Abstract
Immune dysfunction contributes to secondary infection and worse outcomes in sepsis. Regulatory T cells (Tregs) have been implicated in sepsis-induced immunosuppression. Nevertheless, the role of Tregs in secondary infection after sepsis remains to be determined. In the present study, a two-hit model which mimics clinical conditions was used and the potential role of Tregs in secondary Pseudomonas aeruginosa infection post-sepsis was investigated. Results showed that mice were susceptible to secondary P. aeruginosa infection 3 days, but not 7 days, post-cecal ligation and puncture (CLP). The levels of IL-17A, IL-1β, and IL-6 remained low in CLP mice after P. aeruginosa infection, while the levels of IL-10 increased significantly. Additionally, increased number of Tregs in both lung and spleen was observed in “two-hit” mice. Injection with PC61 (anti-CD25) mAb reduced the number of Tregs by 50% in spleen and 60% in lung of septic mice. This partial depletion of Tregs elevated IL-17A, IL-1β, and IL-6 production and decreased IL-10 levels in septic mice with P. aeruginosa infection, leading to lower bacterial load, attenuation of lung injury, and improvement of survival. The present findings demonstrate that Tregs play a crucial role in secondary P. aeruginosa infection after sepsis by modulating the inflammatory response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to microbial infection [1]. Due to the advances in early goal-directed therapy, new antibiotics, and adjunct strategies, more and more septic patients survive the phases of acute circulation failure and organ dysfunction. It has been found that about 60–70% of septic deaths occur in the late phase (≥ 3 days) of the disease and that most late deaths were related to ICU-acquired complications, including nosocomial infections [2]. Recently, a prospective study found that ICU-acquired infections contributed to overall mortality in septic patients [3]. Similarly, our previous study demonstrated that the risk of late death for septic shock patients with secondary infection was about 5.8 times higher than that without secondary infection [4].
It is well-known that, after a transient hyper-inflammatory phase, patients suffering from sepsis rapidly progress to a prolonged immunosuppressive state which is characterized by the defects of both innate and adaptive immune responses [5,6,7,8]. The unique immunosuppressive status of sepsis patients contributes to their susceptibility to secondary infection. CD4+CD25+ regulatory T cells (Tregs) have been shown to be crucial in regulating the immune responses in tumor immunity, transplantation tolerance, and infectious diseases [9,10,11]. Forkhead/winged helix transcription factor p3 (Foxp3) is principally found within the CD4+CD25+Treg cell population and plays an important role in the development and functionality of these cells [12]. Tregs control the immune response by inhibiting the activation of effector T lymphocytes and suppressing the maturation and function of antigen presenting cells [13]. The enhanced suppressive function of CD4+CD25+Tregs has been shown to be associated with fatal outcomes in burn patients [14]. Similar results were also observed in patients with septic shock. Additionally, various clinical studies illustrated that the expansion of CD4+CD25+Tregs and elevated Foxp3 levels were associated with a higher risk of nosocomial infection in critically ill patients including sepsis [15,16,17]. So, we hypothesized that depletion of Tregs might be an effective strategy to prevent secondary infection in sepsis.
It should be noted that, at different phases of sepsis, the same immune cells may have different functions. It has been reported that Foxp3+Tregs are crucial for minimizing host tissue damage when the initial cytokine storm has significantly decreased in septic animals and that these cells are required for recovery from severe sepsis [7]. Moreover, the complete loss of Foxp3+Tregs has been proven to increase the number of deaths from sepsis in animal models [18]. As about 80% of CD25+lymphocytes and 90% of CD4+CD25+T cells express Foxp3, the monoclonal rat anti-mouse CD25 clone PC61 was widely used to deplete Tregs in experimental studies, and a 30–60% reduction in Foxp3+cells in the spleen was observed after PC61 mAb treatment [19,20,21]. So, PC61 mAb was used to deplete Tregs in the present study. We found that Tregs contributed to immunosuppression and secondary infection in sepsis. Depletion of Tregs by PC61 mAb treatment rescues septic mice with secondary Pseudomonas aeruginosa infection. Thus, Tregs may be a potential therapeutic target for limiting secondary infection in sepsis.
MATERIALS AND METHODS
Mice
C57BL/6 female mice, 6–8 weeks old, were purchased from Shanghai Slack Laboratory Animal Co. Ltd. and raised at the Wenzhou Medical University. All mice experiments were conducted in accordance with the guidelines proposed by the Wenzhou Medical University Institutional Animal Care and Use Committee.
“Two-Hit” Mice Model
Cecal ligation and puncture (CLP) was performed as the first hit and intratracheal injection of P. aeruginosa was the second hit. In brief, mice were anesthetized with halothane (5% induction, 2% maintenance) and the abdomen was prepped. Then, a 1-cm midline incision was made and the cecum was exposed. The cecum was punctured through and through using a 27-gauge needle with 50% cecal ligation. Sham animals underwent the same procedure without cecum ligation and puncture. The mice were resuscitated with a subcutaneous injection of 0.5 ml sterile saline. Imipenem-cilastatin (Tienam, 25 mg/kg in 0.5 ml of saline) was given starting 6 hours (h) after surgery and continuing every 12 h for 2 days (d). Over 7 days, the mortalities of the CLP animals with and without antibiotic treatment were approximately 10–20 and 40%, respectively.
P. aeruginosa (ATCC27316) were prepared as previously described [22]. In brief, P. aeruginosa was grown overnight at 37 °C until reaching a stationary phase. Cells were resuspended in sterile saline and the bacterial concentration was calculated with the DENSIMAT method. At 3 and 7 days post-CLP, surviving mice were anesthetized again with mixture of ketamine and xylazine. Mice were held in a “head-up” position and their tracheas were exposed. Then, 20 μl of P. aeruginosa suspension (4 × 105 colony-forming unit [CFU]) was slowly injected via the trachea. Mice receiving an equal volume of sterile saline were used as controls.
Depletion of Tregs
Evidence illustrated that 200 μg/mouse PC61 mAb was enough to maintain a persistent low number of Tregs in septic mice [23]. In the present study, mice were i.p. injected with 200 μg of PC61 (BioLegend, San Diego, CA) or rat IgG1 (BioLegend, San Diego, CA) in 200 μl PBS 24 h before P. aeruginosa infection. Then, the changes in proportion of Tregs in spleen and the number of Foxp3+ cells in lung were analyzed.
Lung Tissue and Bronchoalveolar Lavage Fluid Collection
The mice were sacrificed by overdose anesthesia 24 h after intratracheal injection of P. aeruginosa or saline. Lung tissues and bronchoalveolar lavage fluids (BALFs) were collected immediately. The whole lung tissues were perfused with 4% paraformaldehyde and saved in paraformaldehyde for an additional 36 h for histologic examination. BALFs were collected by lavaging the lung tissue with 2 × 0.5 ml saline. In brief, the trachea was exposed again and 0.5 ml sterile saline was slowly injected into the lungs and retrieved carefully. The bronchoalveolar lavage operation was conducted twice for each mouse.
Cell Preparation and Analysis of Spleen Tregs
Lymphocytes were isolated from single cell suspensions of spleens from sham and septic mice using lymphoprep according to the manufacturer’s instructions. In order to analyze the proportion of CD4+CD25+Foxp3+Treg cells, splenic lymphocytes (1 × 106) were stained with anti-mouse CD4-FITC antibody and CD25-APC antibody (eBioscience, San Diego, CA) or isotype control antibodies for 30 min on ice. For intracellular Foxp3 staining, cells were stained with anti-mouse Foxp3-PE (eBioscience, San Diego, CA) antibody or isotype antibody after permeabilization treatment. Cells were analyzed by flow cytometry using a FACScan (BD Bioscience, Mountain View, CA).
Lung Immunohistochemical Analysis of Foxp3+ Cells
Lung immunohistochemical examination was carried out on formalin-fixed paraffin-embedded tissue with anti-Foxp3 mAb (eBioscience, San Diego, CA) according to the manufacturer’s instructions. The number of Foxp3+ cells was calculated by a pathologist blinded to the study protocol.
Bacteria Culture
In order to evaluate the lung bacterial clearance ability, BALF samples were collected 24 h after P. aeruginosa administration and bacterial culture tests were performed. The number of lung bacterial colonies was calculated by the gradient dilution and plate paint isolation methods. The lung BALFs were collected immediately after the mice were sacrificed and BALFs (100 μl) were spread evenly on the P. aeruginosa selective medium for bacterial culturing. The number of bacterial colonies was observed and calculated 24 h later.
Histopathology of Lung Tissues
Mice lung tissues were fixed in 4% paraformaldehyde for 36 h. Routine histologic techniques were performed to lung specimens. Lungs were embedded in paraffin and 3-μm sections of whole lung were stained with H&E for examination. Qualitative and semi-quantitative histopathological evaluations were carried out by a pathologist blinded to the study protocol.
Cytokine Quantification
IL-1β, IL-6, and IL-17A play crucial roles in host defense against P. aeruginosa infection [24,25,26]. IL-10 has been reported to be an important cytokine mediator of sepsis-induced immunosuppression [27]. Lung tissues were harvested and lung homogenates were prepared for cytokine analysis. IL-1β, IL-6, IL-10, and IL-17A concentrations were analyzed in duplicate using ELISA kits from R&D Systems in accordance with the manufacturer’s guidance.
Statistical Analysis
Data were represented as the mean ± SEM. Differences between two groups were assessed using Student’s t test, and comparisons between more than two groups were done with the Kruskall-Wallis test or one-way analysis of variance (ANOVA). Survival was analyzed by log-rank test. All calculations were made by the Prism 5.0 statistical program (GraphPad software, San Diego, CA). P < 0.05 were accepted as statistically significant.
RESULTS
Time Course Analysis of Susceptibility to P. aeruginosa Infection After Sepsis
As clinical data illustrated that most secondary infections in septic patients were observed 3 days after admission, the susceptibility of mice to P. aeruginosa infection was evaluated 3 and 7 days post-CLP. The mortality of mice was examined every 24 h up to 14 days post-CLP. As shown in Fig. 1a, the mortality of mice infected with P. aeruginosa 3 days post-CLP was higher than that of animals undergoing CLP or P. aeruginosa infection alone. Histological changes were assessed in lung. As shown in Fig. 1c, significantly higher numbers of inflammatory cells, more fluid accumulation, and increased lung histopathology semi-quantitative scores were observed in the mice undergoing CLP followed by P. aeruginosa pneumonia 3 days after surgery compared to the sham mice infected with P. aeruginosa (p < 0.0001). The number of bacterial colonies was calculated 24 h after culturing the BALFs. As expected, no bacterial colonies were observed in BALFs from both septic mice and sham controls. As shown in Fig. 1d, the number of bacterial colonies in septic mice infected with P. aeruginosa 3 days post-CLP was much higher than that of sham mice with P. aeruginosa infection (4420 ± 1273 CFU versus 200 ± 72 CFU) (p < 0.0001). Nevertheless, when challenged with P. aeruginosa 7 days after surgery, there were no statistically significant differences in the histopathological alterations, mortalities, and the number of bacterial colonies between septic mice and sham controls (Fig. 1b–d).
Time course analysis of susceptibility to P. aeruginosa infection after sepsis. Survival curves of septic mice with secondary P. aeruginosa infection 3 days (a) and 7 days (b) post-CLP. Septic mice and sham controls were infected with P. aeruginosa 3 or 7 days after surgery. Results of three independent studies were combined. n = 10 per group. c Pathological score of lung tissue in each group 24 h after secondary infection. Pathological scores of lung tissues in six groups were shown (n = 8). d Lung colony-forming units (CFUs). BAL fluids of each groups were collected for culturing and the number of bacterial colonies was counted 24 h later (n = 8). PA, P. aeruginosa; Sal, saline; CLP, cecal ligation and puncture. ***p < 0.001.
Lung Cytokine Levels in Septic Mice with P. aeruginosa Infection
As shown in Fig. 2, there were increased levels of pro-inflammatory cytokines, including IL-1β (p < 0.0001), IL-6 (p < 0.0001), and IL-17A (p < 0.05), in the lungs of septic mice infected with P. aeruginosa 3 days post-CLP when compared to sham mice with P. aeruginosa infection. Meanwhile, mice infected with P. aeruginosa 3 days post-CLP had increased levels of anti-inflammatory cytokine IL-10 (p < 0.0001) (Fig. 2). However, results showed that there were no statistically significant differences in cytokine levels between CLP mice infected with P. aeruginosa and sham controls with P. aeruginosa infection 7 days after surgery (Fig. 2).
Lung cytokine levels in septic mice with P. aeruginosa infection. CLP mice and sham controls were received P. aeruginosa via the trachea as a second hit 3 or 7 days after surgery. 24 h later, the lungs of each group were collected. The protein levels of IL-17A, IL-1β, IL-6, and IL-10 were measured in tissue lysates by ELISA test (n = 8). PA, P. aeruginosa; Sal, saline; CLP, cecal ligation and puncture. *p < 0.05, ***p < 0.001.
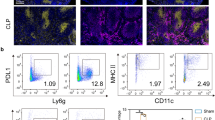
Increased Tregs in Post-Septic Mice After P. aeruginosa Challenge
The number of Tregs in the spleen and lungs was evaluated 24 h after secondary P. aeruginosa infection. The results showed that there was an almost twofold increase in the number of Foxp3+ cells in lung tissues of septic mice after secondary P. aeruginosa infection 3 days post-CLP (11.9 ± 3.843 versus 6.1 ± 3.855, p < 0.0001) (Fig. 3A). The proportion of regulatory T cells in the spleens of CLP mice infected with P. aeruginosa 3 days after surgery was about 1.6 times higher than in CLP mice (8.47 ± 1.259 versus 5.3 ± 1.035%, p < 0.05) (Fig. 3B). Similar results were observed 7 days after surgery (Fig. 3B).
Increased Tregs in post-septic mice after P. aeruginosa challenge. Mice underwent CLP or sham surgery and then received P. aeruginosa via the trachea as a second hit 3 or 7 days after surgery. (A) Immunohistochemical analysis of Foxp3+ cells in lung tissues (image: × 400). (B) Flow cytometric analysis of CD4+CD25+Foxp3+ cells in spleen (n = 8). a, isotype; b, sham + sal.; c, sham + PA; d, CLP + sal. 3d; e, CLP + PA 3d; f, CLP + sal. 7d; g, CLP + PA 7d. PA, P. aeruginosa; Sal, saline; CLP, cecal ligation and puncture. *p < 0.05, **p < 0.01, ***p < 0.001.
Depletion of Tregs Improved Survival of Septic Mice After Secondary Infection
Given the above results that the increased number of Tregs was associated with secondary P. aeruginosa infection in sepsis, PC61 mAb (200 μg/mouse, i.p.) was used to deplete the Tregs in septic mice. Results showed that injection with PC61 mAb reduced the number of Tregs by about 50% in spleen (7.96 ± 1.418 versus 3.992 ± 1.114%) and 60% in lung (15.1 ± 6.935 versus 5.9 ± 2.331%) of septic mice (Fig. 4B) (p < 0.001). Additionally, depletion Tregs with PC61 mAb significantly improved survival of septic mice with P. aeruginosa infection (p < 0.05) (Fig. 5a).
The number of Foxp3+ cells in lung and regulatory T cells in spleen of mice treated with PC61 mAb or IgG1 mAb. Mice that underwent CLP were infected with P. aeruginosa 3 days after surgery. PC61 mAb or IgG1 mAb was administrated 24 h before P. aeruginosa infection. (A) Immunohistochemical analysis of Foxp3+ cells in lung tissues (image: × 400). (B) Flow cytometric analysis of CD4+CD25+ cells (a) and CD4+CD25+Foxp3+ cells (b) in spleen. PA, P. aeruginosa; Sal, saline; CLP, cecal ligation and puncture. ***p < 0.001, compared with lgG1 mAb group.
Decreased susceptibility of septic mice to P. aeruginosa infection after Treg depletion. Mice undergoing CLP were infected with P. aeruginosa 3 days after surgery. PC61 mAb or IgG1 mAb was administrated 24 h before P. aeruginosa infection a survival curves of mice treated with PC61 mAb or IgG1 mAb (n = 10). b Lung colony-forming units (CFUs). BAL fluids of mice treated with PC61 mAb and IgG1 mAb were collected for culturing. The number of bacterial colonies was counted 24 h later (n = 8). c Pathological score of lung tissue in each group 24 h after secondary infection. Pathological scores of lung tissues in two groups were shown (n = 8). PA, P. aeruginosa; Sal, saline. *p < 0.05, ***p < 0.001, compared with lgG1 mAb group.
Decreased Susceptibility of Septic Mice to P. aeruginosa Infection After Treg Depletion
As shown in Fig. 5b, the number of bacterial colonies in lung was decreased in mice treated with PC61 mAb compared with IgG1-treated mice (2237 ± 1170 CFU versus 4775 ± 1026 CFU) (p < 0.01). After Treg depletion, the morphology of the lung tissue was observed by histological examination. As shown in Fig. 5c, decreased numbers of inflammatory cells, less fluid accumulation, and lower lung histopathology semi-quantitative scores were observed in mice treated with PC61 mAb compared to IgG1-treated mice (7 ± 1.07 versus 2.5 ± 0.53, p < 0.0001).
Lung Cytokine Levels in Mice After Treg Depletion
The protein levels of IL-17A, IL-1β, IL-6, and IL-10 were measured in lung tissue by using ELISA kits. As indicated in Fig. 6, the production of IL-1β, IL-6, and IL-17A was significantly increased after Treg depletion in septic mice that underwent P. aeruginosa pneumonia (p < 0.05). Additionally, the protein levels of IL-10 in lung were decreased after Treg depletion (p < 0.001) (Fig. 6).
Lung cytokine levels in mice after Treg depletion. Septic mice were infected with P. aeruginosa 3 days post-CLP. PC61 mAb or IgG1 mAb was given 24 h before P. aeruginosa infection. The protein levels of IL-17A, IL-1β, IL-6, and IL-10 were measured in tissue lysates by ELISA test (n = 8). *p < 0.05, **p < 0.01, ***p < 0.001, compared with lgG1 mAb group.
DISCUSSION
Sepsis is one of the leading causes of death in critically ill patients. Due to the improved treatment strategies, more and more septic patients survive the initial hyper-inflammatory phase and enter a sustained immunosuppressive phase. The immune dysfunction in sepsis lead to the host cannot effectively control the primary infection. More importantly, septic patients who survive the early phase of the disease are at high risk for secondary nosocomial infection [28]. Evidence illustrated that nosocomial infections occurred in 13.5% of sepsis ICU admissions, and the incidence of secondary infection reached 39% in patients with septic shock [29, 30]. Recently, secondary infection has been proved to be the major cause of death among patients with sepsis [4], and effective prevention of secondary infection has become a potential measure to further reduce the mortality of the disease.
There are several factors associated with the incidence of secondary infection in septic patients, including advanced age, the severity of the disease, and the length of ICU stay. Nevertheless, these factors are relatively objective causes of secondary infection and it is hard to control them in clinical work. As mentioned above, immune dysfunction in sepsis is associated with increased incidence of secondary infection. Previous studies have shown that the production of IL-10 contributed to the secondary infection in sepsis [27]. In the present study, the levels of IL-10 in mice infected with P. aeruginosa 3 days post-CLP increased significantly. It is reported that IL-17 exerts a protective effect in the airway by favoring the clearance of pathogens during acute P. aeruginosa infection [31]. Our results demonstrated that the levels of IL-17A in lung increased significantly after acute P. aeruginosa infection in sham-operated mice. However, secondary P. aeruginosa infection 3 days post-CLP did not cause an increase in IL-17A levels. Similarly, at the same time point, the levels of IL-1β and IL-6, which are critical for the host defense against pneumonia due to P. aeruginosa, remained low in mice which underwent CLP followed by P. aeruginosa infection. Additionally, significant increases in the number of bacterial colonies were observed in post-CLP mice after P. aeruginosa infection while the inflammatory cell infiltration in lung was increased, as consistently observed in previous studies. These seemingly contradictory results may be explained by the fact that the neutrophils and macrophages which are recruited to the lung in septic patients with secondary infection appear to have impaired abilities to secrete pro-inflammatory cytokines and clear pathogens [27, 32].
It has been demonstrated that Tregs were associated with the defects in immune cell responses in sepsis. Increased levels of pro-inflammatory cytokines and enhanced immune cell responses after the depletion of Tregs were observed in many studies [33,34,35]. In the present study, the levels of CD4+CD25+Foxp3+Tregs in spleen and Foxp3+ cells in lung were increased in post-septic mice after secondary infection, indicating that the expansion of these cells may contribute to secondary infection in sepsis. Accordingly, Tregs were depleted using the monoclonal rat anti-mouse CD25 clone PC61. Although not all Foxp3+T cells express CD25, PC61 mAb treatment can cause a 30–60% reduction in Foxp3+Tregs and enhance the host immune response against tumors and pathogens [19,20,21]. After PC61 mAb treatment, the levels of IL-1β, IL-6, and IL-17A were increased and the production of IL-10 was decreased significantly in the “two-hit” model. Due to the improvement of immune response after Treg depletion in sepsis, the lung bacterial clearance ability was enhanced. As a result, the inflammatory cell recruitment to lung and the mortality of the animals were decreased.
Sepsis is a highly complex and non-linear process. If immunotherapy is applied during the wrong phase of the sepsis, it may worsen outcomes by causing excessive inflammation or by impairing the immune response. Unfortunately, the markers used to guide immunotherapy treatment are still lacking, although HLA-DR, PD-L1, and others are currently under consideration [36,37,38] . While a single biomarker does not reflect the overall degree of immunosuppression, the shift in host susceptibility to pathogens may be a useful method to evaluate the immune status of sepsis in experimental studies. In the present study, increased susceptibility to P. aeruginosa infection 3 days post-CLP and elevated levels of Tregs were observed. So, Tregs were depleted before secondary infection and improved host response to P. aeruginosa in septic mice was observed. Additionally, an antibiotic was used to help CLP mice survive the early phase of sepsis to mimic clinical conditions in our study.
CONCLUSION
In conclusion, our current findings demonstrate that Tregs contribute to secondary infection in sepsis by modulating the inflammatory response. While more research is needed, our work suggests that Tregs may be an effective target for the development of drugs that prevent secondary infection and reduce mortality in sepsis.
References
Hotchkiss, R.S., and I.E. Karl. 2003. The pathophysiology and treatment of sepsis. New England Journal of Medicine 348 (2): 138–150.
Daviaud, F., D. Grimaldi, A. Dechartres, J. Charpentier, G. Geri, N. Marin, J.D. Chiche, A. Cariou, J.P. Mira, and F. Pène. 2015. Timing and causes of death in septic shock. Annals of Intensive Care 5 (1): 16.
van Vught, L.A., P.M. Klein Klouwenberg, C. Spitoni, B.P. Scicluna, M.A. Wiewel, J. Horn, M.J. Schultz, P. Nürnberg, M.J. Bonten, and O.L. Cremer. 2016. Incidence, risk factors, and attributable mortality of secondary infections in the intensive care unit after admission for sepsis. Jama 315 (14): 1469–1479.
Zhao, G.J., D. Li, Q. Zhao, J.X. Song, X.R. Chen, G.L. Hong, M.F. Li, B. Wu, and Z.Q. Lu. 2016. Incidence, risk factors and impact on outcomes of secondary infection in patients with septic shock: an 8-year retrospective study. Scientific Reports 6: 38361.
Hotchkiss, Richard S., Guillaume Monneret, and Didier Payen. 2013. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infectious Diseases 13 (3): 260–268.
Jiang, L.N., Y.M. Yao, and Z.Y. Sheng. 2012. The role of regulatory T cells in the pathogenesis of sepsis and its clinical implication. Journal of Interferon & Cytokine Research the Official Journal of the International Society for Interferon & Cytokine Research 32 (8): 341–349.
Delano, M.J., and P.A. Ward. 2016. Sepsis-induced immune dysfunction: can immune therapies reduce mortality? Journal of Clinical Investigation 126 (1): 23–31.
Hotchkiss, R.S., G. Monneret, and D. Payen. 2013. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nature Reviews Immunology 13 (12): 862–874.
Son, C.H., J.H. Bae, D.Y. Shin, H.R. Lee, W.S. Jo, K. Yang, and Y.S. Park. 2015. Combination effect of regulatory T-cell depletion and ionizing radiation in mouse models of lung and colon cancer. International Journal of Radiation Oncology Biology Physics 92 (2): 390–398.
Morita, R., Y. Hirohashi, and N. Sato. 2012. Depletion of Tregs in vivo: a promising approach to enhance antitumor immunity without autoimmunity. Immunotherapy 4 (11): 1103–1105.
Onyilagha, C., I. Okwor, S. Kuriakose, R. Singh, and J. Uzonna. 2014. Low-dose intradermal infection with trypanosoma congolense leads to expansion of regulatory T cells and enhanced susceptibility to reinfection. Infection & Immunity 82 (3): 1074–1083.
Zhao, G.J., Z.Q. Lu, L.M. Tang, Z.S. Wu, D.W. Wang, J.Y. Zheng, and Q.M. Qiu. 2012. Curcumin inhibits suppressive capacity of naturally occurring CD4+CD25+ regulatory T cells in mice in vitro. International Immunopharmacology 14 (1): 99–106.
Nascimento, D.C., J.C. Alvesfilho, F. Sônego, S.Y. Fukada, M.S. Pereira, C. Benjamim, D.S. Zamboni, J.S. Silva, and F.Q. Cunha. 2010. Role of regulatory T cells in long-term immune dysfunction associated with severe sepsis. Critical Care Medicine 38 (8): 1718–1725.
Huang, Li Feng, Yong Ming Yao, Ning Dong, Yan Yu, Li Xin He, and Zhi Yong Sheng. 2010. Association between regulatory T cell activity and sepsis and outcome of severely burned patients: a prospective, observational study. Critical Care 14 (1):R3.
Li, J., M. Li, L. Su, H. Wang, K. Xiao, J. Deng, Y. Jia, G. Han, and L. Xie. 2015. Alterations of T helper lymphocyte subpopulations in sepsis, severe sepsis, and septic shock: a prospective observational study. Inflammation 38 (3): 995–1002.
Conway, Morris A., N. Anderson, M. Brittan, T.S. Wilkinson, D.F. Mcauley, J. Antonelli, C. Mcculloch, L.C. Barr, K. Dhaliwal, and R.O. Jones. 2013. Combined dysfunctions of immune cells predict nosocomial infection in critically ill patients. British Journal of Anaesthesia 111 (5): 778–787.
Saito, K., T. Wagatsuma, H. Toyama, Y. Ejima, K. Hoshi, M. Shibusawa, M. Kato, and S. Kurosawa. 2008. Sepsis is characterized by the increases in percentages of circulating CD4+CD25+ regulatory T cells and plasma levels of soluble CD25. Tohoku Journal of Experimental Medicine 216 (1): 61–68.
Kühlhorn, F., M. Rath, K. Schmoeckel, K. Cziupka, H.H. Nguyen, P. Hildebrandt, T. Hünig, T. Sparwasser, J. Huehn, and C. Pötschke. 2013. Foxp3+ regulatory T cells are required for recovery from severe sepsis. PLoS One 8 (5): e65109.
Huss, D.J., A.F. Pellerin, B.P. Collette, A.K. Kannan, L. Peng, A. Datta, B.T. Wipke, and J.D. Fontenot. 2016. Anti-CD25 monoclonal antibody Fc variants differentially impact Treg cells and immune homeostasis(1). Immunology 148 (3): 276–286.
Couper, Kevin N., Daniel G. Blount, J. Brian De Souza, Isabelle Suffia, Yasmine Belkaid, and Eleanor M. Riley. 2007. Incomplete depletion and rapid regeneration of Foxp3+ regulatory T cells following anti-CD25 treatment in malaria-infected mice. Journal of Immunology 178 (7): 4136–4146.
Tenorio, Eda Patricia, Jonadab Efraín Olguín, Jacquelina Fernández, Pablo Vieyra, and Rafael Saavedra. 2015. Reduction of Foxp3+ cells by depletion with the PC61 mAb induces mortality in resistant BALB/c mice infected with Toxoplasma gondii. BioMed Research International 2010 (5):786078.
Flanders, S.A., H.R. Collard, and S. Saint. 2006. Nosocomial pneumonia: state of the science. American Journal of Infection Control 34 (2): 84–93.
Nicholas, Wisnoski, Chung Chun-Shiang, Chen Yaping, Huang Xin, and Ayala Alfred. 2007. The contribution of CD4+ CD25+ T-regulatory-cells to immune suppression in sepsis. Shock 27 (3): 251.
Liu, J., Y. Feng, K. Yang, Q. Li, L. Ye, L. Han, and H. Wan. 2011. Early production of IL-17 protects against acute pulmonary Pseudomonas aeruginosa infection in mice. Fems Immunology & Medical Microbiology 61 (2): 179–188.
Cole, N., M. Krockenberger, S. Bao, K.W. Beagley, A.J. Husband, and M. Willcox. 2001. Effects of exogenous interleukin-6 during Pseudomonas aeruginosa corneal infection. Infection & Immunity 69 (6): 4116–4119.
Karmakar, Mausita, Yan Sun, Amy G. Hise, Arne Rietsch, and Eric Pearlman. 2012. IL-1β processing during Pseudomonas aeruginosa infection is mediated by neutrophil serine proteases and is independent of NLRC4 and Caspase-1. Journal of Immunology 189 (9):4231–4235.
Muenzer, J.T., C.G. Davis, K. Chang, R.E. Schmidt, W.M. Dunne, C.M. Coopersmith, and R.S. Hotchkiss. 2010. Characterization and modulation of the immunosuppressive phase of sepsis. Infection & Immunity 78 (4): 1582–1592.
Hall, Mark W., Nina L. Knatz, Carol Vetterly, Steven Tomarello, Mark D. Wewers, Hans Dieter Volk, and Joseph A. Carcillo. 2011. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Medicine 37 (3): 525–532.
Tao, L., B. Hu, V.D. Rosenthal, X. Gao, and L. He. 2011. Device-associated infection rates in 398 intensive care units in Shanghai, China: International Nosocomial Infection Control Consortium (INICC) findings. International Journal of Infectious Diseases 15 (11): e774–e780.
Rosenthal, V.D., D.G. Maki, R. Salomao, C.A. Moreno, Y. Mehta, F. Higuera, L.E. Cuellar, O.A. Arikan, R. Abouqal, and H. Leblebicioglu. 2006. Device-associated nosocomial infections in 55 intensive care units of 8 developing countries. Annals of Internal Medicine 145 (8): 582–591.
Bayes, H.K., N.D. Ritchie, and T.J. Evans. 2016. Interleukin-17 is required for control of chronic lung infection caused by Pseudomonas aeruginosa. Infection & Immunity 84 (12): 3507–3516.
Steinhauser, M.L., C.M. Hogaboam, S.L. Kunkel, N.W. Lukacs, R.M. Strieter, and T.J. Standiford. 1999. IL-10 is a major mediator of sepsis-induced impairment in lung antibacterial host defense. Journal of Immunology 162 (1): 392.
Ataera, H., H.M. Simkins, E. Hyde, J. Yang, I.F. Hermans, T.R. Petersen, and F. Ronchese. 2013. The control of CD8+ T cell responses is preserved in perforin-deficient mice and released by depletion of CD4+CD25+ regulatory T cells. Journal of Leukocyte Biology 94 (4): 825–833.
Sawant, D.V., D.M. Gravano, P. Vogel, P. Giacomin, D. Artis, and D.A. Vignali. 2014. Regulatory T cells limit induction of protective immunity and promote immune pathology following intestinal helminth infection. Journal of Immunology 192 (6): 2904–2912.
Joller, Nicole, Ester Lozano, Patrick R. Burkett, Bonny Patel, Sheng Xiao, Chen Zhu, Junrong Xia, Tze G. Tan, Esen Sefik, and Vijay Yajnik. 2014. Treg cells expressing the co-inhibitory molecule TIGIT selectively inhibit pro-inflammatory Th1 and Th17 cell responses. Immunity 40 (4): 569–581.
Yan, Zhang, Zhou Ying, Jingsheng Lou, Jinbao Li, Lulong Bo, Keming Zhu, Xiaojian Wan, Xiaoming Deng, and Zailong Cai. 2010. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Critical Care 14 (6):R220.
Cajander, S., A. Bäckman, E. Tina, K. Strålin, B. Söderquist, and J. Källman. 2013. Preliminary results in quantitation of HLA-DRA by real-time PCR: a promising approach to identify immunosuppression in sepsis. Critical Care 17 (5): R223.
Zhao, Guang Ju, Yong Ming Yao, Lu Zhong Qiu, Guang Liang Hong, Xiao Mei Zhu, Wu Yao, Wei Wang Da, Ning Dong, Yu Yan, and Zhi Yong Sheng. 2012. Up-regulation of mitofusin-2 protects CD4 + T cells from HMGB1-mediated immune dysfunction partly through Ca 2+ -NFAT signaling pathway. Cytokine 59 (1): 79–85.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All mice experiments were conducted in accordance with the guidelines proposed by the Wenzhou Medical University Institutional Animal Care and Use Committee.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Zhi-qiang Hu and Yong-ming Yao contributed equal efforts to this study.
Rights and permissions
About this article
Cite this article
Hu, Zq., Yao, Ym., Chen, W. et al. Partial Depletion of Regulatory T Cells Enhances Host Inflammatory Response Against Acute Pseudomonas aeruginosa Infection After Sepsis. Inflammation 41, 1780–1790 (2018). https://doi.org/10.1007/s10753-018-0821-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0821-8