h. n = 6 independent experiments.
DISCUSSION
Ang-II Activates the NLRP3 Inflammasome in CF
First, we confirm that in CF LPS and ATP, the classic first and second signals, respectively, can activate the NLRP3 inflammasome and induce IL-1β secretion [6, 7]. It has been suggested that under pathological conditions, high levels of ATP are released from necrotic cells, and through its interaction with the P2X7 receptor would act as a pro-inflammatory damage signal, inducing the NLRP3 inflammasome assembly and activation [23]. Our study revealed that ATP induced an increase in caspase-1 activity, although it did not show effects on pro-\(IL-1\beta\) expression levels.
Ang-II, a peptide with significant effects in CF, is also known to act as a second signal that can activate the NLRP3 inflammasome in CF [5, 22], endothelial cells [24], and smooth muscle cells [25], although the mechanism was not fully studied. In this sense, Ang-II possessed two of the three basic characteristics necessary to activate the NLRP3 inflammasome: the variations in the osmolarity of the cellular environment and the increase in ROS. The first characteristic would be due to the increase in intracellular Ca2+ concentration and the second due to an increase in NADPH oxidase activity.
In CF, NLRP3 inflammasome activation triggered by Ang-II was demonstrated by three different strategies. Firstly, we showed inflammasome protein colocalization, which is a molecular process that must occur to assemble this protein complex. We showed that Ang-II or LPS + Ang-II stimuli induced NLRP3 and ASC mobilization to the perinuclear sector, achieving its colocalization, a phenomenon that agrees with the literature [26]. Secondly, we demonstrated the activation of caspase-1, which is a key event in the activation of the NLRP3 inflammasome that is responsible for the cleavage of pro-IL-1β into IL-1β. We showed that either Ang-II alone or LPS + Ang-II were able to increase caspase-1 activity. These results suggest that Ang II acts as signal 2 able to induce NLRP3 inflammasome assembly, in an independent manner of signal 1. In this regard, IL-1β secretion is the last event that reveals that the inflammasome complex is assembled and activated [27]. We showed that Ang-II alone did not induce IL-1β secretion; this result agrees with Bracey et al. [22], and it is explained because Ang-II does not induce pro-IL-1β synthesis; however, LPS + Ang-II induced IL-1β secretion. In summary, the three results show that Ang-II, by itself, can assemble the inflammasome activating caspase-1, but by itself cannot induce IL-1β secretion, unless pro-\(IL-1\beta\) is present.
Furthermore, in CF NLRP3 inflammasome activation has been described to be related to TGF-β regulation; thus, in CF deficient in NLRP3, there is altered cell differentiation and activation of R-Smad in response to TGF-β [22]. In this sense, previously we showed that there is an increase in caspase-1 expression in CF when stimulated with TGF-β1 [17]. Finally, caspase-1 has been indicated to participate in pyroptosis presented by immune cells [28]; however, we did not find pyroptosis under the stimuli conditions used in this work.
Concerning the time required to quantify IL-1β secretion induced by ATP, it has been shown that in immune-type cells, at 30 min after stimulus, the concentrations were greater than 1000 pg/mL. However, our results showed that detectable \(IL-1\beta\) levels were reached much later, after 16 h of stimulation. IL-1β secretion likely occurs in a shorter timeframe than that studied in our experiments in CF, but technically the detection of this cytokine requires a longer time. Also, it is possible that in CF, the de novo production of pro-\(IL1\beta\) will take a long time for the pro-\(IL-1\beta\) protein to accumulate to significant levels and that the IL-1β secretion is slow and independent of the stimuli used. This could mean that at the cardiac level, there are two phenomena about activation of the NLRP3 inflammasome and IL-1β secretion: one that would be acute, due especially to the activity of immune cells, and another one that could be chronic and undetectable, which would be due to the inflammatory action by non-immune cells, such as CF. Both phenomena could eventually lead to alterations in cardiac tissue and cardiac function.
Ang-II Activates Caspase-1
In other cell types, other inflammasomes have been described, which can actively participate in IL-1β secretion [29]. Our results showed that NLRP3 siRNA prevented LPS + Ang-II-induced IL-1β secretion in CF. This result is complementary to our previous experiment on NLRP3 and ASC colocalization induced by Ang-II. In summary, it is clear that participation in NLRP3 is crucial for IL-1β secretion and would be the main Ang-II-activated inflammasome in CF. However, it has also been described that other enzymes can cleave pro-IL-1β, such as MMP-9, PR3, cathepsin G, and elastase, among others [30]. In this respect, our results with ac-YVAD-cmk allowed us to confirm the participation of caspase-1 in IL-1β secretion induced by Ang-II. Taken together, our data show that it is the NLRP3 inflammasome that is activated by Ang-II and that, effectively, the enzyme that cleaves pro-IL-1β is caspase-1.
Ang-II Through AT1R/PLC/IP3/Ca.2+ Activates Caspase-1
Our main aim was to elucidate the signaling pathways in CF that couple Ang-II to NLRP3 assembly and caspase-1 activation. First, using losartan it was established that Ang-II must interact with the AT1R. Then, using U73122 and 2-APB, it was established that PLC activation and IP3 genesis activate IP3R, triggering Ca2+ exit from the endoplasmic reticulum and increasing its concentration in the cytosol. Likewise, the use of BAPTA-AM allowed us to demonstrate that Ca2+ was key in the activation of caspase-1 induced by Ang-II. These facts reaffirm that signaling: AT1R/PLC/IP3R/Ca2+ would be the sequence of events that would trigger the assembly of the NLRP3 inflammasome and caspase-1 activation. In this regard, Ca2+ has previously been shown to be an important second messenger that activates the NLRP3 inflammasome [31]. Wang et al. [32] and Usui et al. [33] showed in mouse macrophages that Ang-II increased \(IL-1\beta\) secretion, and this effect was blocked by losartan, demonstrating that AT1R activation was involved in the activation of the NLRP3 inflammasome [32, 33]. However, none of these studies demonstrated that the assembly and activation of the NLRP3 inflammasome by Ang-II is dependent on the signaling cascade that increases intracellular Ca2+. In this respect, fluxes of Ca2+ related to the intracellular and endoplasmic reticulum have been shown to also activate the NLRP3 inflammasome [34], which coincides with our findings. Here, we elucidate a critical role for Ca2+ mobilization in the activation of the NLRP3 inflammasome by Ang-II. We demonstrate that blocking Ca2+ mobilization inhibits the assembly and activation of the NLRP3 inflammasome complex. Besides that, during Ang-II stimulation, Ca2+ signaling is pivotal in promoting NLRP3 activation and \(IL-1\beta\) secretion. Therefore, we established that Ang-II triggers activation of the NLRP3 inflammasome through a Ca2+-dependent mechanism; however, we cannot discard ROS as another possible mechanism by which the NLRP3 inflammasome is activated in CF. In this sense, Zhang et al. [35] showed that Ang-II initiates the epithelial-mesenchymal transition of hepatocytes by activating the NOX-derived ROS-induced NLRP3 inflammasome [35]; and Zhao et al. [36] demonstrated an important role for the NLRP3 inflammasome in mediating podocyte injury and mitochondrial dysfunction induced by a ROS-dependent mechanism [36].
Secretion of IL-1β by a Non-classical Protein Secretion Mechanism
In CF, there are surprisingly no studies on the IL-1β secretory mechanism, especially since it lacks the classical sequence of protein secretion. Using brefeldin A, it was demonstrated that the endoplasmic reticulum/Golgi system was not involved, discarding the classical route of protein secretion [37]. However, some studies indicate that it could be due to an alternative secretion mechanism [38, 39], including the use of carriers, evaginations of the plasma membrane, and the formation of different vesicles [38,39,40,41]. However, one of the most striking possibilities is the formation of autophagic-type vesicles, which would be responsible for favoring the meeting of the components of the NLRP3 inflammasome system in a small space that allows the transport of IL-1β towards the cellular exterior. Bafilomycin A1 prevented IL-1β secretion suggesting a role for vesicles that express the V-ATPase H+ pump. There is a possibility that the vesicular mechanism is not the only one involved in IL-1β secretion, and there could be others not ruled out parallel methods of transport and IL-1β secretion in CF. These results would lead us to think that, as in other studies, there could be a spectrum of methods of IL-1β secretion and that this would depend on the type and time of stimulus used [38]. As mentioned above, immune cells may lose the continuity and fluidity of the plasma membrane, due to the concomitant phenomenon of pyroptosis, so this loss could promote the release of IL-1β and other substances into the extracellular environment. However, CF did not undergo pyroptosis, so the extrusion of IL-1β was driven by an active process. Recent work suggests that one of the roles of Ca2+ would not necessarily be to activate the NLRP3 inflammasome, but rather to favor vesicular secretion of IL-1β towards the extracellular environment in models with low pyroptosis [42]. Additionally, Galliher-Beckley et al. [43] stated that in human monocytes, caspase-1 activation and IL-1β secretion would be distinct and separable events [43]; concerning this fact, and according to the results presented, Ang-II would induce both events: both caspase-1, and if pro-\(IL-1\beta \beta\) is present, then IL-1β secretion could be also triggered.
Conclusion
The findings (summarized in Fig. 6) suggest that Ang-II, through the activation of the AT1R, stimulates the transduction signaling pathways involving PLC and IP3R, which leads to the exit of Ca2+ from the endoplasmic reticulum into the cytosol, resulting in activation of the NLRP3 receptor, allowing its oligomerization and recruitment of ASC and pro-caspase-1. The enzyme would auto-activate to form active caspase-1 and be able to cleave pro-IL-1β into IL-1β. Finally, this active cytokine was secreted through vesicular transport.
Proposed model. In CF Ang-II activates AT1R/PLC/IP3 + DAG/Ca2+ released from the endoplasmic reticulum towards the cytosol, a phenomenon key to the activation of the NLRP3 inflammasome. The assembly of the NLRP3 inflammasome leads to caspase-1 activation. This converts pro-IL-1β into active IL-1β. Finally, this cytokine would be secreted into the extracellular medium by an alternative mechanism of vesicular transport.
PROJECTIONS
It is important to mention that the review by Butts et al. [44] states that the NLRP3 inflammasome is a promising drug target in heart failure [44]. We believe that this work could go in that same direction. Ang-II at the cardiac level could generate the preassembly of the NLRP3 inflammasome in resident heart cells, and once damage occurred, no matter how small, complete activation of the system could be generated, which would lead to the secretion of IL-1β. This cytokine would act in a paracrine fashion inducing hypertrophy and apoptosis of the cardiomyocytes and in the long-term driving cardiac remodeling.
Availability of Data and Materials
All data generated or analyzed and materials used during this study are included in this published article and its supplementary figure files.
References
Forrester, S.J., G.W. Booz, C.D. Sigmund, T.M. Coffman, T. Kawai, V. Rizzo, R. Scalia, and S. Eguchi. 2018. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiological Reviews 98: 1627–1738. https://doi.org/10.1152/physrev.00038.2017.
Wu, Y., Y. Li, C. Zhang, Y. Wang, W. Cui, H. Li, and J. Du. 2014. S100a8/a9 released by CD11b+Gr1+ neutrophils activates cardiac fibroblasts to initiate angiotensin II–induced cardiac inflammation and injury. Hypertension 63: 1241–1250. https://doi.org/10.1161/HYPERTENSIONAHA.113.02843.
Wang, M., L. Jiang, R.E. Monticone, and E.G. Lakatta. 2014. Proinflammation: the key to arterial aging. Trends in Endocrinology & Metabolism 25: 72–79. https://doi.org/10.1016/j.tem.2013.10.002.
Cheng, Z.J., H. Vapaatalo, and E. Mervaala. 2005. Angiotensin II and vascular inflammation. Med Sci Monit 11: Ra194–205.
Gan, W., J. Ren, T. Li, S. Lv, C. Li, Z. Liu, and M. Yang. 2018. The SGK1 inhibitor EMD638683, prevents angiotensin II–induced cardiac inflammation and fibrosis by blocking NLRP3 inflammasome activation. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1864: 1–10. https://doi.org/10.1016/j.bbadis.2017.10.001.
Kawaguchi, M., M. Takahashi, T. Hata, Y. Kashima, F. Usui, H. Morimoto, A. Izawa, Y. Takahashi, J. Masumoto, J. Koyama, M. Hongo, T. Noda, J. Nakayama, J. Sagara, and Taniguchi, S.i. and Ikeda, U. 2011. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation 123: 594–604. https://doi.org/10.1161/CIRCULATIONAHA.110.982777.
Sandanger, Ø., T. Ranheim, L.E. Vinge, M. Bliksøen, K. Alfsnes, A.V. Finsen, C.P. Dahl, E.T. Askevold, G. Florholmen, G. Christensen, K.A. Fitzgerald, E. Lien, G. Valen, T. Espevik, P. Aukrust, and A. Yndestad. 2013. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia–reperfusion injury. Cardiovascular Research 99: 164–174. https://doi.org/10.1093/cvr/cvt091.
Palmer, J.N., W.E. Hartogensis, M. Patten, F.D. Fortuin, and C.S. Long. 1995. Interleukin-1 beta induces cardiac myocyte growth but inhibits cardiac fibroblast proliferation in culture. The Journal of Clinical Investigation. https://doi.org/10.1172/JCI117956.
Abbate, A., S. Toldo, C. Marchetti, J. Kron, B.W. Van Tassell, and C.A. Dinarello. 2020. Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circulation Research 126: 1260–1280. https://doi.org/10.1161/CIRCRESAHA.120.315937.
Takahashi, M. 2019. Role of NLRP3 inflammasome in cardiac inflammation and remodeling after myocardial infarction. Biological and Pharmaceutical Bulletin 42: 518–523. https://doi.org/10.1248/bpb.b18-00369.
Xiao, H., H. Li, J.-J. Wang, J.-S. Zhang, J. Shen, X.-B. An, C.-C. Zhang, J.-M. Wu, Y. Song, X.-Y. Wang, H.-Y. Yu, X.-N. Deng, Z.-J. Li, M. Xu, Z.-Z. Lu, J. Du, W. Gao, A.-H. Zhang, Y. Feng, and Y.-Y. Zhang. 2018. IL-18 cleavage triggers cardiac inflammation and fibrosis upon β-adrenergic insult. European Heart Journal 39: 60–69. https://doi.org/10.1093/eurheartj/ehx261.
Abderrazak, A., T. Syrovets, D. Couchie, K. El Hadri, B. Friguet, T. Simmet, and M. Rouis. 2015. NLRP3 inflammasome: from a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biology 4: 296–307. https://doi.org/10.1016/j.redox.2015.01.008.
Man, S.M., and T.-D. Kanneganti. 2015. Regulation of inflammasome activation. Immunological Reviews 265: 6–21. https://doi.org/10.1111/imr.12296.
Diaz-Araya, G., R. Vivar, C. Humeres, P. Boza, S. Bolivar, and C. Munoz. 2015. Cardiac fibroblasts as sentinel cells in cardiac tissue: receptors, signaling pathways and cellular functions. Pharmacological Research 101: 30–40. https://doi.org/10.1016/j.phrs.2015.07.001.
Aránguiz-Urroz, P., D. Soto, A. Contreras, R. Troncoso, M. Chiong, J. Montenegro, D. Venegas, C. Smolic, P. Ayala, W.G. Thomas, S. Lavandero, and G. Díaz-Araya. 2009. Differential participation of angiotensin II type 1 and 2 receptors in the regulation of cardiac cell death triggered by angiotensin II. American Journal of Hypertension 22: 569–576. https://doi.org/10.1038/ajh.2009.32.
Vivar, R., C. Soto, M. Copaja, F. Mateluna, P. Aranguiz, J.P. Muñoz, M. Chiong, L. Garcia, A. Letelier, W.G. Thomas, S. Lavandero, and G. Díaz-Araya. 2008. Phospholipase C/protein kinase C pathway mediates angiotensin II-dependent apoptosis in neonatal rat cardiac fibroblasts expressing AT1 receptor. Journal of Cardiovascular Pharmacology. https://doi.org/10.1097/FJC.0b013e318181fadd.
Boza, P., P. Ayala, R. Vivar, C. Humeres, F.T. Cáceres, C. Muñoz, L. García, M.A. Hermoso, and G. Díaz-Araya. 2016. Expression and function of toll-like receptor 4 and inflammasomes in cardiac fibroblasts and myofibroblasts: IL-1β synthesis, secretion, and degradation. Molecular Immunology 74: 96–105. https://doi.org/10.1016/j.molimm.2016.05.001.
Turner, N.A. 2016. Inflammatory and fibrotic responses of cardiac fibroblasts to myocardial damage associated molecular patterns (DAMPs). Journal of Molecular and Cellular Cardiology 94: 189–200. https://doi.org/10.1016/j.yjmcc.2015.11.002.
Bolívar, S., R. Santana, P. Ayala, R. Landaeta, P. Boza, C. Humeres, R. Vivar, C. Muñoz, V. Pardo, S. Fernandez, R. Anfossi, and G. Diaz-Araya. 2017. Lipopolysaccharide activates Toll-like receptor 4 and prevents cardiac fibroblast-to-myofibroblast differentiation. Cardiovascular Toxicology 17: 458–470. https://doi.org/10.1007/s12012-017-9404-4.
Olivares-Silva, F., R. Landaeta, P. Aranguiz, S. Bolivar, C. Humeres, R. Anfossi, R. Vivar, P. Boza, C. Munoz, V. Pardo-Jimenez, C. Peiro, C.F. Sanchez-Ferrer, and G. Diaz-Araya. 2018. Heparan sulfate potentiates leukocyte adhesion on cardiac fibroblast by enhancing Vcam-1 and Icam-1 expression. Biochimica et Biophysica Acta, Molecular Basis of Disease 1864: 831–842. https://doi.org/10.1016/j.bbadis.2017.12.002.
Humeres, C., R. Vivar, P. Boza, C. Munoz, S. Bolivar, R. Anfossi, J.M. Osorio, F. Olivares-Silva, L. Garcia, and G. Diaz-Araya. 2016. Cardiac fibroblast cytokine profiles induced by proinflammatory or profibrotic stimuli promote monocyte recruitment and modulate macrophage M1/M2 balance in vitro. Journal of Molecular and Cellular Cardiology. https://doi.org/10.1016/j.yjmcc.2016.10.014.
Bracey, N.A., B. Gershkovich, J. Chun, A. Vilaysane, H.C. Meijndert, J.R. Wright Jr., P.W. Fedak, P.L. Beck, D.A. Muruve, and H.J. Duff. 2014. Mitochondrial NLRP3 protein induces reactive oxygen species to promote Smad protein signaling and fibrosis independent from the inflammasome *. Journal of Biological Chemistry 289: 19571–19584. https://doi.org/10.1074/jbc.M114.550624.
Gombault, A., L. Baron, and I. Couillin. 2013. ATP release and purinergic signaling in NLRP3 inflammasome activation. Frontiers in Immunology. https://doi.org/10.3389/fimmu.2012.00414.
Li, J., X. Teng, S. Jin, J. Dong, Q. Guo, D. Tian, and Y. Wu. 2019. Hydrogen sulfide improves endothelial dysfunction by inhibiting the vicious cycle of NLRP3 inflammasome and oxidative stress in spontaneously hypertensive rats. Journal of Hypertension 37: 1. https://doi.org/10.1097/HJH.0000000000002101.
Zhou, B., Y. Qiu, N. Wu, A.-D. Chen, H. Zhou, Q. Chen, Y.-M. Kang, Y.-H. Li, and G.-Q. Zhu. 2020. FNDC5 attenuates oxidative stress and NLRP3 inflammasome activation in vascular smooth muscle cells via activating the AMPK-SIRT1 signal pathway. Oxidative Medicine and Cellular Longevity 2020: 6384803. https://doi.org/10.1155/2020/6384803.
Bryan, N.B., A. Dorfleutner, Y. Rojanasakul, and C. Stehlik. 2009. Activation of inflammasomes requires intracellular redistribution of the apoptotic speck-like protein containing a caspase recruitment domain. The Journal of Immunology 182: 3173. https://doi.org/10.4049/jimmunol.0802367.
Jo, E.-K., J.K. Kim, D.-M. Shin, and C. Sasakawa. 2016. Molecular mechanisms regulating NLRP3 inflammasome activation. Cellular & Molecular Immunology 13: 148–159. https://doi.org/10.1038/cmi.2015.95.
Liang, Y.-D., W.-J. Bai, C.-G. Li, L.-H. Xu, H.-X. Wei, H. Pan, X.-H. He, and D.-Y. Ouyang. 2016. Piperine suppresses pyroptosis and interleukin-1β release upon ATP triggering and bacterial infection. Frontiers in Pharmacology. https://doi.org/10.3389/fphar.2016.00390.
Guo, H., J.B. Callaway, and J.P.Y. Ting. 2015. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nature medicine 21: 677–687. https://doi.org/10.1038/nm.3893.
Netea, M.G., A. Simon, F. van de Veerdonk, B.-J. Kullberg, J.W.M. Van der Meer, and L.A.B. Joosten. 2010. IL-1β processing in host defense: beyond the inflammasomes. PLOS Pathogens 6: e1000661. https://doi.org/10.1371/journal.ppat.1000661.
Rossol, M., M. Pierer, N. Raulien, D. Quandt, U. Meusch, K. Rothe, K. Schubert, T. Schöneberg, M. Schaefer, U. Krügel, S. Smajilovic, H. Bräuner-Osborne, C. Baerwald, and U. Wagner. 2012. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nature communications 3: 1329–1329. https://doi.org/10.1038/ncomms2339.
Wang, F., L. Huang, Z.Z. Peng, Y.T. Tang, M.M. Lu, Y. Peng, W.J. Mel, L. Wu, Z.H. Mo, J. Meng, and L.J. Tao. 2014. Losartan inhibits LPS + ATP-induced IL-1beta secretion from mouse primary macrophages by suppressing NALP3 inflammasome. Die Pharmazie 69: 680–684. https://doi.org/10.1691/ph.2014.3926.
Usui, F., K. Shirasuna, H. Kimura, K. Tatsumi, A. Kawashima, T. Karasawa, K. Yoshimura, H. Aoki, H. Tsutsui, T. Noda, J. Sagara, and Taniguchi, S.i. and Takahashi, M. 2015. Inflammasome activation by mitochondrial oxidative stress in macrophages leads to the development of angiotensin II–induced aortic aneurysm. Arteriosclerosis, Thrombosis, and Vascular Biology 35: 127–136. https://doi.org/10.1161/ATVBAHA.114.303763.
Horng, T. 2014. Calcium signaling and mitochondrial destabilization in the triggering of the NLRP3 inflammasome. Trends in Immunology 35: 253–261. https://doi.org/10.1016/j.it.2014.02.007.
Zhang, L.-L., S. Huang, X.-X. Ma, W.-Y. Zhang, D. Wang, S.-Y. Jin, Y.-P. Zhang, Y. Li, and X. Li. 2016. Angiotensin(1–7) attenuated angiotensin II-induced hepatocyte EMT by inhibiting NOX-derived H2O2-activated NLRP3 inflammasome/IL-1β/Smad circuit. Free Radical Biology and Medicine 97: 531–543. https://doi.org/10.1016/j.freeradbiomed.2016.07.014.
Zhao, M., M. Bai, G. Ding, Y. Zhang, S. Huang, Z. Jia, and A. Zhang. 2018. Angiotensin II stimulates the NLRP3 inflammasome to induce podocyte injury and mitochondrial dysfunction. Kidney Diseases 4: 83–94. https://doi.org/10.1159/000488242.
Malhotra, V. 2013. Unconventional protein secretion: an evolving mechanism. The EMBO Journal 32: 1660–1664. https://doi.org/10.1038/emboj.2013.104.
Lopez-Castejon, G., and D. Brough. 2011. Understanding the mechanism of IL-1β secretion. Cytokine & Growth Factor Reviews 22: 189–195. https://doi.org/10.1016/j.cytogfr.2011.10.001.
Eder, C. 2009. Mechanisms of interleukin-1β release. Immunobiology 214: 543–553. https://doi.org/10.1016/j.imbio.2008.11.007.
Qu, Y., L. Franchi, G. Nunez, and G.R. Dubyak. 2007. Nonclassical IL-1β secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. The Journal of Immunology 179: 1913. https://doi.org/10.4049/jimmunol.179.3.1913.
Piccioli, P., and A. Rubartelli. 2013. The secretion of IL-1β and options for release. Seminars in Immunology 25: 425–429. https://doi.org/10.1016/j.smim.2013.10.007.
Katsnelson, M., L. Rucker, H. Russo, and G. Dubyak. (2015). K+ Efflux agonists induce NLRP3 inflammasome activation independently of Ca2+ signaling. Journal of immunology (Baltimore, Md. : 1950) 194. https://doi.org/10.4049/jimmunol.1402658.
Galliher-Beckley, A.J., L.-Q. Lan, S. Aono, L. Wang, and J. Shi. 2013. Caspase-1 activation and mature interleukin-1β release are uncoupled events in monocytes. World journal of biological chemistry 4: 30–34. https://doi.org/10.4331/wjbc.v4.i2.30.
Butts, B., R.A. Gary, S.B. Dunbar, and J. Butler. 2015. The importance of NLRP3 inflammasome in heart failure. Journal of Cardiac Failure 21: 586–593. https://doi.org/10.1016/j.cardfail.2015.04.014.
Acknowledgements
We thank ANID and ACCDiS institutions for funding this work.
Funding
This work was supported by the FONDECYT (grant 1170425 and grant 1210627 to G. Díaz-Araya) and FONDAP ACCDiS grant 15130011.
Author information
Authors and Affiliations
Contributions
Jenaro Espitia-Corredor and Pía Boza: formal analysis, investigation, validation, writing–original draft, and writing–review and editing. Claudio Espinoza-Pérez, José Miguel Lillo, Constanza Rimassa-Taré, Victor Machuca, José Osorio-Sandoval, Raúl Vivar, Samir Bolívar, and Viviana Pardo-Jiménez: Formal analysis and investigation. Concepción Peiró, Carlos F. Sánchez-Ferrer, and Guillermo Díaz-Araya: Conceptualization, writing–review and editing, and supervision.
Corresponding author
Ethics declarations
Ethics Approval
The Bioethics Committee of the Faculty of Chemical and Pharmaceutical Sciences of the University of Chile with the code CBE2012-20 approved the management and care of laboratory animals used for the isolation of cardiac fibroblasts.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Espitia-Corredor, J.A., Boza, P., Espinoza-Pérez, C. et al. Angiotensin II Triggers NLRP3 Inflammasome Activation by a Ca2+ Signaling-Dependent Pathway in Rat Cardiac Fibroblast Ang-II by a Ca2+-Dependent Mechanism Triggers NLRP3 Inflammasome in CF. Inflammation 45, 2498–2512 (2022). https://doi.org/10.1007/s10753-022-01707-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-022-01707-z