Abstract
We aimed to investigate serum and gingival crevicular fluid levels of myeloperoxidase, interleukin-17, and interleukin-23 before and after nonsurgical periodontal therapy in generalized aggressive periodontitis patients and compare to those in healthy controls. Interleukin-17, interleukin-23, and myeloperoxidase levels were measured by enzyme-linked immunosorbent assay in gingival crevicular fluid and serum samples taken from 19 systemically healthy generalized aggressive periodontitis patients and 22 healthy controls. In addition, the levels of IL-17, IL-23, and myeloperoxidase were reassessed at 3 months after periodontal therapy in the generalized aggressive periodontitis (GAP) group. Periodontal clinical parameters were also evaluated at baseline and 3 months post-therapy. The investigated molecule levels in serum decreased significantly at 3 months as a result of the therapy (p = 0.014 for IL-17, p = 0.000 for IL-23, and p = 0.001 for myeloperoxidase (MPO)). Significant reductions were also observed in gingival crevicular fluid (GCF) IL-17, IL-23, and MPO levels at 3 months after therapy (p = 0.000 for all molecules). However, the GCF levels of IL-17, IL-23, and MPO in GAP patients were still higher than those in the controls at 3 months (p = 0.001). A significant decrease in the local and systemic levels of IL-17, IL-23, and MPO based on the therapy might indicate the role of these mediators for tissue destruction in periodontal tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Generalized aggressive periodontitis (GAP) is a rapidly progressive, rarely seen disease that systemically affects healthy individuals at an early age, resulting in severe bone and attachment loss, with a distinct tendency to run in families [1]. Specific types of bacteria are essential for the initiation and progression of periodontal diseases. However, tissue destruction may result from an imbalance between host-destructive and host-protective mechanisms, initiated by an infection [2, 3]. Periodontal lesions are characterized by dense lymphoid infiltrates containing CD4+ (cluster of differentiation) T-helper (Th) cells [4]. The involvement of Th1 and Th2 cells, which have different profiles of cytokine secretion, is accepted in the pathogenesis and prognosis of periodontal diseases [5]. The disruption of Th1 and Th2 cytokine profiles results in inflammatory/immune diseases [6]. However, recently, a distinct T-helper cell lineage (Th17) has also been identified [7]. These lymphocytes are thought to play a key role in the pathogenesis of cell-mediated tissue damage caused by autoimmunity [8] and protective immune responses against a variety of microbial infections [9]. Interleukin-17 (IL-17) is a proinflammatory cytokine and is produced by Th17 cells and neutrophils [9–11]. IL-17 expression was first reported in gingival tissues of periodontitis patients in 2004 [12]. IL-17 has already been shown to be prominently involved in the activation and recruitment of neutrophils to inflammatory sites [13]. The first studies regarding the association of IL-17 with periodontitis assessed IL-17 levels in gingival biopsies [12, 14], gingival crevicular fluid (GCF) [15], and peripheral blood [16, 17]. These studies helped in clarifying the potential role of IL-17 in periodontal tissue breakdown and resulted in intervention studies. Later, there are studies reporting increased levels of IL-17 in chronic [8, 18–20] and aggressive periodontitis [5, 20, 21]. IL-17 stimulates a variety of cell types, including endothelial and epithelial cells and fibroblasts, to produce inflammatory mediators such as IL-1β, IL-6, TNF-α, matrix metalloproteinases, and chemokines [22, 23]. IL-17 is also involved in osteoclastogenesis, by inducing the receptor activator of the NF-κB ligand (RANKL) in osteoblastic cells [24]. The cytokine levels in inflamed gingival tissues in periodontitis are higher than those in healthy control tissues [25, 26]. Similarly, the amount of IL-17 in GCF [25, 27] and serum IL-17 levels [16] has been found to be significantly higher in periodontitis patients.
The production and development of Th17 cells is dependent on IL-23, IL-6, and transforming growth factor-β [28]. IL-23 is a recently identified proinflammatory cytokine that belongs to the IL-12 heterodimeric cytokine family and is produced by activated antigen-presenting cells, such as dendritic cells and macrophages, activated endothelial cells, monocytes, and Th cells [29, 30]. This cytokine is responsible for the differentiation and expansion of Th17 cells [7]. In addition, IL-23 stimulates Th17 cells to produce IL-17, regulates antibody production, activates natural killer cells, and controls regulatory T cells [31]. The IL-23/IL-17 pathway is currently an important area in immunology research for its role in the development of chronic inflammation and in host defenses against bacterial infections [32]. The IL-23/IL-17 immune pathway is activated in periodontitis lesions, and this axis may be essential to understand the progression of periodontitis [18, 25]. In addition, the pathology of GAP has been suggested to be an interesting model for examining the role of Th17 cells in periodontal disease [15].
IL-17 has a protective effect via mobilization of neutrophils against Porphyromonas gingivalis-induced bone loss [33] and may increase the activity of proteolytic enzymes such as myeloperoxidase (MPO), which facilitates the development of inflammation [13]. MPO is a major constituent of the azurophilic granules of polymorphonuclear neutrophils (PMNs), and it oxidizes chloride ions to the strong oxidant hypochlorous acid, the most bactericidal oxidant produced by neutrophils [34]. MPO, considered as a marker of neutrophil activation, has been found to be elevated in inflamed regions [35]. MPO also contributes directly to the activation of proteases (by inhibiting antiproteases), which cause damage to connective tissue [36, 37]. Levels of MPO have been evaluated in GCF [38, 39], blood [40, 41], and gingival tissue [42–44]. Being an indicator of PMN infiltration, GCF MPO is associated with the severity of periodontal disease [45]. MPO content has also been shown to reflect the presence of systemic inflammation, rather than a local inflammatory condition [46].
Periodontal therapy has been shown to yield a prominent change in GCF MPO levels in GAP patients [47] and in chronic periodontitis (CP) patients [38]. However, the results regarding IL-17 and IL-23 are controversial. Nonsurgical periodontal treatment of CP patients may reduce the expression of IL-17 [19]. Duarte et al. [20] evaluated IL-17 and IL-23 levels in the serum of GAP and CP patients before and after scaling and root planing (SRP) and reported significantly higher levels of L-17 in the GAP group than in the CP group and healthy controls at baseline. In addition, the level of IL-17 decreased significantly at 6 months post-therapy in the GAP group, similar to the results of Zhao et al. [19]. In contrast, Ay et al. [48] reported no significant difference in the amount of IL-17 in GCF between the GAP group and healthy controls. A noteworthy increase in IL-23 concentrations in diseased periodontal sites suggests its possible role in the pathogenesis of periodontal destruction [18, 49]. However, Duarte et al. [20] did not find any significant differences in serum levels of IL-23 among GAP, CP, and periodontally healthy (PH) groups at baseline or after SRP.
We hypothesized that the presence of GAP, a severe infectious and inflammatory disease, may be associated with increased levels of proinflammatory mediators in GCF and serum due to their local and systemic effects. In addition, nonsurgical periodontal therapy could decrease local and systemic levels of these mediators. Thus, we investigated the levels of MPO, IL-17, and IL-23 in serum and GCF and their correlations with clinical findings, before and after nonsurgical periodontal therapy in GAP patients, when compared with PH controls.
MATERIALS AND METHODS
Study Population and Study Design
The study protocol was approved by the Ethical Committee of Istanbul University (Approval no: 2011/1993-861), and the methods were carried out in accordance with the guidelines of the Declaration of Helsinki (version 2008). The aims and methods of the study and potential risks and benefits were explained and informed consent was obtained from all subjects. In total, 19 previously untreated GAP patients and 22 PH patients were recruited between June 2011 and May 2013 at the Periodontology Department, Faculty of Dentistry, Istanbul University. The selection of GAP patients was made based on the 1999 Classification of Periodontal Disease [50]. Patients in the GAP group had a family history of ≥1 other family member presenting with or having a history of severe periodontal problems, were <35 years of age, had three teeth other than the first molars and incisors showing a minimum of 5 mm attachment loss (AL), and had radiographic evidence of advanced alveolar bone loss. PH subjects were selected on the basis of having a mean probing depth (PD) and AL ≤3 mm, a mean gingival index (GI) <1, and no radiographical alveolar bone loss. In addition, all study participants had never smoked and were not obese [51] (BMI <30 kg/m2; BMI classification, 2012). PH subjects were age- and gender-matched to the GAP patients. Inclusion criteria were being systemically healthy and having not had any previous periodontal therapy. All participants had more than 20 teeth. Exclusion criteria included antibiotic therapy in the last 6 months, any smoking habit at any period, any kind of regular medication, pregnancy, and lactation.
Clinical Periodontal Measurements and Periodontal Therapy
Recorded periodontal parameters were as follows: plaque index (PI) [52], GI [53], bleeding on probing (BoP), PD, and clinical attachment level (CAL). All measurements were performed at six sites on each tooth (mesio-buccal, mid-buccal, disto-buccal, mesio-lingual, mid-lingual, and disto-lingual), excluding third molars, using a periodontal probe (Williams, Hu-Friedy, Chicago, IL). All periodontal parameters were recorded at baseline and 3 months after therapy by the same calibrated examiner (EC). An intraexaminer correlation coefficient of 0.85 for PD measurements indicated that examiner reliability was high. Nonsurgical periodontal treatment consisting of SRP and reinforced oral hygiene instructions was accomplished in 24 h in two sequential visits. No periodontal intervention was carried out in the periodontally healthy controls.
GCF Sampling
GCF samples were collected from the four deepest and nonadjacent periodontal pockets of GAP patients 1 week after clinical measurements. GCF samples from PH controls were collected from four nonadjacent and noninflamed sites. If necessary, supragingival plaque was removed carefully from the interproximal surfaces with a sterile curette; the study sites were gently dried using an air syringe and were isolated by cotton rolls. Filter paper strips (PerioPaper, Oraflow, Smithtown, NY) were placed gently into the gingival sulcus/periodontal pocket until a minimum of resistance was felt, and left there for 30 s. Strips visibly contaminated with blood were discarded. Four strips were obtained from all participants, pooled, and stored −80 °C until the laboratory analyses.
Serum Sampling
Blood (5 mL) was obtained from the antecubital vein by venipuncture and serum was separated by centrifugation (3000 rpm, 10 min, cool). Separated serum samples were collected into microcentrifuge tubes and stored at −80 °C.
IL-17, IL-23, and MPO Analyses
Enzyme-linked immunosorbent assays (ELISA) were used for the quantitative detection of IL-17A/F (Diamicrone, France), IL-23 (Diamicrone, France), and MPO (eBioscience, Vienna, Austria) levels in GCF and serum. First, 350 μL phosphate-buffered saline (PBS) 0.05 % (w/v)-Tween-20 buffer was added to each microcentrifuge tube containing strips. The day before the analyses, GCF samples were kept at +4 °C overnight on a shaking platform for the elution of GCF from the strips. On the day of analysis, each sample was vortexed for 1 min before processing. Then, 50 μL aliquots were used for IL-17 analyses. These aliquots were diluted 2-fold for serum and 4-fold for GCF IL-17 analyses. One hundred microliter aliquots without any dilutions were used for both serum and GCF IL-23 analyses; 100 μL aliquots were used and 50-fold dilutions were prepared for serum and GCF MPO analyses. Dilution factors were multiplied by the concentration read from a standard curve, generated by plotting the average absorbance of each standard on the vertical axis versus the corresponding IL-17 standard concentration on the horizontal axis. The minimum detection limits were 6.6 pg/mL, <20 pg/mL, and 0.03 ng/mL for IL-17, IL-23, and MPO, respectively.
Statistical Analyses
The minimum required sample size was determined to be 19 patients for each group with 99 % power and a 0.05 significance level for PI, GI, and BoP. The SPSS software (ver. 17 for Windows) was used to analyze the study data. One-way analysis of variance (ANOVA) was used to determine the presence/absence of intergroup differences in age. The Shapiro-Wilk normality test was used to determine whether the data were normally distributed.
The clinical periodontal parameters of PI, GI, PD, and BoP were normally distributed, so a paired samples t test was used to analyze the change between baseline and 3 months based on the therapy. To detect differences between GAP patients and healthy controls in terms of BoP and PD at baseline and 3 months, an independent samples t test was used. Because PI, GI, and CAL were not normally distributed, the Mann-Whitney U test was used for intergroup analyses of these parameters. As CAL was not normally distributed, the change between baseline and 3 months was compared using the Wilcoxon test. For intragroup analyses of the biochemical data, a paired samples t test was used to detect changes in the levels of IL-17 and MPO in serum and MPO in GCF, at 3 months when compared with baseline. As the levels of IL-17 and IL-23 in GCF and IL-23 in serum were not normally distributed, the Wilcoxon test was used. For intergroup analyses of the biochemical data, an independent samples t test was used to detect the differences in the levels of IL-17 in serum at baseline and the levels of IL-23 in GCF and MPO in serum at 3 months when compared with the healthy controls. The baseline levels of IL-17, IL-23, and MPO in GCF; IL-23 and MPO in serum; IL-17 in GCF; and IL-17 and IL-23 in serum at 3 months were compared with the healthy control values using the Mann-Whitney U test. To analyze the correlations between periodontal data and levels of IL-17, IL-23, and MPO in both serum and GCF, Spearman’s rho correlation analyses were used.
RESULTS
Table 1 presents the demographic parameters of the study groups. All participants were age- and sex-matched, besides being nonsmokers in both the GAP and healthy control groups.
Table 2 presents periodontal parameters of the study groups at baseline. As expected, all periodontal parameters, including GI, PD, CAL, accumulation of supragingival plaque, and percentage of sites with BoP in the GAP group, were significantly higher than those in the control group (p < 0.05). In addition, Table 2 shows the changes in clinical periodontal parameters in GAP patients according to SRP and a comparison of baseline and post-therapy scores of these parameters (PI, GI, PD, CAL, BoP) versus the control group. There were significant differences between the baseline scores of periodontal parameters of GAP patients and healthy controls (p = 0.001 for PI and p < 0.001 for GI, PD, CAL, and BoP). Periodontal clinical parameters were expectedly improved after therapy (p = 0.001 for PI and p < 0.001 for GI, CAL, PD, and BoP). However, all clinical parameters were still higher than those in the control group (p < 0.001).
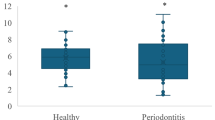
Table 3 reports the serum levels of IL-17 and IL-23 and the MPO examined in the groups. Significantly higher IL-23, IL-17, and MPO levels were observed in the GAP group than in healthy controls (all p < 0.001) (Fig. 1a–c). IL-17, IL-23, and MPO levels decreased significantly at 3 months as a result of the SRP (p = 0.014 for IL-17, p < 0.001 for IL-23, and p = 0.001 for MPO). Although significant reductions were observed in the GAP patients based on therapy, IL-17 and IL-23 were still higher than those in the healthy controls. However, the serum MPO levels in GAP patients approached the levels of the healthy controls after therapy (p > 0.05).
The GCF levels of the molecules examined are presented in Table 4. GAP patients had significantly higher levels of IL-17, IL-23, and MPO than those in the healthy controls at baseline (all p < 0.001) (Fig. 2a–c). Significant reductions were observed in all three at 3 months after therapy (all p < 0.001). Although there were significant reductions due to therapy in the GAP patients, the levels of IL-17, IL-23, and MPO were still higher than those in the controls at 3 months (all p < 0.001).
Spearman’s rho correlation analysis revealed no significant correlations between the clinical parameters and the levels of IL-17, IL-23, or MPO in serum or GCF at baseline or at 3 months after therapy.
DISCUSSION
There is not enough data to conclude that the levels of IL-17, IL-23, and MPO have direct relationships with aggressive periodontitis. However, several studies have reported higher levels of these cytokines in the sera and GCF samples from such patients [16, 21, 54]. We evaluated the levels of IL-17, IL-23, and MPO in GAP patients before and after periodontal therapy in both GCF and serum and demonstrated that the levels of each in both serum and GCF were significantly higher during the course of GAP and then decreased simultaneously with the resolution of periodontal inflammation.
Aggressive periodontitis is characterized by rapid attachment and bone loss in young adults with no apparent systemic disease [50]. Local production of proinflammatory cytokines causes the breakdown of periodontal tissue [54]. The local exacerbated immune responses may cause an increased systemic inflammatory response. The proinflammatory cytokine IL-17 has been shown to play a role in many inflammatory conditions, such as autoimmune diseases, metabolic disorders, and some cancers [9, 55, 56]. IL-17 is a proinflammatory cytokine produced by Th17 cells, while IL-23 plays an essential role in maintaining and expanding the Th17 cell population [57]. Lester et al. [18] demonstrated elevated levels of IL-23 at sites of clinical attachment loss. However, there is no detailed information on the precise role of IL-23 in periodontal diseases. Reports from different studies show conflicting results [20, 25, 58]. Although IL-23 was reported to decrease after periodontal therapy [49], there are other studies reporting almost no change in the levels of IL-23 after therapy [20, 58, 59]. In this study, IL-23 levels decreased significantly in GCF as well as in serum, confirming a relationship between local and systemic inflammatory responses. According to our results, the decreased levels of IL-23 in parallel with IL-17 in both GCF and serum in GAP patients after therapy may provide information about the role of these two proinflammatory cytokines in the progression of periodontitis.
Interventional studies have reported consistently significant decreases based on the therapy applied in IL-17 [19, 20, 58]. However, the results regarding IL-23 levels from different reports are comparatively inconsistent [20, 58, 59]. Among them, Santos et al. [58] demonstrated a significant decrease in IL-17 levels and no change in IL-23 levels after therapy in type 2 diabetic patients, regardless of whether disinfection was performed full mouth or partial mouth. There are fewer studies on aggressive periodontitis cases that have assessed the molecules evaluated in this study. Duarte et al. [20] reported significantly higher levels of IL-17 in the serum of GAP patients versus chronic periodontitis patients and periodontally healthy controls, with significant decreases in IL-17 levels and no significant change in IL-23 levels after therapy.
Significantly higher levels of IL-17 and IL-23 in GAP patients have been demonstrated to be contributing factors in progressive attachment and bone loss [16, 20]. However, these increases might also be associated with the host response to the multiplying microbial load and, thus, prevent further tissue loss [33]. In this study, we found significantly lower levels of these proinflammatory cytokines in GAP patients as a result of successful SRP. These data are consistent with the findings of Duarte et al. [20] in part because no significant differences in the levels of IL-23 were found among the groups between baseline and after therapy in their study. Levels of IL-23 in both serum and GCF were also decreased after therapy. The significant difference between GAP patients and healthy controls at baseline disappeared after therapy (p > 0.05). In this study, we observed that serum levels of IL-17 and IL-23 and GCF levels of IL-17 in subjects with GAP remained elevated when compared with the levels observed in healthy controls although there were significant reductions when compared with baseline. This limited systemic and local response to therapy suggests only a partial restoration of systemic and local inflammatory load in subjects with GAP. Individuals with GAP seem to have a preexisting local and systemic inflammatory load probably related to the host susceptibility trait. As reported in the review by Kulkarni and Kinane, familial aggregation of cases of GAP indicates that there may be a significant genetic component involved in the susceptibility to this disease [60].
MPO has been reported to be correlated with worse clinical status in periodontitis [38]. This enzyme has been suggested to be involved in the pathogenesis of periodontal disease [61, 62]. Increased activity of MPO at sites of periodontal disease and decreased activity after treatment may support a role for MPO in destructive periodontal diseases [37, 63]. Recently, Ozcaka et al. [40] reported increased concentrations of MPO in smokers with chronic periodontitis, although they observed no differences with the nonsmoker group. They suggested that the increase might be associated with an increased risk of periodontal tissue destruction. Nizam et al. [64] evaluated MPO levels in different periodontitis patient groups and healthy controls; although they found higher amounts of neutrophilic enzymes in periodontitis patients, there was no difference in the enzyme profile between GAP and CP patients. Our findings showing significantly higher local and systemic MPO levels in GAP patients than healthy controls are consistent with their data. In addition, the present results confirm that the levels of IL-17 in serum and GCF are increased in GAP patients, and this is accompanied by increases in IL-23 and MPO in serum and GCF.
To analyze the overall clinical and biochemical data of this study, despite reductions in serum and GCF IL-17, IL-23, and MPO, the levels remained higher than those in healthy controls, while we observed significant improvement in clinical parameters. These elevated levels could be the result of the genetic predisposition of aggressive periodontitis cases as speculated by Shaddox et al. [65].
In conclusion, the levels of two proinflammatory cytokines, IL-17 and IL-23, and the neutrophil enzyme MPO were significantly higher in aggressive periodontitis patients than in healthy controls at baseline, suggesting a role in the pathogenesis of this disease. Significant decreases in the levels, both locally and systemically, were seen after therapy, possibly indicating specific roles for these mediators in the active inflammation of periodontal tissues.
Limitation of the Study
We have recruited a limited number of subjects in the study. The limited sample size unfortunately limits the generalizability of the results. Further studies with a larger sample size are necessary to confirm the findings of this study.
References
Lindhe, J., N.P. Lang, and K. Karring. 2003. Periodontology and implant dentistry, 4th ed, 217. Oxford: Blackwell Munksgaard.
Page, R.C., and K.S. Kornman. 1997. The pathogenesis of human periodontitis: an introduction. Periodontology 2000(14): 9–11.
Sahingur, S.E., and R.E. Cohen. 2004. Analysis of host responses and risk for disease progression. Periodontology 2000(34): 57–583. Review.
Page, R.C., S. Offenbacher, H.E. Schroeder, G.J. Seymour, and K.S. Kornman. 1997. Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontology 2000(14): 216–248.
Mosmann, T., H. Cherwinski, M. Bond, M. Giedlin, and R. Coffman. 1986. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. Journal of Immunology 136: 2348–2357.
Houri-Haddad, Y., A. Wilensky, and L. Shapira. 2007. T-cell phenotype as a risk factor for periodontal disease. Periodontology 2000(45): 67–75.
Harrington, L.E., P.R. Mangan, and C.T. Weaver. 2006. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Current Opinion in Immunology 18: 349–356.
Steinman, L. 2007. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nature Medicine 13: 139–145. Review.
Tesmer, L.A., S.K. Lundy, S. Sarkar, and D.A. Fox. 2008. Th17 cells in human disease. Immunological Reviews 223: 87–113. doi:10.1111/j.1600-065X.2008.00628.x.
Ferretti, S., O. Bonneau, G.R. Dubois, C.E. Jones, and A. Trifilieff. 2003. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. The Journal of Immunology 170: 2106–2112.
Kramer, J.M., and S.L. Gaffen. 2007. Interleukin-17: a new paradigm in inflammation, autoimmunity, and therapy. Journal of Periodontology 78: 1083–1093.
Johnson, R.B., N. Wood, and F.G. Serio. 2004. Interleukin-11 and IL-17 and the pathogenesis of periodontal disease. Journal of Periodontology 75: 37–43.
Eskan, M.A., R. Jotwani, T. Abe, J. Chmelar, J.H. Lim, S. Liang, P.A. Ciero, J.L. Krauss, F. Li, M. Rauner, L.C. Hofbauer, E.Y. Choi, K.J. Chung, A. Hashim, M.A. Curtis, T. Chavakis, and G. Hajishengallis. 2012. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nature Immunology 13: 465–473. doi:10.1038/ni.2260.
Ito, H., T. Honda, H. Domon, T. Oda, T. Okui, R. Amanuma, T. Nakajima, and K. Yamazaki. 2005. Gene expression analysis of the CD4+ T-cell clones derived from gingival tissues of periodontitis patients. Oral Microbiology and Immunology 20: 382–386.
Vernal, R., N. Dutzan, A. Chaparro, J. Puente, M. Antonieta Valenzuela, and J. Gamonal. 2005. Levels of interleukin-17 in gingival crevicular fluid and in supernatants of cellular cultures of gingival tissue from persons with chronic periodontitis. Journal of Clinical Periodontology 32: 383–389.
Schenkein, H.A., T.E. Koertge, C.N. Brooks, R. Sabatini, D.E. Purkall, and J.G. Tew. 2010. IL-17 in sera from patients with aggressive periodontitis. Journal of Dental Research 89: 943. doi:10.1177/0022034510369297.
Ozcaka, O., A. Nalbantsoy, and N. Buduneli. 2011. Interleukin-17 and interleukin-18 levels in saliva and plasma of patients with chronic periodontitis. Journal of Periodontal Research 46: 592–598.
Lester, S.R., J.L. Bain, R.B. Johnson, and F.G. Serio. 2007. Gingival concentrations of interleukin-23 and -17 at healthy sites and at sites of clinical attachment loss. Journal of Periodontology 78: 1545–1550.
Zhao, L., Y. Zhou, Y. Xu, Y. Sun, L. Li, and W. Chen. 2011. Effect of non-surgical periodontal therapy on the levels of Th17/Th1/Th2 cytokines and their transcription factors in Chinese chronic periodontitis patients. Journal of Clinical Periodontology 38: 509–516.
Duarte, P.M., M. da Rocha, E. Sampaio, M.J. Mestnik, M. Feres, L.C. Figueiredo, M.F. Bastos, and M. Faveri. 2010. Serum levels of cytokines in subjects with generalized chronic and aggressive periodontitis before and after non-surgical periodontal therapy: a pilot study. Journal of Periodontology 81: 1056–1063.
Borch, T.S., M. Lobner, K. Bendtzen, P. Holmstrup, and C.H. Nielsen. 2009. Decreased interleukin-2 responses to Fusobacterium nucleatum and Porphyromonas gingivalis in generalized aggressive periodontitis. Journal of Periodontology 80: 800–887.
Takahashi, K., T. Azuma, H. Motohira, D.F. Kinane, and S. Kitetsu. 2005. The potential role of interleukin-17 in the immunopathology of periodontal disease. Journal of Clinical Periodontology 32: 369–374.
Beklen, A., T. Sorsa, and Y. Konttinen. 2009. Toll-like receptors 2 and 5 in human gingival epithelial cells co-operate with T cell cytokine interleukin-17. Oral Microbiology and Immunology 24: 38–42.
Sato, K., A. Suematsu, K. Okamoto, A. Yamaguchi, Y. Morishita, Y. Kadono, S. Tanaka, T. Kodama, S. Akira, Y. Iwakura, D.J. Cua, and H. Takayanagi. 2006. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. The Journal of Experimental Medicine 203: 2673–2682.
Ohyama, H., N. Kato-Kogoe, A. Kuhara, F. Nishimura, K. Nakasho, K. Yamanegi, N. Yamada, M. Hata, J. Yamane, and N. Terada. 2009. The involvement of IL-23 and the Th17 pathway in periodontitis. Journal of Dental Research 88: 633–638.
Beklen, A., M. Ainola, M. Hukkanen, C. Gurgan, T. Sorsa, and Y.T. Konttinen. 2007. MMPs, IL-1, and TNF are regulated by IL-17 in periodontitis. Journal of Dental Research 86: 347–351.
Buduneli, N., E. Buduneli, and N. Kutukculer. 2009. Interleukin-17, RANKL, and osteoprotegerin levels in gingival crevicular fluid from smoking and nonsmoking patients with chronic periodontitis during initial periodontal treatment. Journal of Periodontology 80: 1274–1280.
Zelante, T., A. De Luca, P. Bonifazi, C. Montagnoli, S. Bozza, S. Moretti, M.L. Belladonna, C. Vacca, C. Conte, P. Mosci, F. Bistoni, P. Puccetti, R.A. Kastelein, M. Kopf, and L. Romani. 2007. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. European Journal of Immunology 37: 2695–2706.
Watford, W.T., B.D. Hissong, J.H. Bream, Y. Kanno, L. Muul, and J.J. O’Shea. 2004. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunological Reviews 202: 139–156.
Kastelein, R.A., C.A. Hunter, and D.J. Cua. 2007. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annual Review of Immunology 25: 221–42. Review.
Tan, Z.Y., K.W. Bealgey, Y. Fang, Y.M. Gong, and S. Bao. 2009. Interleukin-23: immunological roles and clinical implications. The International Journal of Biochemistry & Cell Biology 41: 733–735. Review.
Iwakura, Y., and H. Ishigame. 2006. The IL-23/IL-17 axis in inflammation. Journal of Clinical Investigation 116: 1218–1222.
Yu, J.J., M.J. Ruddy, G.C. Wong, C. Sfintescu, P.J. Baker, J.B. Smith, R.T. Evans, and S.L. Gaffen. 2007. An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood 109: 3794–3802.
Sorsa, T., L. Tjäderhane, Y.T. Konttinen, A. Lauhio, T. Salo, H.M. Lee, L.M. Golub, D.L. Brown, and P. Mäntylä. 2006. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Annals of Medicine 38: 306–321.
Bradley, P.P., D.A. Priebat, R.D. Christensen, and G. Rothstein. 1982. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. Journal of Investigative Dermatology 78: 206–209.
Travis, J., R. Pike, T. Imamura, and J. Potempa. 1994. The role of proteolytic enzymes in the development of pulmonary emphysema and periodontal disease. American Journal of Respiratory and Critical Care Medicine 150: 143–146. Review.
Yamalik, N., F. Caglayan, K. Kilinc, A. Kilinc, and C. Tumer. 2000. The importance of data presentation regarding gingival crevicular fluid myeloperoxidase and elastase-like activity in periodontal disease and health status. Journal of Periodontology 71: 460–467.
Hernandez, M., J. Gamonal, T. Tervahartiala, P. Mäntylä, O. Rivera, A. Dezerega, N. Dutzan, and T. Sorsa. 2010. Associations between matrix metalloproteinase-8 and -14 and myeloperoxidase in gingival crevicular fluid from subjects with progressive chronic periodontitis: a longitudinal study. Journal of Periodontology 81: 1644–1652.
Marcaccini, A.M., C.A. Meschiari, L.R. Zuardi, T.S. de Sousa, M. Taba Jr., J.M. Teofilo, A.L. Jacob-Ferreira, J.E. Tanus-Santos, A.B. Novaes Jr., and R.F. Gerlach. 2010. Gingival crevicular fluid levels of MMP-8, MMP-9, TIMP-2, and MPO decrease after periodontal therapy. Journal of Clinical Periodontology 37: 180–190.
Ozcaka, O., N. Bicakci, P. Pussinen, T. Sorsa, T. Köse, and N. Buduneli. 2011. Smoking and matrix metalloproteinases, neutrophil elastase and myeloperoxidase in chronic periodontitis. Oral Diseases 17: 68–76.
Meschiari, C.A., A.M. Marcaccini, B.C. Santos Moura, L.R. Zuardi, J.E. Tanus-Santos, and R.F. Gerlach. 2013. Salivary MMPs, TIMPs, and MPO levels in periodontal disease patients and controls. Clinical Chimica Acta 421: 140–146.
Holzhausen, M., L.C. Spolidorio, and N. Vergnolle. 2005. Proteinase-activated receptor-2 (PAR2) agonist causes periodontitis in rats. Journal of Dental Research 84: 154–159.
Gomes, D.A., J.R. Pires, E.P. Zuza, M.N. Muscara, B.S. Herrera, L.C. Spolidorio, B.E. Toledo, and D.M. Spolidorio. 2009. Myeloperoxidase as inflammatory marker of periodontal disease: experimental study in rats. Immunological Investigations 38: 117–122. doi:10.1080/08820130802457503.
Akman, S., V. Canakci, A. Kara, U. Tozoglu, T. Arabaci, and I.M. Dagsuyu. 2013. Therapeutic effects of alpha lipoic acid and vitamin C on alveolar bone resorption after experimental periodontitis in rats: a biochemical, histochemical, and stereologic study. Journal of Periodontology 84: 666–674.
Sorsa, T., L. Tjäderhane, and T. Salo. 2004. Matrix metalloproteinases (MMPs) in oral diseases. Oral Diseases 10: 311–318.
Queiroz-Junior, C.M., C.M. Pacheco, A.H. Fonsec, A. Klein, M.V. Caliari, and J.N. de Francischi. 2009. Myeloperoxidase content is a marker of systemic inflammation in a chronic condition: the example given by the periodontal disease in rats. Mediators of Inflammation 2009: 760837. doi:10.1155/2009/760837.
Kaner, D., J.P. Bernimoulin, B.M. Kleber, W.R. Heizmann, and A. Friedmann. 2006. Gingival crevicular fluid levels of calprotectin and myeloperoxidase during therapy for generalized aggressive periodontitis. Journal of Periodontal Research 41: 132–139.
Ay, Z.Y., G. Yılmaz, M. Ozdem, H. Koçak, R. Sütçü, E. Uskun, M.Ö. Tonguç, and F.Y. Kırzıoğlu. 2012. The gingival crevicular fluid levels of interleukin-11 and interleukin-17 in patients with aggressive periodontitis. Journal of Periodontology 83: 1425–1431.
Himani, G.S., M.L. Prabhuji, and B.V. Karthikeyan. 2014. Gingival crevicular fluid and interleukin-23 concentration in systemically healthy subjects: their relationship in periodontal health and disease. Journal of Periodontal Research 49: 237–245.
Armitage, G.C. 1999. Development of a classification system for periodontal diseases and conditions. Annals of Periodontology 4: 1–6.
BMI classification. Global database on body mass index. World Health Organization. 2006. Retrieved July 27, 2012. http://apps.who.int/bmi/index.jsp?introPage=intro_3.html
Silness, J., and H. Löe. 1964. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontologica Scandinavica 22: 121–135.
Mendonca, A.C., V.R. Santos, F.V. Ribeiro, J.A. Lima, T.S. Miranda, M. Feres, and P.M. Duarte. 2012. Surgical and non-surgical therapy with systemic antimicrobials for residual pockets in type 2 diabetics with chronic periodontitis: a pilot study. Journal of Clinical Periodontology 39: 368–376. doi:10.1111/j.1600-051X.2012.01860.x.
Graves, D. 2008. Cytokines that promote periodontal tissue destruction. Journal of Periodontology 79: 1585–1591. doi:10.1902/jop.2008.080183. Review.
Chabaud, M., F. Fossiez, J. Taupin, and P. Miossec. 1998. Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. The Journal of Immunology 161: 409–414.
Matusevicius, D., P. Kivisakk, B. He, N. Kostulas, V. Ozenci, S. Fredrikson, and H. Link. 1999. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Multiple Sclerosis Journal 5: 101–104.
Mendonca, A.C., V.R. Santos, F.V. Ribeiro, J.A. Lima, T.S. Miranda, M. Feres, and P.M. Duarte. 2012. Surgical and non-surgical therapy with systemic antimicrobials for residual pockets in type 2 diabetics with chronic periodontitis: a pilot study. Journal of Clinical Periodontology 39: 368–376.
Santos, V.R., F.V. Ribeiro, J.A. Lima, T.S. Miranda, M. Feres, M.F. Bastos, and P.M. Duarte. 2012. Partial- and full-mouth scaling and root planing in type 2 diabetic subjects: a 12-mo follow-up of clinical parameters and levels of cytokines and osteoclastogenesis-related factors. Journal of Periodontal Research 47: 45–54.
de Ribeiro, F.V., A.C. Mendonca, V.R. Santos, M.F. Bastos, L.C. Figueiredo, and P.M. Duarte. 2011. Cytokines and bone-related factors in systemically healthy patients with chronic periodontitis and patients with type 2 diabetes and chronic periodontitis. Journal of Periodontology 82: 1187–1196. doi:10.1902/jop.2011.100643.
Kulkarni, C., and D.F. Kinane. 2014. Host response in aggressive periodontitis. Periodontology 2000 65(1): 79–91.
Smith, Q.T., J.E. Hinrichs, and R.S. Melnyk. 1986. Gingival crevicular fluid myeloperoxidase at periodontitis sites. Journal of Periodontal Research 21: 45–55.
Cao, C.F., and Q.T. Smith. 1989. Crevicular fluid myeloperoxidase at healthy, gingivitis and periodontitis sites. Journal of Clinical Periodontology 16: 17–20.
Wei, P.F., K.Y. Ho, Y.P. Ho, Y.M. Wu, Y.H. Yang, and C.C. Tsai. 2004. The investigation of glutathione peroxidase, lactoferrin, myeloperoxidase and interleukin-1 beta in gingival crevicular fluid: implications for oxidative stress in human periodontal diseases. Journal of Periodontal Research 39: 287–293.
Nizam, N., P. Gumus, J. Pitkänen, T. Tervahartiala, T. Sorsa, and N. Buduneli. 2014. Serum and salivary matrix metalloproteinases, neutrophil elastase, myeloperoxidase in patients with chronic or aggressive periodontitis. Inflammation 37: 1771–1778. doi:10.1007/s10753-014-9907-0.
Shaddox, L.M., P.F. Gonçalves, A. Vovk, N. Allin, H. Huang, W. Hou, et al. 2013. LPS-induced inflammatory response after therapy of aggressive periodontitis. Journal of Dental Research 92(8): 702–708.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cifcibasi, E., Koyuncuoglu, C., Ciblak, M. et al. Evaluation of Local and Systemic Levels of Interleukin-17, Interleukin-23, and Myeloperoxidase in Response to Periodontal Therapy in Patients with Generalized Aggressive Periodontitis. Inflammation 38, 1959–1968 (2015). https://doi.org/10.1007/s10753-015-0176-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-015-0176-3