Abstract
Integrin-α9 (ITGA9) and its corresponding ligands are involved in inflammatory and immune responses. The present study aimed to investigate whether ITGA9 participates in the development of chronic periodontitis (ChP) and to explore the underlying mechanisms. We collected gingival tissue and gingival crevicular fluid in vivo from patients to determine the levels of ITGA9 and its ligands. We cultured primary periodontal ligament cells (PDLCs) in vitro and applied small interfering RNA to knock down ITGA9 in order to analyze the changes of inflammatory cytokines and explore the related cellular signaling pathways. The expression level of ITGA9 was significantly higher in the gingiva of patients with ChP than that of healthy individuals. ITGA9 knockdown in the PDLCs inhibited the secretion of interleukin (IL)-1β, IL-6, and IL-8. Western blot analysis indicated that this change could be attributed to the regulation of the mitogen-activated protein kinase (MAPK) signaling pathway. ITGA9 plays a regulatory role in the homeostasis of ChP. The results of the present study provide potential insights into the treatment of periodontitis.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Chronic periodontitis (ChP) is a global inflammatory disease infecting almost half of the population over 30 in the USA [1, 2]. This chronic infectious disease is caused by bacterial plaques stemming from an imbalance between microorganisms and the host’s immune–inflammatory reaction, leading to the destruction of periodontal tissue and, often, relapse after conventional treatment [3, 4]. It not only leads to the resorption of alveolar bone, loss of teeth, and disorder of occlusion but also affects the host’s immune system [5,6,7].
Integrin-α9 (ITGA9) is a type of receptor that interacts with its corresponding ligands present in the extracellular matrix, including tenascin-C, osteopontin, and vascular endothelial growth factor-C [8,9,10]. It also participates in the activation of self-directed proliferation and proinflammatory properties of fibroblast-like synoviocytes enhanced via the focal adhesion kinase pathway [11], mediation of the interaction between prostate cancer and the bone microenvironment with tenascin-C [12], and regulation of the suppression of integrin α3β1 by α9β1 in the epidermis to control the paracrine resolution of wound angiogenesis [13]. Furthermore, in the mouse model, ITGA9 is involved in the development of autoimmune diseases [14]. Previous reports have indicated that the integrin family in periodontal ligament mesenchymal cells primarily promotes tissue formation and regeneration as a matrix [15]. However, research on the role of ITGA9 in ChP is still lacking.
We collected excised gingival tissue and gingival crevicular fluid (GCF) to analyze the differences in the expression of ITGA9 and its ligands between patients with and without ChP while determining the content of tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1β in the GCF. In this study, we used periodontal ligament cells (PDLCs) to further investigate the role of ITGA9 in an inflammatory environment and explore the underlying molecular mechanisms.
MATERIALS AND METHODS
Collection of Gingival Tissues
This clinical study was approved by the Medical Ethics Committee of the Wuhan University School of Stomatology (authorized c-14/2014). The present study was conducted in accordance with the Declaration of Helsinki and its guidelines and regulations.
Gingival tissues were collected from seven patients with ChP and ten patients with altered passive eruption or dentition defects requiring implant surgery. Each patient signed an informed consent form prior to surgery. Patients with periodontitis had a pocket depth of ≥ 5 mm and bleeding or suppuration upon probing after 6 weeks of supportive treatment in accordance with the surgical indications of ChP [16]. Patients with altered passive eruption or dentition defects were diagnosed as periodontally healthy. Subjects with smoking habits, hypertension, diabetes, or any systemic infectious diseases were excluded. After saline washing, the samples were fixed with 4% paraformaldehyde for 24 h, washed, dehydrated, and paraffin pack-buried. The samples were stained with hematoxylin and eosin (HE) and immunohistochemistry (IHC) and then analyzed.
Collection of GCF
In studying GCF, 66 patients between 18 and 50 years old were included. In accordance with the diagnostic criteria for ChP [17], the present study included 34 patients with grades B and C, stages III and IV periodontitis, and at least one pocket depth of ≥ 5 mm in the first molar. A total of 32 healthy individuals without periodontitis were eligible as controls (PD < 3 mm; no attachment loss, bleeding, or suppuration upon probing). The exclusion criteria included receiving dental treatment during or 3 months prior to the study, use of antibiotics, decompensated systemic disease, pregnant or lactating women, and aggressive periodontitis. In contrast to the gingival samples, patients whose GCF was collected had received no dental-related treatment within the past 3 months. A chromatography paper (Whatman, Little Chalfont, Buckinghamshire, UK) was cut into 2 mm × 8 mm specifications and placed in sterile Eppendorf (EP) tubes after high-temperature, high-pressure sterilization. We selected first molars with the most serious inflammation, removed saliva with cotton balls, dried the tooth surface and removed the underarm plaque. A filter strip was inserted in the gingiva sulcus at a depth of 2–3 mm for 30 s in proximal and distal sites of each tooth. Each site was taken twice, and the sampling interval of the same site was over 3 min to allow sufficient secretion. All operations were performed gently to prevent bleeding. Four filter strips per patient were placed together in an EP tube containing 500 mL of sterile phosphate-buffered saline (PBS) buffer (Hyclone, Marlborough, MA, USA).
HE Staining, IHC, and Histological Observation
The paraffin-embedded gingival samples were cut into 4-μm-thick sections. Then, the sections were dewaxed and rehydrated, and a portion of the serial sections was used for HE staining. The antigen for IHC was repaired via microwave using a pH 6.0 citric acid buffer. An endogenous peroxidase inhibitor was used for 10 min (ZSGB-Bio, Beijing, China) and blocked with animal non-immune serum (goat) for 1 h (MXB Biotechnologies, Fuzhou, China). The tissue range was incubated overnight with anti-ITGA9 (1:100 dilution; Proteintech, Wuhan, China) at 4 °C. The slides were treated with a secondary antibody (ZSGB-Bio, Beijing, China) for 1 h and reacted with avidin–biotin–peroxidase complexes. Finally, the sections were counterstained with hematoxylin, dehydrated, and fixed on glass slides. The completed tissue sections were scanned with a digital slide scanner (Pannoramic P250; 3D HISTECH, Budapest, Hungary) and examined using the CaseViewer software. The gingival tissue from the healthy and periodontitis groups was photographed, and the number of cells positive for ITGA9 was counted using ImageJ. The average of three random fields of view for each section (× 400 magnification) was obtained. The data of all samples were summarized for statistical analysis.
Determination of Tenascin-C, Osteopontin, TNF-α, IL-6, and IL-1β in GCF
The samples were fully eluted via centrifugation at 12,000 rpm for 15 min at 4 °C. Afterward, the supernatant was obtained and stored at − 80 °C for further analysis. The level of tenascin-C in the GCF was determined using a Human Tenascin-C ELISA Kit (EK1170, Boster, China) according to the manufacturer’s instructions.
The concentrations of osteopontin, TNF-α, IL-6, and IL-1β were measured using Cytometric Bead Array multifactor kits via flow cytometry (BioLegend, LEGENDplex Multi-Analyte Flow Assay Kit, San Diego, CA, USA). The LEGENDplex v8.0 software was used to analyze the results.
Isolation of Human PDLCs
Periodontal ligament tissue was extracted from 10 teeth, including premolars or third molars, from eight healthy donors aged 12–22 years at the Department of Oral and Maxillofacial Surgery, Hospital of Stomatology, Wuhan University.
After washing each tooth with PBS, the tissue on the middle root was scraped with a scalpel and cut into pieces smaller than 2 mm × 2 mm. The samples were digested with type I collagenase (Sigma, St. Louis, MO, USA) for 2 h at 37 °C before being spread evenly on the bottom wall of T25; they were maintained in alpha MEM with 1% penicillin and streptomycin plus and 20% fetal bovine serum (ScienCell, San Diego, CA, USA) under humidified conditions (37 °C, 5% CO2).
Cellular Immunofluorescence
Cells were inoculated onto slides and fixed with 4% paraformaldehyde in 50–60% confluency, ruptured by incubation with 1% Triton X-100 for 15 min, and blocked with bovine serum albumin for 1 h at 37 °C. Next, the cells were incubated with a primary antibody to ITGA9 (1:100; Proteintech, China) overnight at 4 °C. Afterward, the cells were washed and incubated with a secondary antibody conjugated to Cy3 (Beyotime, Shanghai, China) for 1 h, and the nuclei were dyed with 4′,6-diamidino-2-phenylindole (ZSGB-BIO, Beijing, China) for 3 min. Immunofluorescence was observed under a Zeiss fluorescence microscope, and images were captured.
ITGA9 Silencing by Small Interfering RNA (siRNA) in PDLCs
The PDLCs were transfected with PepMute (SignaGen, MD, USA) delivering either ITGA9 siRNA (5′-CGCUGGAAGAACAUCUACUAU-3′ and 5′-AUAGUAGAUGUUCUUCCAGCG-3′) or negative control siRNA encoding non-specific sequences for 24 h. When the PDLCs reached 50–60% confluency, the transfection efficiency was observed via real-time polymerase chain reaction (RT-PCR) (Applied Biosystems QuantStudio 6). Then, human TNF-α (Life Technologies, Carlsbad, CA, USA) was added to the medium (20 ng/mL) for 48 h.
RNA Isolation and Quantitative RT-PCR
Total RNA was isolated from the PDLCs using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and reverse-transcribed into first-strand cDNA (Takara, Kusatsu, Shiga, Japan) by an EP Mastercycler nexus in accordance with the manufacturer’s instructions. The generated cDNA was used as a template for RT-PCR using SYBR Green Master (Takara). Quantitative RT-PCR was performed in triplicate using the Applied Biosystems QuantStudio 6 with the SYBR Premix Ex Taq II Kit (Takara) to analyze the expression of ITGA9 (forward, 5′-CCAGATGCCCAGGGTCTACT-3′; reverse, 5′-GGGCCACATAGTGACGACAC-3′), IL-1β (forward, 5′-GCACGATGCACCTGTACGAT-3′; reverse, 5′-TGGAGAACACCACTTGTTGC-3′), IL-6 (forward, 5′-CATCACCATCTTCCAGGAG-3′; reverse, 5′-AGGCTGTTGTCATACTTCTC-3′), IL-8 (forward, 5′-CCAAGGAAAACTGGGTGCAGAG-3′; reverse, 5′-GGCACAGTGGAACAAGGACTTG-3′), tenascin-C (forward, 5′-CCGCAGAGGTGACATGTCAA-3′; reverse, 5′-CTCCATTCCAGGGTGATGCT-3′), and osteopontin (forward, 5′-ACCCTTCCAAGTAAGTCCAACG-3′; reverse, 5′-GGTGAGAATCATCAGTGTCATCTAC-3′) genes. The relative mRNA expressions of ITGA9, IL-1β, IL-6, and IL-8 were expressed as fold changes in the gene expression normalized by GAPDH, which was used as a housekeeping gene (forward, 5′-CTTTGGTATCGTGGAAGGACTC-3′; reverse, 5′-CAGTAGAGGCAGGGATGATGTT-3′). The gene expressions were calculated using the ΔΔCT method.
Western Blot Analysis
The cells were harvested in protein lysis buffer (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with intact protease inhibitor tablets (Roche Diagnostics, Indianapolis, IN, USA). The protein concentrations were measured via the bicinchoninic acid protein assay (Beyotime, Shanghai, China). Equal amounts of proteins (20 μg) were loaded onto each lane, separated by 10% SDS polyacrylamide gel, and transferred to a polyvinylidene difluoride membrane (Roche, UK) via a wet transfer system. The membrane was blocked by a buffer containing 5% nonfat milk and incubated overnight at 4 °C with the following primary antibodies: β-actin (1:10,000; Proteintech, China), ITGA9 (1:500; Proteintech), IL-6 (1:1000; Proteintech), IL-1β (1:1000; ABclonal, China), P38 (1:1000; CST, USA), phospho-p38 (1:1000; CST), phospho-p44/42 mitogen-activated protein kinase (MAPK) (Erk1/2) (1:1000; CST), p44/42 MAPK (Erk1/2) (1:1000; CST), phospho-stress-activated protein kinase/c-Jun NH2-terminal kinase (SAPK/JNK) (1:1000; CST) ,and SAPK/JNK (1:1000; CST). The membrane was washed thrice with Tris-buffered saline containing 0.05% Tween-20 and incubated with horseradish peroxidase-conjugated anti-rabbit (1:8000; Proteintech) or anti-mouse (1:10,000; Proteintech) secondary antibody for 1 h. The proteins were detected using the Enhanced Chemiluminescence Detection Reagents (Advansta, CA, USA) or the SuperSignal West Femto Trial Kit (Thermo Fisher Scientific, Waltham, MA, USA) and then exposed to the Odyssey LI-COR scanner.
Statistical Analysis
Data were analyzed using the Prism 6 software (GraphPad software, San Diego, CA, USA) and expressed as mean ± SD or mean ± SEM. All experiments were repeated at least thrice. The statistical analysis was performed using one-way ANOVA, and the difference between the periodontitis and control groups was analyzed using the t test. A P value < 0.05 was considered statistically significant (****, P < 0.0001; ***, P < 0.001; **, P < 0.01; *, P < 0.05; Ns, P > 0.05).
RESULTS
Elevated ITGA9 Expression Detected in the Gingiva of ChP Patients
The HE staining results showed a considerable inflammatory cell (macrophages and lymphocytes) infiltration and increased capillary network in the gingiva of patients with ChP. Numerous capillaries contained inflammatory substances. By contrast, the subepithelial connective tissue of the control group consisted of sequential and regular gingival fibroblasts (Fig. 1a).
Histological analysis of integrin-α9 (ITGA9) in the gingival samples of healthy controls and chronic periodontitis (ChP) patients. Gingival tissues were taken from 10 healthy individuals and 7 ChP patients, and 4 μm serial sections were obtained from each paraffin block. a Representative images of HE staining in gingival tissues from healthy controls and ChP groups. b Immunohistochemistry (IHC) staining for ITGA9 in gingival tissues from the two groups. c The percentage of ITGA9 was compared via IHC analysis; the average of three random fields of view of each section was obtained at × 400 magnification, and the difference was statistically significant (****, P < 0.0001).
The results of the IHC image scan suggested that the expression of ITGA9 increased on the cell membrane in the gingival epithelium and connective tissue (Fig. 1b, c).
Tenascin-C, TNF-α, and IL-1β Increased in the GCF of Patients with ChP, but Osteopontin Expression Showed No Significant Change
The ELISA and Cytometric Bead Array detection kits showed that the expression of tenascin-C was higher in the ChP group (P < 0.05), whereas no difference was observed in the osteopontin. A statistical difference in TNF-α (P < 0.05) and IL-1β (P < 0.01) was observed between the ChP and control groups. The mean expression level of IL-6 was higher in the ChP group but was not considered as a significant difference, as indicated by the results of the t test (P = 0.2028) (Fig. 2a, b and Table 1).
Determination of tenascin-C, osteopontin, tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1β in the gingival crevicular fluid (GCF). A total of 66 GCF samples (32 healthy individuals and 34 patients with newly diagnosed chronic periodontitis [ChP]) were collected. The data were analyzed via one-way ANOVA, and both groups were compared using an unpaired t test. The mean ± SEM of each group was calculated and represented using GraphPad version 6.0. Significant differences were indicated as follows: **P < 0.01; *, P < 0.05; and Ns, P > 0.05. a Tenascin-C was detected via ELISA kits, and the other four cytokines, i.e. osteopontin, TNF-α, IL-6, and IL-1β, were detected by Cytometric Bead Array multifactor kits. b Two sets of heat maps were sorted on the basis of tenascin-C concentration. Data were transformed using log2. Warm and cool colors indicate high and low concentrations, respectively.
PDLCs Expressed More ITGA9 and Its Ligands Under TNF-α Stimulation
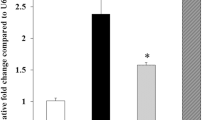
PDLCs isolated from the periodontal ligament contained ITGA9 on the membrane, as shown by the cellular immunofluorescence (Fig. 3a, c). The RT-PCR showed that ITGA9, tenascin-C, and osteopontin increased after TNF-α stimulation, suggesting that PDLCs produced more ITGA9 and ligands in an inflammatory environment (Fig. 3b).
Integrin-α9 (ITGA9) and its ligands were increased during tumor necrosis factor (TNF)-α stimulation of periodontal ligament cells (PDLCs). a Simple model of periodontal tissue. The periodontal ligament is located between the cementum and alveolar bone. b After 12 h of stimulation with TNF-α (20 ng/mL), the levels of ITGA9, tenascin-C, and osteopontin were analyzed via quantitative real-time polymerase chain reaction; GAPDH was used as an internal reference. The data represent three separate experiments with no fewer than three samples each. The values are presented as mean ± SD. ****, P < 0.0001; **, P < 0.01; *, P < 0.05. c The expression of ITGA9 in PDLCs was observed via an immunofluorescence analysis.
ITGA9 Regulated the Secretion of Inflammatory Factors in PDLCs Through the MAPK Signaling Pathway
After transfection with siRNA for 24 h, the expression of ITGA9 decreased to 40% in the PDLCs (Fig. 4a, b), and the secretion of IL-1β, IL-6, and IL-8 significantly decreased after stimulation by TNF-α (Fig. 4c, d). Western blot analysis showed that p-Erk1/2, p-JNK, and p-p38 were significantly inhibited after ITGA9 silencing (Fig. 4e). Thus, ITGA9 and its ligands may be responsible for secreting downstream inflammatory factors in PDLCs via the MAPK signaling pathway.
Integrin-α9 (ITGA9) regulates the secretion of the interleukin (IL) family in periodontal ligament cells (PDLCs) through the mitogen-activated protein kinase (MAPK) signaling pathway. a, b ITGA9 was silenced for 24 h using small interfering RNA, and the expression level was down-regulated to approximately 40% according to real-time polymerase chain reaction (RT-PCR) and Western blot analysis. c, d The PDLCs that knocked down ITGA9 were stimulated by human tumor necrosis factor (TNF)-α (20 ng/mL) for 48 h; the expressions of IL-1β, IL-,6 and IL-8 were measured via quantitative RT-PCR, and GAPDH was used as an internal reference. e The MAPK signaling pathway was inactivated to varying degrees during the down-regulation of ITGA9, as shown by the results of Western blot analysis. The data represent three separate experiments with no fewer than three samples each. The values are presented as mean ± SD. ****, P < 0.0001; ***, P < 0.001.
DISCUSSION
In our study, ITGA9 was regarded as a novel membrane receptor of the inflammatory response in periodontitis. Our results showed that ITGA9 spurs PDLCs to release the IL family and that the MAPK signaling pathway is also involved. Overall, the present study suggested that the knockdown of ITGA9 may be conducive to reducing the secretion of inflammatory cytokines in the periodontium.
ChP is a global disease that affects patients’ daily life [18], and its treatment requires long-term maintenance [4]. In 1994, ITGA9 was discovered [19] and has since been reported to be involved in specific inflammation and immune responses, such as those observed in prostate cancer or autoimmune diseases, with its corresponding ligands [12, 14, 20]. Furthermore, blockade of ITGA9 is a promising approach to treat synovial inflammation in rheumatoid arthritis [11]. However, the function of ITGA9 in oral diseases is rarely explored, which became the focus of the current research.
Regarding the principal treatment of ChP, a re-evaluation was conducted after supportive treatment for 6 weeks to reassess the necessity of surgery [21]. At this point, clinical inflammation had mostly subsided [22]. However, the results of HE staining still showed pathological changes in the subepithelial connective tissue of patients with ChP, and widespread inflammatory cell infiltration and tissue destruction were observed [23]. More importantly, the higher expression of ITGA9 in the ChP group revealed that this integrin is involved in the local inflammatory environment and may play a functional role in the periodontal microenvironment.
In contrast to the gingival sample, the GCF was obtained from newly diagnosed patients who had not undergone treatment, as the GCF secreted by individuals with ChP was more representative of the disease. In the present study, TNF-α and IL-1β increased in the ChP group, consistent with other studies on GCF [24, 25]. However, IL-6 showed no significant difference, likely because of the small sample size. A previous study has shown that IL-6 secretion in ChP patients is higher than that in healthy people [22]. In the lateral direction of three typical inflammatory factors during the test [26], the results were consistent with the peripheral blood serum of healthy individuals: the secretion of IL-1β was the highest in the GCF and peripheral serum, followed by IL-6, whereas TNF-α secretion was minimal [27]. Osteopontin showed no change between the two groups, but tenascin-C, a classic ligand for ITGA9, presented a significant difference, suggesting that ITGA9 and tenascin-C may be involved in the homeostasis of ChP.
The PDLCs are one of the most important cell types in the periodontium, constantly forming new fibers and cementum and reconstructing the alveolar bone [28]. They are crucial for the repair and regeneration of periodontal tissue and participate in the development of ChP [29, 30]. The TNF-α-stimulated PDLCs led to a higher expression of ITGA9, similar to the changes in the gingiva with ChP, and the up-regulation of tenascin-C was consistent with the GCF results. Tenascin-C could be secreted into the extracellular matrix, interact with ITGA9 on the membranes of adjacent cells, and promote the production of inflammatory mediators [31]. In addition, siRNAs that inhibit ITGA9 synthesis attenuated the production of TNF-α-mediated proinflammatory mediators (IL-1β, IL-6, and IL-8). One of the key players linking these pathways may be MAPK signaling, which showed relatively decreased p-Erk1/2/total p-Erk1/2, p-JNK/total JNK, and p-38/total p-38 ratios, suggesting that ITGA9 signaling is involved in cellular responses to TNF-α in the periodontal ligament.
The current drugs for ChP are often accompanied by supportive or surgical treatment [32, 33] and have an antibacterial focus [34]. In the present study, the knockdown of ITGA9 reduced the secretion of inflammatory mediators in PDLCs. An excessive interleukin factor response may cause a chronic inflammatory lesion, leading to the loss of periodontal ligament and alveolar bone [35]. At present, host modulation therapy is used as an adjunct to conventional periodontal treatment to ameliorate the destructive aspects of the host inflammatory response [36]. As the integrin family is present on the cell membrane as a specific extracellular matrix receptor, topical injection of its antagonist could have a blocking effect [11] and exert a smaller effect on the whole body. Thus, investigating the function of ITGA9 in periodontitis could offer clinical benefits in periodontal treatment in the future. Certainly, the conclusions of this dissertation require further study to obtain further validation.
References
Eke, P.I., B.A. Dye, L. Wei, G.D. Slade, G.O. Thornton-Evans, W.S. Borgnakke, G.W. Taylor, R.C. Page, J.D. Beck, and R.J. Genco. 2015. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. Journal of Periodontology 86 (5): 611–622.
Tonetti, M.S., S. Jepsen, L. Jin, and J. Otomo-Corgel. 2017. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. Journal of Clinical Periodontology 44 (5): 456–462.
Vieira Colombo, A.P., C.B. Magalhães, F.A. Hartenbach, R. Martins do Souto, and C. Maciel da Silva-Boghossian. 2016. Periodontal-disease-associated biofilm: A reservoir for pathogens of medical importance. Microbial Pathogenesis 94: 27–34.
Axelsson, P., B. Nystrom, and J. Lindhe. 2004. The long-term effect of a plaque control program on tooth mortality, caries and periodontal disease in adults. Results after 30 years of maintenance. Journal of Clinical Periodontology 31 (9): 749–757.
Branschofsky, M., T. Beikler, R. Schäfer, T.F. Flemming, and H. Lang. 2011. Secondary trauma from occlusion and periodontitis. Quintessence International 42 (6): 515–522.
Morishita, M., W. Ariyoshi, T. Okinaga, M. Usui, K. Nakashima, and T. Nishihara. 2013. A. actinomycetemcomitans LPS enhances foam cell formation induced by LDL. Journal of Dental Research 92 (3): 241–246.
Gaudilliere, D.K., et al. 2019. Systemic Immunologic Consequences of Chronic Periodontitis. Journal of Dental Research: 22034519857714.
Yokosaki, Y., et al. 1998. Identification of the ligand binding site for the integrin alpha9 beta1 in the third fibronectin type III repeat of tenascin-C. The Journal of Biological Chemistry 273 (19): 11423–11428.
Yokosaki, Y., N. Matsuura, T. Sasaki, I. Murakami, H. Schneider, S. Higashiyama, Y. Saitoh, M. Yamakido, Y. Taooka, and D. Sheppard. 1999. The integrin alpha(9)beta(1) binds to a novel recognition sequence (SVVYGLR) in the thrombin-cleaved amino-terminal fragment of osteopontin. The Journal of Biological Chemistry 274 (51): 36328–36334.
Liao, Y.F., P.J. Gotwals, V.E. Koteliansky, D. Sheppard, and L. van de Water. 2002. The EIIIA segment of fibronectin is a ligand for integrins alpha 9beta 1 and alpha 4beta 1 providing a novel mechanism for regulating cell adhesion by alternative splicing. The Journal of Biological Chemistry 277 (17): 14467–14474.
Emori, T., et al. 2017. Constitutive activation of integrin alpha9 augments self-directed hyperplastic and proinflammatory properties of fibroblast-like synoviocytes of rheumatoid arthritis. Journal of Immunology 199 (10): 3427–3436.
San Martin, R., R. Pathak, A. Jain, S.Y. Jung, S.G. Hilsenbeck, M.C. Piña-Barba, A.G. Sikora, K.J. Pienta, and D.R. Rowley. 2017. Tenascin-C and integrin alpha9 mediate interactions of prostate cancer with the bone microenvironment. Cancer Research 77 (21): 5977–5988.
Longmate, W.M., S.P. Lyons, S.V. Chittur, K.M. Pumiglia, L. van de Water, and C. DiPersio. 2017. Suppression of integrin alpha3beta1 by alpha9beta1 in the epidermis controls the paracrine resolution of wound angiogenesis. The Journal of Cell Biology 216 (5): 1473–1488.
Matsumoto, N., et al. 2017. A novel alpha9 integrin ligand, XCL1/lymphotactin, is involved in the development of murine models of autoimmune diseases. Journal of Immunology 199 (1): 82–90.
Barczyk, M., A.I. Bolstad, and D. Gullberg. 2013. Role of integrins in the periodontal ligament: organizers and facilitators. Periodontol 2000 63 (1): 29–47.
American Academy of P. 2002. The American Academy of Periodontology statement regarding gingival curettage. J Periodontol 73 (10): 1229–1230.
Dietrich, T., P. Ower, M. Tank, N.X. West, C. Walter, I. Needleman, F.J. Hughes, R. Wadia, M.R. Milward, P.J. Hodge, I.L.C. Chapple, and British Society of Periodontology. 2019. Periodontal diagnosis in the context of the 2017 classification system of periodontal diseases and conditions - implementation in clinical practice. British Dental Journal 226 (1): 16–22.
Albandar, J.M. 2002. Global risk factors and risk indicators for periodontal diseases. Periodontol 2000 29: 177–206.
Palmer, E.L., et al. 1993. Sequence and tissue distribution of the integrin alpha 9 subunit, a novel partner of beta 1 that is widely distributed in epithelia and muscle. The Journal of Cell Biology 123 (5): 1289–1297.
Danussi, C., L. del Bel Belluz, E. Pivetta, T.M. Modica, A. Muro, B. Wassermann, R. Doliana, P. Sabatelli, A. Colombatti, and P. Spessotto. 2013. EMILIN1/alpha9beta1 integrin interaction is crucial in lymphatic valve formation and maintenance. Molecular and Cellular Biology 33 (22): 4381–4394.
Drisko, C.H. 2001. Nonsurgical periodontal therapy. Periodontol 2000 25: 77–88.
Morrison, E.C., S.P. Ramfjord, and R.W. Hill. 1980. Short-term effects of initial, nonsurgical periodontal treatment (hygienic phase). Journal of Clinical Periodontology 7 (3): 199–211.
Cortes-Vieyra, R., C. Rosales, and E. Uribe-Querol. 2016. Neutrophil functions in periodontal homeostasis. Journal of Immunology Research 2016: 1396106.
Subbarao, K.C., et al. 2019. Gingival crevicular fluid: An overview. Journal of Pharmacy & Bioallied Sciences 11 (Suppl 2): S135–S139.
Toyman, U., et al. 2015. Evaluation of gingival crevicular fluid levels of tissue plasminogen activator, plasminogen activator inhibitor 2, matrix metalloproteinase-3 and interleukin 1-beta in patients with different periodontal diseases. Journal of Periodontal Research 50 (1): 44–51.
Garbers, C., S. Aparicio-Siegmund, and S. Rose-John. 2015. The IL-6/gp130/STAT3 signaling axis: Recent advances towards specific inhibition. Current Opinion in Immunology 34: 75–82.
Contasta, I., P. Pellegrini, A.M. Berghella, and D. Adorno. 2001. Cell cycle control in cellular homeostasis during the immune response: Interactions between TH1, TH2 cytokines, and Bcl2 and p53 molecules. Cancer Biotherapy & Radiopharmaceuticals 16 (1): 63–71.
Isaka, J., A. Ohazama, M. Kobayashi, C. Nagashima, T. Takiguchi, H. Kawasaki, T. Tachikawa, and K. Hasegawa. 2001. Participation of periodontal ligament cells with regeneration of alveolar bone. Journal of Periodontology 72 (3): 314–323.
Gould, T.R., A.H. Melcher, and D.M. Brunette. 1980. Migration and division of progenitor cell populations in periodontal ligament after wounding. Journal of Periodontal Research 15 (1): 20–42.
McCulloch, C.A., and S. Bordin. 1991. Role of fibroblast subpopulations in periodontal physiology and pathology. Journal of Periodontal Research 26 (3 Pt 1): 144–154.
Yokosaki, Y., E.L. Palmer, A.L. Prieto, K.L. Crossin, M.A. Bourdon, R. Pytela, and D. Sheppard. 1994. The integrin alpha 9 beta 1 mediates cell attachment to a non-RGD site in the third fibronectin type III repeat of tenascin. The Journal of Biological Chemistry 269 (43): 26691–26696.
Haffajee, A.D., S.S. Socransky, and J.C. Gunsolley. 2003. Systemic anti-infective periodontal therapy. A systematic review. Annals of Periodontology 8 (1): 115–181.
Herrera, D., B. Alonso, R. León, S. Roldán, and M. Sanz. 2008. Antimicrobial therapy in periodontitis: The use of systemic antimicrobials against the subgingival biofilm. Journal of Clinical Periodontology 35 (8 Suppl): 45–66.
Emingil, G., B. Han, G. Ozdemir, T. Tervahartiala, C. Vural, G. Atilla, H. Baylas, and T. Sorsa. 2012. Effect of azithromycin, as an adjunct to nonsurgical periodontal treatment, on microbiological parameters and gingival crevicular fluid biomarkers in generalized aggressive periodontitis. Journal of Periodontal Research 47 (6): 729–739.
Nibali, L., S. Fedele, F. D'Aiuto, and N. Donos. 2012. Interleukin-6 in oral diseases: A review. Oral Diseases 18 (3): 236–243.
Preshaw, P.M. 2018. Host modulation therapy with anti-inflammatory agents. Periodontol 2000 76 (1): 131–149.
Funding
The current work was supported by the National Natural Science Foundation of China (No. 81870776 and No. 81570946).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(XLS 25 kb)
Rights and permissions
About this article
Cite this article
Xu, S., Jiang, C., Liu, H. et al. Integrin-α9 and Its Corresponding Ligands Play Regulatory Roles in Chronic Periodontitis. Inflammation 43, 1488–1497 (2020). https://doi.org/10.1007/s10753-020-01226-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01226-9