Abstract
The reparative and immunoregulatory properties of mesenchymal stromal cells (MSCs) have made them attractive candidates for cellular therapy. However, the underlying mechanism of the effects of transplanted MSCs on allergic asthma remains elusive. Here, we show that administration of MSCs isolated from human bone marrow provoked a pronounced polarization in alveolar macrophages to M2 subtypes, rather than induced an increase in the total macrophage number, and efficiently inhibited hallmark features of asthma, including airway hyperresponsiveness and eosinophilic accumulation. Moreover, transforming growth factor beta (TGF-β) signaling pathway appeared to mediate the effects of MSCs on macrophage polarization and subsequently the inhibition of hallmark features of asthma. Inhibition of TGF-β signaling was sufficient to inhibit the macrophage polarization in response to MSCs and consequently reserved the inhibitory effects of macrophage polarization on hallmark features of asthma. Collectively, our data demonstrate that human MSCs have immunosuppressive activity on asthma, which is mediated by TGF-β-signaling-dependent alveolar macrophage polarization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Initially identified in the bone marrow, mesenchymal stromal cells (MSCs) have the stem-cell-like ability to expand in culture and differentiate into osteoblasts, chondrocytes, and adipocytes. Beyond their multilineage potential, MSCs have been shown as a highly immunosuppressive cell population with extensive immunomodulatory properties [1]. Clinical evidence further demonstrates that administration of MSCs is capable of promoting hematopoietic engraftment and tissue repair [1, 2]. When MSCs are intravenously delivered, they are predominnantly relocalized in the lungs, where they secrete anti-inflammatory molecules to exert local and distal effects [3]. Therefore, pulmonary conditions could benefit from the immunosuppressive and anti-inflammatory activity of MSCs in the lungs, as demonstrated in many animal studies.
Asthma is characterized by bronchial inflammation, hyperresponsive airways, and airflow obstruction caused by increased mucus secretion. Most asthma cases are classified as allergic asthma, in which the sensitized immune system reacts adversely to subsequent inhalation exposure to innocuous environmental triggers [4, 5]. Animal asthma models have shown that administration of human or mouse bone marrow derived MSCs during airway challenge may suppress allergic responses by producing transforming growth factor beta (TGF-β) or IFN-gamma [6], but the precise molecular mechanism remains largely unknown.
Inflammatory macrophages (marked by F4/80 expression) can regulate bronchial inflammation, hyperresponsive airways, and airflow obstruction [7]. Besides the classically activated macrophages (also called M1 macrophages), which respond to inflammatory stimuli and appear early on, there are also the alternatively activated macrophages (M2 macrophages), which appear later, to mediate humoral immunity and tissue repair [8, 9]. M2 macrophages are known to secrete a wide range of chemokines, enzymes, and growth factors, to promote neovascularization, fibrosis, and tissue repair [10–12]. However, the effects of M2-polarized macrophages on asthma models have not been systematically investigated.
Here, we sought to determine the critical cellular responses that mediate the therapeutic effects of human MSCs. First, we show that administration of MSCs isolated from human bone marrow provoked a pronounced polarization in alveolar macrophages to a M2 subtypes, rather than induced an increase in the total macrophage number, and efficiently inhibited hallmark features of asthma, including airway hyperresponsiveness and eosinophilic accumulation. Moreover, transforming growth factor beta (TGF-β) signaling pathway appeared to mediate the effects of MSCs on macrophage polarization and subsequently the inhibition of hallmark features of asthma. Inhibition of TGF-β signaling was sufficient to inhibit the macrophage polarization in response to MSCs and consequently reserved the inhibitory effects of macrophage polarization on hallmark features of asthma. Collectively, our data demonstrate that human MSCs have immunosuppressive activity on asthma, which is mediated by TGF-β-signaling-dependent alveolar macrophage polarization.
MATERIALS AND METHODS
MSCs Isolation, Culturing, Differentiation, and Labeling
The MSCs were isolated and grown in culture as described [13, 14]. Briefly, plugs of human bone marrow from healthy donors were dispersed in Dulbecco's modified Eagle’s medium (DMEM) and then centrifuged at 900 g for 5 min. The pellets were resuspended and plated at 105 cells/cm2 in DMEM containing 10 % FBS. Thereafter, a recombinant lentivirus expressing eGFP under the control of CMV promoter (Invivogen: Itsint) efficiently infected MSCs at MOI 100 and resulted in nearly 100 % infection efficiency. After 10 passages’ selection of green fluorescent protein (GFP)-expressing infected cells, a positive clone was selected after subjected to chondrogenetic, osteogenic, and adipogenic differentiation assays to confirm a MSC phenotype. For chondrogenetic induction, 2.5 × 105 MSCs were induced with 5-ml chondrogenetic induction medium containing 10 μg TGF-β1 (R&D), 50 μg insulin growth factor 1 (R&D), and 2 mg/ml dexamethasone (DMSO, Sigma) followed by centrifugation at 500 g for 5 min. The cell pellets were maintained in the chondrogenetic induction medium for 14 days and subjected to Alcian blue staining. For osteogenic induction, cells were digested and seeded onto a 24-well plate at a density of 104 cells/well, and then maintained in osteogenic induction medium containing 10 nM vitamin D3 (Sigma) and 10 mM beta-phosphoglycerol and 0.1 μM DMSO for 14 days and were subjected to Von kossa staining. For adipogenic induction, cells were digested and seeded onto a 24-well plate at a density of 104 cells/well, and then maintained in the adipogenic induction medium containing 0.5 mM 3-isobutyl-1-methylxanthine (IBMX), 200 μM indomethacin, 10 μM insulin, and 1 μM dexamethasone (Sigma) for 14 days and subjected to Oil red O staining.
Mouse Handling
All mouse experiments were approved by the IACUC of Tongji University School of Medicine. Only 10-week-old male NOD/SCID mice were used for in vivo experiments.
Ovalbumin-Induced Allergic Asthma Model
Ten week-old male NOD/SCID mice were sensitized with an i.p. injection of 50 μg ovalbumin (OVA, grade V; Sigma-Aldrich, USA) with 2 mg aluminum hydroxide gel (Alum; Sigma-Aldrich). Control mice received phosphate-buffered saline (PBS) with 2 mg Alum. On day 7, 106 MSCs with/without 1 μg/kg body weight TGF-β receptor I inhibitor, SB-431542, were administrated in 200 μl sterile Dulbecco’s PBS (Invitrogen) via tail vein injections [10, 15]. On days 10, 11, 12, and 13, mice were challenged with 50 μg OVA by intranasal administration under light anesthesia.
Airway Hyperresponsiveness
Airway hyperresponsiveness (AHR) was measured by restrained invasive plethysmography 1 day after the last intranasal OVA challenge. Mice were anesthetized, after which a small incision was made to expose the trachea, and a cannula was inserted to connect to an inline nebulizer and ventilator. Mice were then challenged with aerosolized PBS followed by increasing doses of methacholine (Sigma-Aldrich). Airway resistance and dynamic compliance (Cdyn) were determined by analysis of pressure and flow waveforms (Buxco).
Bronchoalveolar Lavage, Lung Digestion, and Isolation of Macrophages or MSCs
Mice were euthanized by pentobarbitone overdose 2 days after the last intranasal OVA challenge. Bronchoalveolar lavage fluid (BALF) was obtained by instilling three washes of 0.4 ml PBS with 0.1 % BSA. BALF was centrifuged at 470 g for 5 min, and cells were enumerated and labeled for flow cytometric analysis. For lung digestion, lungs were perfused with PBS, after which 0.8 ml 300 U/ml collagenase type I (Sigma-Aldrich) and 50 U/ml DNase I (Roche) in RPMI 1640 was injected into the trachea. Lungs were then removed, minced into small pieces, and digested at 37 °C for 30 min. Lung pieces were disrupted with a syringe plunger, filtered through a nylon mesh, and centrifuged. The cell pellet was resuspended in RBC lysis buffer, washed, enumerated, and labeled for flow cytometric analysis. Macrophages were analyzed by flow cytometry with specific fluorescence-conjugated antibodies (F4/80 and CD163, all from BD), and MSCs were analyzed with direct GFP fluorescence. Data were collected on a FACSCalibur with CellQuest software and analyzed using FlowJo software. The percentage expression of each marker on MSCs was determined by the percentage of positive events, as determined by the isotype-matched negative control.
Western Blot
Purified macrophages or MSCs by FACS were lysed in RIPA buffer before the proteins were extracted for Western blot. Primary antibodies for Western blot are rabbit TGF-β1, TGF-β receptor I (TBRI), phosphorylated TGF-β receptor I (pTBRI), and alpha-tubulin (all purchased from Cell Signaling). Secondary antibody is HRP-conjugated anti-rabbit (Jackson Labs, USA). Images shown in the figure were representative from five individuals.
Statistics
Statistical analysis was performed with the unpaired two-tailed Student’s t test (for comparison between two groups), one-way ANOVA with the Tukey posttest (for comparison between three or more groups), or repeated-measures ANOVA with the Dunnett posttest (for AHR dose–response curves), using GraphPad Prism software. Data were represented as mean ± SD and were considered significant if p < 0.05.
RESULTS
Isolation, Culturing, and Differentiation of Primary Human MSCs
Primary human MSCs were isolated, labeled with GFP, and expanded in culture (Fig. 1a) [13, 14]. Differentiation assays including Von kossa staining to evaluate osteogenic induction, Oil red O staining to evaluate adipogenic induction, and Alcian blue staining to evaluate chondrogenetic induction were performed to confirm MSC phenotype (Fig. 1b–d).
MSC culture and differentiation assays. a GFP-labeled MSCs in culture. b–d MSC differentiation assay: Von kossa staining (b) to evaluate osteogenic induction, Oil red O staining (c) to evaluate adipogenic induction, and Alcian blue staining (d) to evaluate chondrogenetic induction. Scale bars are 50 μm.
MSCs Reduced Hallmarks of the Asthma
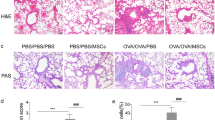
In order to evaluate the effect of transplantation of MSCs on allergic asthma, we used a well-established mouse allergic asthma model to study the effects of human MSCs (Fig. 2a) [16, 17]. In this model, mice were first sensitized to alum-adsorbed OVA, and then exposed to repeated airway provocation to develop AHR, as demonstrated by a dose-dependent increase in lung resistance (Rl) (Fig. 2b) and decrease in Cdyn in response to a cholinergic stimulus (methacholine) (Fig. 2c). We found that administration of MSCs just prior to acute allergen challenge significantly inhibited AHR (Fig. 2b, c). Another hallmark feature of OVA-induced allergic asthma, eosinophilic accumulation in the pulmonary airways, is also evaluated. Analysis of BALF from OVA-sensitized mice showed that the influx of inflammatory eosinophils increased to about 60 % of total BALF cells (Fig. 2d). Increased eosinophils were also found in lung digests in OVA-sensitized mice (Fig. 2e). In MSC-treated mice, we found a threefold decrease in the proportion of eosinophils recovered in the BALF and lungs after OVA challenge (Fig. 2d, e), suggesting that MSCs significantly reduced hallmarks of the asthma.
MSCs reduced hallmarks of the asthma. a Experimental schematic for assessing the effects of MSC transplantation on OVA inhalation challenge. b, c Rl (b) and Cdyn (c) in response to increasing doses of methacholine of unsensitized mice (PBS), OVA-sensitized mice (OVA), and OVA-sensitized mice treated with MSCs (OVA + MSC). d, e Percentage of BALF eosinophils (d) and lung eosinophils (e). NS no significance. *p < 0.05.
MSCs Induced Macrophage Polarization but not Changes in Number in OVA-Treated Lung
Since macrophages have been extensively reported to play critical roles during inflammatory modulation, we were prompted to check whether transplantation of MSCs may affect macrophage recruitment and polarization. We thus digested the lung and analyzed the macrophages by flow cytometry based on a pan-macrophage marker F4/80. We also differentiated macrophage subtypes based on a specific M2 macrophage marker, CD163 [10]. We found that transplantation of MSCs did not change the number of the total recruited macrophages in the OVA-treated lung but polarized most CD163-negative M1 macrophages into CD163-positive M2 macrophages (Fig. 3a–c).
MSCs induced macrophage polarization but not changes in number in OVA-treated lung. a, b Representative flow cytometry of digested lung for analysis of F4/80 and CD163 in the OVA-treated lung, with or without MSCs at D14. c Quantification of the percentage of macrophages in the lung and their M1/M2 subtype distribution. NS no significance. *p < 0.05.
MSC-Induced Polarization of Macrophages is Directed by TGF-β Signaling
To further elucidate the signaling pathways that control this MSC-triggered macrophage polarization, we isolated purified MSCs and macrophages from the lung by flow cytometry, taking advantage of their expression of GFP or F4/80, respectively (Fig. 4a). Interestingly, we found that MSCs expressed high levels of TGF-β1, while macrophages expressed high levels of TGF-β receptor I (TBRI) and its phosphorylated form (pTBRI), suggesting that TGF-β signaling pathway may play a role in the MSC-triggered macrophage polarization (Fig. 4b) [10]. To confirm the causal link, we gave the OVA-treated mice a combination of MSCs and 1 μg/kg body weight of SB-431542 (SB) [10], a specific TGFβ receptor I inhibitor, to inhibit TGF-β signaling (Fig. 2a). We found that application of SB significantly inhibited the polarization of macrophages by MSC transplantation, suggesting that the interplay between MSCs and macrophages is indeed via TGF-β signaling (Fig. 4c, d).
MSC-induced polarization of macrophages is directed by TGF-β signaling. a Representative flow cytometry plot for purifying MSCs and macrophages from the lung, taking advantage of their expression of GFP, and F4/80, respectively. b Western blot showed that MSCs expressed high levels of TGF-β1, while macrophages expressed high levels of TGF-β receptor I (TBRI) and its phosphorylated form (pTBRI). c, d Application of SB-431542 (SB), a specific TGF-β receptor I inhibitor, significantly inhibited the polarization of macrophages by MSC transplantation, without affecting total macrophages, showing in flow cytometry (c), and by quantification (d). NS no significance. *p < 0.05.
Inhibition of TGF-β Signaling Prevented Macrophage Polarization (SB), Which Prevented the Therapeutic Effect of MSC Transplantation
Moreover, inhibition of TGF-β signaling not only inhibited MSC-triggered macrophage polarization but also significantly abolished the therapeutic effect of MSCs on the hallmarks of the allergic asthma (Fig. 5a–d). Thus, our data demonstrate that human MSCs have immunosuppressive activity on asthma, which is mediated by TGF-β-signaling-dependent alveolar macrophage polarization.
Inhibition of TGF-β signaling prevented macrophage polarization, which prevented the therapeutic effect of MSC transplantation. a, b Rl (b) and Cdyn (c) in response to increasing doses of methacholine of unsensitized mice (PBS), OVA-sensitized mice treated with MSCs (OVA+MSC), and OVA-sensitized mice treated with MSCs and SB (OVA+MSC+SB). d, e Percentage of BALF eosinophils (d) and lung eosinophils (e). NS no significance. *p < 0.05.
DISCUSSION
MSCs have been shown to have highly immunosuppressive properties [1]. MSCs predominantly relocalized in the lungs when intravenously delivered, and they may secrete anti-inflammatory molecules that have local and distal effects [3]. Thus, pulmonary conditions could benefit from the immunosuppressive and anti-inflammatory activity of MSCs in the lungs. Allergic asthma is characterized by bronchial inflammation, hyperresponsive airways, and airflow obstruction, in which the sensitized immune system reacts adversely to subsequent inhalation exposure to innocuous environmental triggers [4, 5]. Animal asthma models have shown that administration of human or mouse bone marrow MSCs during airway challenge suppressed allergic responses [6], but the underlying mechanism remains largely unknown.
Inflammatory cells, and macrophages in particular (marked by F4/80 expression) can regulate bronchial inflammation, hyperresponsive airways, and airflow obstruction [7]. Besides the classically activated macrophages (also called M1 macrophages), which respond to inflammatory stimuli and appear early on, there are also the alternatively activated macrophages (M2 macrophages), which appear later, to mediate humoral immunity and tissue repair [8, 9]. M2 macrophages are known to secrete a wide range of chemokines, enzymes, and growth factors, to promote neovascularization, fibrosis, and tissue repair [10–12]. However, the polarization of macrophages in response to MSCs and their effects on asthma models have not been systematically investigated.
Here, we sought to determine the critical cellular and molecular responses that mediate the therapeutic effects of human MSCs on allergic asthma. First, we show that administration of MSCs isolated from human bone marrow provoked a pronounced anti-asthma effect, consistent with previous reports. Then, we analyzed local macrophages and their polarization. We found that the alveolar macrophages did not change their number in response to MSCs but were polarized predominantly to M2 subtypes. Moreover, the polarized M2 macrophages appeared to be responsible for the inhibition of the hallmark features of asthma, including airway hyperresponsiveness and eosinophilic accumulation. Furthermore, TGF-β signaling pathway appeared to mediate the effects of MSCs on macrophage polarization and subsequently the inhibition of hallmark features of asthma, in which MSCs released TGF-β1, which may bind to the receptor in macrophages to trigger downstream cascades to induce M2 polarization. We did check other TGF-β receptor ligands, and we did not find pronounced changes in them. Thus, inhibition of TGF-β signaling was sufficient to inhibit the macrophage polarization in response to MSCs and consequently reserved the inhibitory effects of macrophage polarization on hallmark features of asthma.
Collectively, our data demonstrate that human MSCs have immunosuppressive activity on asthma, which is mediated by TGF-β-signaling-dependent alveolar macrophage polarization. Therefore, TGF-β signaling, as well as polarized macrophages, appears to be a novel therapeutic target for allergic asthma.
References
Zipori, D. 2004. Mesenchymal stem cells: harnessing cell plasticity to tissue and organ repair. Blood Cells, Molecules & Diseases 33: 211–215.
Uccelli, A., L. Moretta, and V. Pistoia. 2008. Mesenchymal stem cells in health and disease. Nature Reviews Immunology 8: 726–736.
Fischer, U.M., M.T. Harting, F. Jimenez, W.O. Monzon-Posadas, H. Xue, S.I. Savitz, et al. 2009. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells and Development 18: 683–692.
Holgate, S.T. 2008. Pathogenesis of asthma. Clinical and Experimental Allergy 38: 872–897.
Holgate, S.T. 2008. The airway epithelium is central to the pathogenesis of asthma. Allergology International: Official Journal of the Japanese Society of Allergology 57: 1–10.
Bonfield, T.L., M. Koloze, D.P. Lennon, B. Zuchowski, S.E. Yang, and A.I. Caplan. 2010. Human mesenchymal stem cells suppress chronic airway inflammation in the murine ovalbumin asthma model. American Journal of Physiology - Lung Cellular and Molecular Physiology 299: L760–L770.
Sica, A., and A. Mantovani. 2012. Macrophage plasticity and polarization: in vivo veritas. Journal of Clinical Investigation 122: 787–795.
Martinez, F.O., L. Helming, and S. Gordon. 2009. Alternative activation of macrophages: an immunologic functional perspective. Annual Review of Immunology 27: 451–483.
Gordon, S., and F.O. Martinez. 2010. Alternative activation of macrophages: mechanism and functions. Immunity 32: 593–604.
Xiao, X., I. Gaffar, P. Guo, J. Wiersch, S. Fischbach, L. Peirish, et al. 2014. M2 macrophages promote beta-cell proliferation by up-regulation of SMAD7. Proceedings of the National Academy of Sciences of the United States of America 111: E1211–E1220.
Ricardo, S.D., H. van Goor, and A.A. Eddy. 2008. Macrophage diversity in renal injury and repair. Journal of Clinical Investigation 118: 3522–3530.
Pull, S.L., J.M. Doherty, J.C. Mills, J.I. Gordon, and T.S. Stappenbeck. 2005. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proceedings of the National Academy of Sciences of the United States of America 102: 99–104.
Tropel, P., D. Noel, N. Platet, P. Legrand, A.L. Benabid, and F. Berger. 2004. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Experimental Cell Research 295: 395–406.
Zhou, B., X.C. Cao, Z.H. Fang, C.L. Zheng, Z.B. Han, H. Ren, et al. 2007. Prevention of diabetic microangiopathy by prophylactic transplant of mobilized peripheral blood mononuclear cells. Acta Pharmacologica Sinica 28: 89–97.
Inman, G.J., F.J. Nicolas, J.F. Callahan, J.D. Harling, L.M. Gaster, A.D. Reith, et al. 2002. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Molecular Pharmacology 62: 65–74.
Lee, H.H., E.H. Meyer, S. Goya, M. Pichavant, H.Y. Kim, X. Bu, et al. 2010. Apoptotic cells activate NKT cells through T cell Ig-like mucin-like-1 resulting in airway hyperreactivity. Journal of Immunology 185: 5225–5235.
Pichavant M, Goya S, Hamelmann E, Gelfand EW, Umetsu DT. Animal models of airway sensitization. Current Protocols in Immunology 2007; Chapter 15:Unit 15 18.
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (81100018) and The Young Scientist Grant of Shanghai Health Bureau (2010Y039).
Conflict of Interest
The authors have declared that no competing interests exist.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, X., Xie, S., Lu, K. et al. Mesenchymal Stem Cells Alleviate Experimental Asthma by Inducing Polarization of Alveolar Macrophages. Inflammation 38, 485–492 (2015). https://doi.org/10.1007/s10753-014-9954-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-014-9954-6