Abstract
The aim of our study was to elucidate the function and signaling pathway of found in inflammatory zone 1 (FIZZ1) in airway remodeling in asthma. We used a mice model sensitized and challenged by ovalbumin (OVA) to evaluate the expression of FIZZ1, type I collagen, and fibronectin-1 in the airway in asthma. To investigate the signaling pathway regulated by FIZZ1, we treated a cultured murine lung epithelium cell-12 (MLE-12) with FIZZ1 recombination protein, silenced the expression of FIZZ1 with FIZZ1-shRNA in vitro, and then detected phosphorylated phosphatase and tensin homolog deleted on chromosome 10 (PTEN) and expression of type I collagen and fibronectin-1 (FN-1) by Western blotting. In addition, we increased the expression of PTEN by PTEN plasmid transfection then detected the expression of type I collagen and fibronectin-1 in MLE-12 by Western blot analysis and immunofluorescence cytochemistry technology, respectively. First, the expression of FIZZ1, type I collagen, and fibronectin-1 was significantly elevated in the lungs of OVA-challenged mice compared with saline-treated control animals. Secondly, the phosphorylation of PTEN was decreased in MLE-12 treated with FIZZ1 recombination protein in vitro. On the contrary, the phosphorylation of PTEN was increased in MLE-12 cells transfected with FIZZ1-shRNA. Thirdly, results of the Western blot analysis and immunofluorescence cytochemistry showed that expression of type I collagen and fibronectin-1 was increased in cells treated with FIZZ1 recombination protein, while the levels of type I collagen and fibronectin-1 were significantly decreased in cells transfected with PTEN plasmid. FIZZ1 may be a critical cytokine in airway remodeling in asthma. This study indicates that targeting FIZZ1 and/or PTEN may be a new therapeutic strategy for asthma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
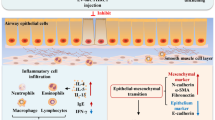
Bronchial asthma is a chronic lung disease which is characterized by chronic airway inflammation, smooth muscle dysfunction, and airway remodeling [1]. More recently, it has become clear that airway remodeling is an important part of the pathogenesis of asthma, as well as the basis of asthmatic airway pathological changes [2, 3]. Although much information about the presence of airway remodeling in asthma has been learned in the past years, the natural history of the process is not fully understood. It is conceivable that airway remodeling in asthma contributes to asthma exacerbations and even death because of airway obstruction caused by smooth muscle contraction, airway edema, and mucus plugging. Many factors contribute to airway remodeling in asthma, such as thymic stromal lymphopoietin (TSLP) [4] and interleukin 33 (IL-33) [5]. Holgate et al. [6] proposed that airway remodeling was initiated by damage on the airway epithelium that extended and amplified to deeper mucous membranes then caused the differentiation of myofibroblasts which are located in the subcutaneous reticular layer, leading to airway remodeling [7].
The noteworthy feature of airway remodeling in asthma is subepithelial fibrosis, and this process includes thickening of the subepithelial reticularis basement membrane. The thickened basement membrane is the outcome of extracellular matrix deposition, primarily including collagens I, III, and V, and noncollagenous matrix deposition, such as elastin, fibronectin-1, and so on [8, 9].
Found in inflammatory zone 1 (FIZZ1) is one of the novel cysteine-rich secreted proteins, also known as resistin-like molecules (RELMs) which was discovered during lung allergic inflammation [10]. The role of FIZZ1 in lung pathologies has been described. Conversely, Liu et al. reported that FIZZ1 was highly inducible in bleomycin (BLM)-induced lung fibrosis and localized primarily to alveolar epithelial cells [11]. Consistent with its role in promoting pulmonary airway inflammation, previous studies showed that FIZZ1 could stimulate the proliferation of pulmonary vascular smooth muscle cells [12]. Liang et al. [13] have identified that expression of FIZZ1, a member of a family of resistin-like molecules, was increased in the airway epithelium of an ovalbumin (OVA)-induced asthma animal model. Meanwhile, FIZZ1 could enhance the expression of type I collagen and alpha smooth muscle actin (α-SMA) in fibroblasts then further lead to early-stage airway remodeling in asthma [13]. These findings showed that FIZZ1 may play an important role in the process of airway remodeling, and these are consistent with previous reports [14].
The phosphatase and tensin homolog deleted on chromosome 10 (PTEN) gene, as one of the most commonly mutated tumor suppressor genes in humans, manifests a variety of effects on cell growth, migration, death, and differentiation [15]. This gene has drawn interesting concern to its potential role in asthma in recent years. Recent data has confirmed that PTEN expression is attenuated in an asthma animal model and that exogenous PTEN could effectively relieve asthma in the mice model [16, 17], as well as efficiently reduce chronic airway inflammation and remodeling [18].
The role of FIZZ1 in airway remodeling in asthma is still not clear. In this study, we aimed to analyze whether FIZZ1 could induce the expression of type I collagen and fibronectin-1 in asthma by inhibiting the phosphorylation of the PTEN signaling pathway. Therefore, we investigated the function and signal pathway of FIZZ1 in airway remodeling in asthma using a mice model and murine lung epithelium cell-12 lines (MLE-12). In addition, we demonstrated that FIZZ1 is associated with the inhibition of PTEN, resulting in increased expression of type I collagen and fibronectin-1 in airway remodeling in asthma.
MATERIALS AND METHODS
Ethics Statement
The animal experiments were performed according to the Institutional Animal Care and Use Committee of Shandong University. The protocol was approved by the Committee on the Ethics of Animal Experiments of Shandong University. Each mouse was anesthetized with intraperitoneal ketamine (30 mg/kg). All efforts were made to minimize pain and discomfort.
Animal Model
Twenty female BALB/c mice (8 to 10 weeks old, 20 ± 2 g) purchased from the Animal Experiment Center of Shandong University were randomly divided into two groups: the OVA group and the control group. The mice in the OVA group were sensitized with OVA as follows [19]: 100 mg/ml OVA (Sigma-Aldrich, St. Louis, MO, USA) was emulsified with 200 mg/ml aluminum hydroxide (Fuchen Chemistry Inc., Tianjin, China) in saline (Hualu Inc., Shandong, China), and each mouse was injected intraperitoneally with 0.1 ml of the solution at days 1 and 8. From day 15 to 21, the mice were nebulized daily with a 5 % (w/v) OVA solution for 30 min in a 30 cm × 24 cm × 50 cm chamber. The control group received intraperitoneal injections of saline followed by nebulization with saline. The mice were killed, and the lung specimens were removed for immunohistochemistry analysis.
Immunohistochemistry Analysis
Immunohistochemical staining was performed as described previously [4]. Saline- and OVA-sensitized and challenged mice were killed, and the lung lobes were dissected, fixed in 10 % formaldehyde, and processed for immunohistochemistry. Slides (4 μm thick) were dewaxed and rehydrated, and antigen retrieval was performed with 10 mM sodium citrate (pH 6.1) and blocked with 5 % bovine serum albumin for 60 min at room temperature. The sections were incubated with anti-FIZZ1 antibody (Santa Cruz Biotechnology, USA) (1:200), anti-type I collagen antibody (Santa Cruz Biotechnology, USA) (1:300), and anti-fibronectin-1 antibody (Santa Cruz Biotechnology, USA) (l:500) overnight at 4 °C and subsequently with polyclonal immunoglobulins/horseradish peroxidase (HRP) (1:100) for 1 h at room temperature. The nuclei were counterstained with hematoxylin. Control slides were incubated with the same antibodies. Cover slips were mounted with 80 % glycerol (Zsbio, Beijing, China).
The samples were examined using a microscope equipped with a digital camera (Hitachi, Japan). FIZZ1-, type I collagen-, and fibronectin-1-positive areas were quantified by densitometry using the Image-Pro Plus software (Media Cybernetics, USA).
Cell Culture and Transfection
MLE-12 was purchased from a cell bank (ATCC cell bank, USA) and cultured in DMEM/F12 complete medium supplemented with 10 % fetal bovine serum (FBS), 2 mM glutamine, 100 U/ml of penicillin, and 100 μg/ml of streptomycin at 37 °C under conditions of 5 % CO2. After 2 days in culture, adherent cells were consistently 90 % of epithelial morphology. The cells were seeded at 1 × 105 cells per well in six-well culture plates.
Transient Transfection
The FIZZ1-shRNA and pcDNA3.0-PTEN recombinant plasmid of the bacteria Escherichia coli JM109 were amplified in a liquid medium. Plasmid DNA was extracted using the Omega D6950 plasmid extraction kit after dissolving the bacteria, and plasmid concentration was determined to be 0.45 μg/μl.
At 50–70 % confluence, fresh media were replaced the day before treatment and transfection. The MLE-12 cells were treated with FIZZ1 recombination protein (Santa Cruz Biotechnology, USA) according to the manufacturer’s instructions. Transient transfections were performed using Lipofectamine 2000 reagent (Invitrogen Inc., USA), FIZZ1-shRNA plasmid (sc-39724-SH, Santa Cruz Biotechnology, USA) and pcDNA3.0-PTEN recombinant plasmid (Santa Cruz Biotechnology, USA) according to the manufacturer’s instructions. After 48 h of incubation, the cells were harvested for analysis of type I collagen and fibronectin-1.
The control group was cultured and not treated with plasmid. Then cells were collected to analyze protein expression by Western blot analysis and immunofluorescence cytochemistry.
Western Blot Analysis
MLE-12 cells were grown to confluence in six-well plates. After treatment with FIZZ1 recombination protein and transfection with FIZZ1-shRNA and PTEN plasmid, MLE-12 cells were lysed with a cell extraction buffer (Invitrogen Inc., USA), and the total amount of protein was quantitated using the Pierce BCA Protein Assay Kit (Thermo Rad Laboratories, USA). Equal amounts of total protein (30 μg) were separated on 10 ~ 12 % Bis-Tris gels in MOPS SDS Running Buffer (Invitrogen Inc., USA), transferred to a polyvinylidene difluoride membrane (PVDF) (Immobilon, Millipore Corp, Bedford, MA, USA). Then the PVDF membrane was blocked with 5 % skimmed milk in Tris-buffered saline (TBS) (50 mM Tris-Cl, pH 7.5, 150 mM NaCl) for 60 min at room temperature and probed with anti-type I collagen antibody (1:300; Santa Cruz Biotechnology, USA), anti-fibronectin-1 antibody (l:500; Santa Cruz Biotechnology, USA), anti-p-PTEN (1:500; Santa Cruz Biotechnology, USA), and anti-total PTEN (1:500, Santa Cruz, USA) followed by overnight incubation with primary antibodies (type I collagen, fibronectin-1, p-PTEN, t-PTEN) in TBS-T (0.1 % Tween-20 in TBS). The membrane was washed three times with 1× TBS-T for 5 min and then incubated for 1 h with secondary antibodies conjugated to horseradish peroxidase at room temperature. The membrane was exposed to a high-performance autoradiography film (Fuji XR film, Fuji Film Corporation, Tokyo, Japan) and visualized using the ECL system (Santa Cruz, CA, USA). The integrated density value (IDV) of band intensities from the films was analyzed by the ImageQuant 5.2 software (Molecular Dynamics, Sunnyvale, CA, USA).
Immunofluorescence Cytochemistry
The MLE-12 cells cultured on glass slides were fixed with 4 % paraformaldehyde for 10 min, incubated with 0.5 % Triton X-100 for 30 min, and then blocked with 1 % bovine serum albumin for 30 min at room temperature. The slides were incubated with anti-type I collagen, E-cadherin, and fibronectin-1 primary antibodies (1:100) overnight at 4 °C and subsequently with goat anti-rabbit IgG-fluorescein isothiocyanate (FITC) mAb (1:200) for 1 h. DAPI (500 ng/ml in 95 % ethanol) was used to stain the nuclei for 20 s. Cover slips were mounted with 80 % glycerol (Zsbio, Beijing, China). The slides were examined under fluorescence microscopy (Nikon Eclipse TE-2000U, Japan). Control slides were incubated with the same antibodies. The protein-positive areas were quantified by integrated optical density (IOD) using the Image-Pro Plus software (Media Cybernetics, USA).
Data Analysis and Statistics
Data were presented as means ± SD. Continuous variables were tested by analysis of Student’s t test between the groups studied, with p < 0.05 designated as significant difference.
RESULTS
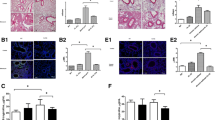
The Expression of FIZZ1, Type I Collagen, and Fibronectin-1 Was Upregulated in the Airways of OVA-Induced Mice
We found that FIZZ1 was highly expressed in the airway of OVA-induced mice compared with the control group. Quantitative analysis showed that FIZZ1 was significantly increased in the OVA group (p < 0.01, Fig. 1a,b). As expected, the airway remodeling markers type I collagen and fibronectin-1 were increased in the bronchial epithelial cells of asthmatic mice compared with the control group (p < 0.05 and p < 0.01, respectively, Fig. 1a,b).
FIZZ1 Promotes Airway Remodeling in Airway Epithelial Cells In Vitro
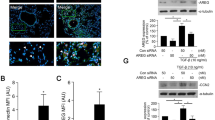
To explore the role of FIZZ1 in airway remodeling in asthma, we tested whether FIZZ1 could induce airway remodeling in mice airway epithelial cells. We used FIZZ1 recombinant protein to treat MLE-12 cells and detected type I collagen and fibronectin-1 expression. Then we observed that FIZZ1 induced high expression of type I collagen and fibronectin-1 in a FIZZ1 dose-dependent manner (p < 0.01 and p < 0.01, respectively, Fig. 2a). To confirm these results, we silenced FIZZ1 with FIZZ1-shRNA in the MLE-12 cells. Both type I collagen and fibronectin-1 expressions were decreased when FIZZ1 was silenced (p < 0.05 and p < 0.05, respectively, Figs. 2b and 3). These results demonstrated that FIZZ1 could promote airway remodeling in asthma in vitro.
Western blot analysis showing that FIZZ1 induced high expression of type I collagen and fibronectin-1 in a FIZZ1 dose-dependent manner (a), both type I collagen and fibronectin-1 expressions were decreased when FIZZ1 was silenced (b), and high expression of PTEN could decrease type I collagen and fibronectin-1 expression (c).
FIZZ1 Promotes Airway Remodeling Through the PTEN Signaling Pathway In Vitro
To identify which factor mediates the expression of type I collagen and fibronectin-1, we first examined whether FIZZ1 could act on the PTEN signaling pathway and detected the phosphorylation of PTEN after FIZZ1-shRNA transfection in MLE-12 cells. The results showed that phosphorylated PTEN was markedly decreased in cells treated with FIZZ1 recombination protein (p < 0.01, Fig. 2a). While silencing FIZZ1, we found that the phosphorylated PTEN was markedly enhanced in the cells (p < 0.05, Fig. 2b). In order to confirm these results, we detected the expression of type I collagen and fibronectin-1 in MLE-12 cells transfected with PTEN plasmid. As shown in the results, the high expression of PTEN could decrease type I collagen and fibronectin-1 expression in cells (p < 0.01 and p < 0.01, respectively, Figs. 2c and 4).
DISCUSSION
Here we delineate the role and signaling pathway of FIZZ1 in airway remodeling in asthma. We demonstrated that FIZZ1 was overexpressed in the bronchial epithelium of mice sensitized and challenged by OVA again, and this is consistent with previous findings [13]. We also demonstrated that overexpression of PTEN could decrease the expression of type I collagen and fibronectin-1 in MLE-12 cells. Therefore, our data suggested that FIZZ1 may play an important role in the process of airway remodeling in asthma through the PTEN signaling pathway.
Asthma is a chronic disease of the airway, and the main pathological changes in asthma are chronic inflammation, airway hyperresponsiveness (AHR), and airway remodeling [1, 20]. Numerous studies on the pathology and morphology aspects of the lung have confirmed these structural features [21, 22]. Airway remodeling was first reported by Huber in 1922 [23]. An important limitation of asthma therapy is the lack of effective methods on any features of airway remodeling which contribute to disease chronicity. Therefore, study on airway remodeling would provide a new strategy for the prevention and treatment of asthma.
In this study, we demonstrated the upregulation of FIZZ1 expression in a mouse asthma model and FIZZ1 overexpression along with the increased synthesis of type I collagen and fibronectin-1. FIZZ1 was actually discovered during lung allergic inflammation and belonged to a novel class of cysteine-rich secreted proteins [10]. Normally, these proteins are expressed in the lung, white adipose tissue, mammary gland, tongue, and heart [24, 25]. Recent studies have revealed that FIZZ1 expression was remarkably increased in hypertrophic, hyperplastic bronchial epithelium and was induced in alveolar epithelial type II cells during allergic pulmonary inflammation in asthma [10, 14].
In the mice model of chronic hypoxia with pulmonary hypertension, the data has suggested that FIZZ1 exhibited angiogenic properties, as well as the ability to stimulate proliferation of pulmonary vascular smooth muscle cells [11, 14]. In our asthma animal model, we demonstrated that the expression of FIZZ1 was highly upregulated in the lungs and significantly stimulated both type I collagen and fibronectin-1 expression which contributed to airway remodeling. Conversely, Liu et al. reported that FIZZ1 was highly inducible during BLM-induced lung fibrosis and localized primarily to alveolar epithelial cells as assessed by cDNA microarray analysis and in situ hybridization [11, 26].
Previous studies have demonstrated that airway remodeling was closely related to proto-oncogenes such as activation of c-fos and c-jun, but fewer studies were done on the role of tumor suppressor gene [27]. PTEN was the first tumor suppressor gene with a dual-specificity phosphatase activity ever found so far. Its expression product is PTEN, a PI3K inhibitor, which has a negative regulatory role in cell growth [28]. The main function of PTEN is to initiate D3 site dephosphorylation of phosphatidylinositol-3-phosphate (PIP3) and to antagonize the PI3K/Akt signaling pathway [27], thus resulting in apoptosis and cell growth inhibition [29, 30].
At the same time, a number of studies in vitro have warranted that the upregulated expression of PTEN could effectively inhibit the migration of human airway smooth muscle [31]. In our study, we detected the phosphorylation level of PTEN by Western blot analysis in MLE-12 cells which were treated with FIZZ1 recombination protein and FIZZ1-shRNA plasmid in vitro. The results showed that upregulated FIZZ1 could inhibit PTEN phosphorylation and the inhibitory effect of FIZZ1 on PTEN phosphorylation was blocked when FIZZ1 was silenced with FIZZ1-shRNA in vitro. We also observed that the overexpression of PTEN could downregulate the expression of type I collagen and fibronectin-1 in the protein level. These results suggested that the increased expression of PTEN may play a key role in airway remodeling by downregulating the expression of type I collagen and fibronectin-1.
These data clearly indicated that FIZZ1 could inhibit the phosphorylation of PTEN, which is consistent with previous reports [16, 32], and then this process could increase the expression of type I collagen and fibronectin-1. The appearance of collagen and fibronectin deposition correlates with the extent of airflow obstruction and may contribute to airway wall thickening, which eventually leads to airway collapse and airflow limitation [33].
Recent reports have indicated that FIZZ1 may induce collagen deposition as well as myofibroblast differentiation in lung tissue [34]. Furthermore, reduplicative intranasal administration of recombinant FIZZ1 protein could cause fibrotic changes in a lung granuloma model [35]. Hence, these studies suggested that FIZZ1 may play several roles in the lung tissue depending on the cell types involved and stage of the inflammatory response or the process of airway remodeling. Our data, as well as that of others, have suggested an early pro-inflammatory and pro-remodeling role of FIZZ1 [34, 35]. Although the approach presented in our study was on the basis of the FIZZ1 function in asthma, it still remains to be determined whether the observed effects of FIZZ1 are specific for airway remodeling or airway inflammation. Also, identification of these changes provides evidence that further work will be required to fully illustrate the multiple functions of FIZZ1 in the lung during airway remodeling in asthma, and further therapeutic development of anti-asthma strategies aimed at the FIZZ1/PTEN axis still needs further study.
In summary, our study has demonstrated that FIZZ1 may play a pivotal role in the process of airway remodeling by regulating the PTEN signaling pathway. The airway epithelium is the forefront of asthma pathophysiology, and we could design different approaches to therapy rather than just to suppress inflammation and modulate immune response in the past by focusing on airways vulnerable to environmental factors. Thereby, it would provide theoretical support to carry out the target treatment of FIZZ1-PTEN-type I collagen and fibronectin-1 signaling pathway for airway remodeling in asthma.
References
Holgate, S.T. 2008. Pathogenesis of asthma. Clinical and Experimental Allergy 38(6): 872–897.
Seow, C.Y., R.R. Schellenberg, and P.D. Pare. 1998. Structural and functional changes in the airway smooth muscle of asthmatic subjects. American Journal of Respiratory and Critical Care Medicine 158(5): 179–186.
Kaminska, M., S. Foley, K. Maghni, et al. 2009. Airway remodeling in subjects with severe asthma with or without chronic persistent airflow obstruction. Journal of Allergy Clinical Immunology 124(1): 45–51.
Wu J, Liu F, Zhao J, Wei Y, Lv J, Dong F, et al. 2012. Thymic stromal lymphopoietin promotes asthmatic airway remodeling in human lung fibroblast cells through STAT3 signalling pathway. Cell biochemistry and function.
Guo, Z., J. Wu, J. Zhao, et al. 2014. IL-33 promotes airway remodeling and is a marker of asthma disease severity. The Journal of Asthma 5: 1–7.
Holgate, S.T., D.E. Davies, P.M. Lackie, et al. 2000. Epithelial mesenchymal interactions in the pathogenesis of asthma. The Journal of Allergy and Clinical Immunology 105: 193–204.
Richter, A., S.M. Puddicombe, J.L. Lordan, et al. 2001. The contribution of interleukin (IL)-4 and IL-13 to the epithelial-mesenchymal trophic unit in asthma. American Journal of Respiratory Cell and Molecular Biology 25(3): 385–391.
Homer, R.J., and J.A. Elias. 2005. Airway remodeling in asthma: therapeutic implications of mechanisms. Physiology 20: 28–35.
Shifren A, Witt C, Christie C, Castro M. 2012. Mechanisms of remodeling in asthmatic airways. Journal of Allergy.
Holcomb, I.N., R.C. Kabakoff, and B. Chan. 2000. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. The EMBO Journal 19: 4046–4055.
Liu, T.J., and Saravana M. Dhanasekaran. 2004. FIZZ1 stimulation of myofibroblast differentiation. The American Journal of Pathology 164: 1315–1326.
Ma, W.L., H. Ye, X.N. Tao, and J.B. Xin. 2005. Dynamic changes of found in inflammatory zone 1 protein and mRNA expression in the lung with experimental pulmonary fibrosis of the rat. Sheng Li Xue Bao 57: 493–497.
Shujuan, W., C.M. Blanca, L. Hongjia, et al. 2008. FIZZ1 plays a crucial role in early stage airway remodeling of OVA-induced asthma. Journal of Asthma 45: 648–653.
Chung, M.J., T. Liu, M. Ullenbruch, and S.H. Phan. 2007. Antiapoptotic effect of found in inflammatory zone (FIZZ) 1 on mouse lung fibroblasts. The Journal of Pathology 2: 180–187.
Hill, R., and H. Wu. 2009. PTEN, stem cells, and cancer stem cells. The Journal of Biological Chemistry 284(18): 11755–11759.
Lee, Y.C. 2004. The role of PTEN in allergic inflammation. Archivum Immunologiae et Therapiae Experimentalis 52: 250–254.
Lee, K.S., S.R. Kim, S.J. Park, et al. 2006. Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) reduces vascular endothelial growth factor expression in allergen-induced airway inflammation. Molecular Pharmacology 69(6): 1829–1839.
Kim, S.R., K.S. Lee, S.J. Park, et al. 2007. PTEN down-regulates IL-17 expression in a murine model of toluene diisocyanate-induced airway disease. Journal of Immunology 179(10): 6820–6829.
Smit, J.J., H. Van Loveren, and M.O. Hoekstra. 2003. Therapeutic treatment with heat-killed Mycobacterium vaccae (SRL172) in a mild and severe mouse model for allergic asthma. European Journal of Pharmacology 470: 193–199.
Davies, D.E., J. Wicks, R.M. Powell, et al. 2003. Airway remodeling in asthma: new insights. Journal of Allery Clinical Immunology 111(2): 215–225.
Hackett, T.L., and D.A. Knight. 2007. The role of epithelial injury and repair in the origins of asthma. Current Opinion in Allergy and Clinical Immunology 7: 63–68.
Vignola, A.M., R. Gagliardo, and A. Siena. 2001. Airway remodeling in the pathogenesis of asthma. Current Opinion in Allergy and Clinical Immunology 1: 85–93.
Pilewski, J.M., and S.M. Albelda. 1995. Cell adhesion molecules in asthma: homing, activation, and airway remodeling. Journal of Respiration Cell Molecular Biology 12: 1–3.
Wagner, K.F., A.K. Hellberg, and S. Balenger. 2004. Hypoxia-induced mitogenic factor has antiapoptotic action and is upregulated in the developing lung: coexpression with hypoxia-inducible factor-2alpha. American Journal of Respiratory Cell and Molecular Biology 31: 276–282.
Patel, S.D., M.W. Rajala, and L. Rossetti. 2004. Disulfide-dependent multimeric assembly of resistin family hormones. Science 304(5674): 1154–1158.
Katsuma, S., K. Nishi, K. Tanigawara, et al. 2001. Molecular monitoring of bleomycin-induced pulmonary fibrosis by cDNA microarray-based gene expression profiling. Biochemical and Biophysical Research Communications 288: 747–751.
Shen, W.H., A.S. Balajee, J. Wang, et al. 2007. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell 128(1): 157–170.
Cai, X.M., B.B. Tao, L.Y. Wang, et al. 2005. Protein phosphatase activity of PTEN inhibited the invasion of glioma cells with epidermal growth factor receptor mutation type 3 expression. International Journal of Cancer 117(6): 905–912.
Jiang, B.H., and L.Z. Liu. 2008. PI3K/PTEN signaling in tumorigenesis and angiogenesis. Biochimica et Biophysica Acta 1784(1): 150–158.
Downes, C.P., N. Perera, S. Ross, et al. 2007. Substrate specificity and acute regulation of the tumor suppressor phosphatase PTEN. Biochemical Society Symposium 74(1): 69–80.
Lan, H., H. Zhong, Y. Gao, et al. 2010. The PTEN tumor suppressor inhibits human airway smooth muscle cell migration. International Journal of Molecular Medicine 26(6): 893–899.
Koul, D., R. Shen, S. Shishodia, et al. 2007. PTEN downregulates AP-1 and targets c-fos in human glioma cells via PI3-kinase/ Akt pathway. Molecular and Cellular Biochemistry 300: 77–87.
Tiddens, H., M. Silverman, and A. Bush. 2000. The role of inflammation in airway disease: remodeling. American Journal of Respiratory and Critical Care Medicine 162: 7–10.
Yamaji-Kegan, K., Q. Su, D.J. Angelini, A.C. Myers, C. Cheadle, and R.A. Johns. 2010. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELMalpha) increases lung inflammation and activates pulmonary microvascular endothelial cells via an IL-4-dependent mechanism. Journal of Immunology 185: 5539–5548.
Ito, T., M. Schaller, T. Raymond, et al. 2009. Toll-like receptor 9 activation is a key mechanism for the maintenance of chronic lung inflammation. American Journal of Respiratory and Critical Care Medicine 180: 1227–1238.
Acknowledgments
We thank Prof. Wenxiang Bi (Institute of Biochemistry and Molecular Biology, School of Medicine, Shandong University) for the excellent technical assistance and Yan Wang for providing us with the histochemistry staining apparatus and technical guidance. This work was supported by grants from the Natural and Science Foundation of China (Grant No. 81070016, 81270072).
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jiping Zhao and Xingai Jiao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhao, J., Jiao, X., Wu, J. et al. FIZZ1 Promotes Airway Remodeling in Asthma Through the PTEN Signaling Pathway. Inflammation 38, 1464–1472 (2015). https://doi.org/10.1007/s10753-015-0121-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-015-0121-5