Abstract
Chronic asthma is characterized by airway hyperresponsiveness, inflammation, and remodeling. Previous studies have shown that mesenchymal stromal/stem cells (MSCs) exert anti-inflammatory effects on asthma via regulation of the immune cells. However, the therapeutic mechanism of MSCs, especially the mechanism of airway remodeling in chronic asthma, remains to be elucidated. Here, we aimed to investigate the therapeutic effect of MSCs on airway remodeling in chronic asthma and explored the mechanisms by analyzing the polarization phenotype of macrophages in the lungs. We established a mouse model of chronic asthma induced by ovalbumin (OVA) and evaluated the effect of MSCs on airway remodeling. The data showed that MSCs treatment before the challenge exerted protective effects on OVA-induced chronic asthma, i.e., decreased the inflammatory cell infiltration, Th2 cytokine levels, subepithelial extracellular matrix deposition, and transforming growth factor β (TGF-β)/Smad signaling. Additionally, we found that MSCs treatment markedly suppressed macrophage M2 polarization in lung tissue. At the same time, MSCs treatment inhibited NF-κB p65 nuclear translocation, ER stress, and oxidative stress in the OVA-induced chronic allergic airway remodeling mice model. In conclusion, these results demonstrated that MSCs treatment prevents OVA-induced chronic airway remodeling by suppressing macrophage M2 polarization, which may be associated with the dual inhibition of ER stress and oxidative stress. This discovery may provide a new theoretical basis for the future clinical application of MSCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Allergic asthma is a common chronic respiratory disorder characterized by airway hyperresponsiveness and inflammatory cell infiltration, affecting 1–18% of people internationally. Allergic airway inflammation consists of various inflammatory cells, including eosinophils, macrophages, mast cells, neutrophils, and T cells [1]. Long-term inflammatory cell infiltration and cytokine secretion drive airway microenvironmental changes, which cause dysregulated tissue repair processes and airway remodeling. Airway remodeling is associated with a progressive decline in lung function, affecting patients’ quality of life, although current treatment options are limited.
Macrophages are the most abundant immune cells in the lungs and play a considerable role in innate and adaptive immunity. Two functionally different macrophage phenotypes have been studied: classically activated type I macrophages (M1) and alternatively activated type II macrophages (M2). M1 macrophages exert pro-inflammatory effects response to inflammatory stimuli in the early stage, while M2 macrophages display anti-inflammatory effects and participate in tissue repair in the later [2]. Accumulating studies indicate that abnormal differentiation and dysfunction of M2 macrophages promote the recruitment and proliferation of fibroblasts and secretion of fibroblast mediators, which are essential causes of pulmonary fibrosis and airway remodeling [3,4,5,6].
Mesenchymal stromal/stem cells (MSCs) are pluripotent stem cells that can be separated from many tissues, such as bone marrow (BM), umbilical cord tissue, and adipose tissue (AD). They provide a new treatment strategy for various diseases because of their simple isolation, expansion potential, and low immunogenicity. Particularly, MSCs are capable of secreting paracrine factors, which can regulate immune response [7]. Accumulating studies have reported that MSCs benefit inflammatory and autoimmune disorders, including inflammatory bowel disease, graft-versus-host disease, and rheumatoid arthritis [8,9,10]. The role of MSCs in relieving allergic airway inflammation has also been demonstrated and is possibly associated with the immune cell regulation capability of MSCs in the lung [11, 12]. Studies have shown that MSCs play a therapeutic role in the treatment of pulmonary fibrosis diseases through M2 macrophage immunomodulation [13]. Although several studies have shown that MSCs can reduce extracellular matrix (ECM) deposition [14, 15], the role and mechanism of MSCs in allergic airway remodeling by regulating macrophage M2 polarization is still unclear.
The endoplasmic reticulum (ER) is an organelle that plays a vital role in biosynthesis, correct protein folding, and post-translational modification of secreted and membrane proteins. An accumulation of unfolded or misfolded proteins causes an ER stress response, activating the unfolded protein response (UPR), an important physiological process in many inflammatory diseases. Current studies find that ER stress and associated signaling networks are becoming important factors in regulating airway inflammation and immune responses in asthma [16]. Increased ROS production is a key feature of asthma [17]. Superoxide dismutase (SODs) and glutathione peroxidase (GSH-Px) are common antioxidant enzymes that can help the body resist the damage of ROS. Heme oxygenase 1 (HO-1) is the target gene of nuclear factor erythroid 2-related factor 2 (Nrf2), the main factor regulating redox homeostasis. Targeting the Nrf2/HO-1 pathway also helps to alleviate reactive oxygen species (ROS)-mediated airway inflammation and remodeling [18]. Studies have shown that MSCs can protect myocardial infarction by regulating macrophage polarization through Nrf2/HO-1 pathway [19]. Currently, it remains unclear whether the ER stress and oxidative stress pathway is involved in MSCs regulation of macrophage polarization in chronic allergic airway remodeling.
Therefore, we aimed to investigate whether MSCs treatment can improve chronic allergic airway remodeling through macrophage polarization, explicitly focusing on MSCs affect the macrophage polarization in allergic airway remodeling through ER stress and oxidative stress.
MATERIALS AND METHODS
Animals
Female BALB/c mice (14 ± 1 g) were purchased from Laboratory Animal Corporation (China). All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Xinhua Hospital, Shanghai Jiao Tong University School of Medicine. All animals had free access to water and food in a specific pathogen-free clean environment (light/dark cycle 12 h/12 h) and were raised for 1 week before the intervention.
Experimental Animal Models and Human BM-MSCs Transplantation
Mice were divided into four groups: control (phosphate-buffered saline [PBS]/PBS/PBS group), control treated with MSCs (PBS/PBS/MSCs group), chronic asthma (ovalbumin (OVA)/OVA/PBS group), and chronic asthma treated with MSCs (OVA/OVA/MSCs group) groups. The experimental chronic asthma mouse model was established as previously described [20]. The chronic asthma group mice were intraperitoneally injected with 20 μg OVA (grade V; Sigma-Aldrich, St. Louis, MO, USA) and 2.25 mg aluminum hydroxide on days 0 and 14 of the experiment. On days 21–27, sensitized mice were given a 5% OVA aerosol challenge for 30 min. Then, three times a week for 8 weeks. The control group mice were treated with an equal volume of PBS. Human bone marrow mesenchymal stem cells (hBM-MSCs, Cyagen Biosciences Inc., Guangzhou, China) were used before the 10th passage. The MSCs treated groups were injected with 5*10^6 cells/mL (200 µL) MSCs on days 20, 22, and 24 via the tail vein, while the PBS treated groups were administered PBS injections (Fig. 1a).
MSCs treatment reduced airway inflammation in chronic asthma mice. a Schematic diagram showing the mouse model of chronic allergic asthma. b Representative H&E staining of lung tissue (200×). Scale bar: 50 μm. c Representative PAS staining of lung tissue (200×). Scale bar: 50 μm. d Semi-quantitative analyses of the inflammatory cell in the lung sections. e Semi-quantitative analyses of the PAS-positive cells in the lung sections. The data represent the means ± standard errors of the mean (SEM). * represents versus the PBS/PBS/PBS group. *p < 0.5, **p < 0.01, ***p < 0.001. # represents versus the OVA/OVA/PBS group. #p < 0.5, ##p < 0.01, ###p < 0.001.
Lung Histology
Lung tissue was fixed with 4% paraformaldehyde overnight. Lung sections were stained with hematoxylin and eosin (H&E), periodic acid–Schiff (PAS), or Masson’s trichrome stain to assess airway inflammation and remodeling. As previously mentioned, amount of inflammation and PAS-positive cells were calculated using a reproducible scoring system [21]. Masson area analysis was performed using ImageJ.
Collection of Serum and Bronchoalveolar Lavage Fluid (BALF)
The serum was obtained by cardiac puncture, centrifuged at 500 g for 15 min, and collected as supernatant. The serum was used for subsequent cytokine detection; 800 µL PBS was used for bronchoalveolar lavage, and the obtained BALF was centrifuged at 500 g for 10 min. The supernatant was collected for subsequent cytokine detection, and the pellet was analyzed by the Wright-Giemsa staining method for cell composition.
Serum and BALF Cytokine Analysis
The cytokine expressions of interleukin (IL)-4, IL-5, and IL-13 in the serum and BALF were assayed using enzyme-linked immunosorbent assay kits from Anogen (Mississauga, Ontario, Canada) in accordance with the manufacturer’s directions.
Real-Time Polymerase Chain Reaction (PCR)
RNA was extracted from the lung tissue using TRIzol reagent (Takara Biotechnology, Dalian, China). After tissue grinding, chloroform, isopropanol, and 75% alcohol were added to achieve RNA precipitation and subsequent synthesis of cDNA using Prime Script RT Master Mix (Takara Biotechnology). Real-time PCR was conducted with TB Green® Premix Ex Taq™ (Takara Biotechnology) on the ABI 7500 System (Applied Biosystems, Foster City, CA, USA) following the manufacturer’s instructions. The relative transcript expressions were determined by the 2-△△CT method, normalized to glyceraldehyde-3-phosphate dehydrogenase. The primers used were as follows: TNF-α F-GCGACGTGGAACTGGCAGAAG; R-GAATGAGAAGAGACATAGGC; IL-6 F-ACTTCCATCCAGTTGCCTTCTTGG; R-TTAAGCCTCCGACTTGTGAAGTGG; IL-1β F-TTCAGGCAGGCAGTATCACTCATTG; R-ACACCAGCTATCATCATCATCC; CD206 F-AGTCAGAACAGACTGCGTGG; R-CCAGAGGGATCGCCTGTTTT; CD68 F-CCTCTTGCTGCCTCTCATCATTGG; R-GGCTGGTAGGTTGATTGTCGTCTG; TGF-β1 F-CACCTGCAAGACCATCGACA; R-CATAGTAGTCCGCTTCGGGC; iNOS F-GGAGTGACGGCAAACATGACT; R-TCGATGCACAACTGGGTGAAC; Arg1 F-CTCCAAGCCAAAGTCCTTAGAG; R-AGGAGCTGTCATTAGGGACATC; COL-I F-GCTCCTCTTAGGGGCCACT; R-CCACGTCTCACCATTGGGG; and COL-III F-CTGTAACATGGAAACTGGGGAAA; R-CCATAGCTGAACTGAAAACCACC. Primer set for GAPDH (CAT# B66130 4) was purchased from Sangon Biotech (Shanghai, China).
Western Blotting
Total protein from the lung was extracted using a lysis solution comprising radioimmunoprecipitation assay buffer, phenylmethylsulfonyl fluoride, and phosphatase inhibitors. The sample protein (30 µg) was loaded in sodium dodecyl sulfate-polyacrylamide gel and isolated by electrophoresis. Subsequently, the target proteins attached to the gel were transferred to polyvinylidene difluoride membranes and blocked with 5% non-fat milk. The membranes were then incubated with antibodies against transforming growth factor β (TGF-β), p-Smad2/3, Smad2/3, GRP-78 (Cell Signaling Technology, USA), tubulin (Abcam, UK), ATF4, HO-1 (Santa Cruz Biotechnology, USA), CHOP (HUABIO, China), and Nrf2 (Proteintech, China), overnight at 4 °C. After being washed thoroughly with tris-buffered saline with Tween-20 (TBST), the membranes were incubated with a secondary antibody for 1 h at room temperature. After the membranes were washed thoroughly with TBST again, an enhanced chemiluminescence kit (Millipore, Billerica, MA) was used to visualize protein expression on the polyvinylidene difluoride membranes.
Immunohistochemistry
Immunohistochemistry was conducted to assess the markers of airway remodeling. After deparaffinization, hydration, and antigen retrieval, the paraffin sections of lung tissue were blocked after 30 min in 3% bovine serum albumin (BSA) at room temperature. Then, the sections were stained with anti-collagen I and anti-collagen III (Servicebio, China) primary antibodies overnight at 4 °C. The antibody dilution ratio in this experiment was 1:200. Subsequently, a secondary antibody was used for the incubation of sections at room temperature for 50 min. The sections were observed under an Olympus microscope at magnification ×200.
Immunofluorescence
Lung paraffin slices were retrieved using citrate antigen retrieval solution (pH 6.0). Next, the slices were blocked with BSA for 30 min in the dark. The slices from each mice group were subjected to anti-CD206 (Servicebio, China) and anti-NF-κB p65 (Servicebio, China) primary antibodies overnight at 4 °C. The antibody dilution ratio in this experiment was 1:100. The next day, the slices were incubated with a fluorescent-conjugated secondary antibody for 50 min. The nuclei were stained with 4′,6-diamidino-2-phenylindole in the dark for 10 min. The slices were observed using an Olympus microscope at magnification ×400.
Statistical Analyses
All data in this experiment were analyzed using the GraphPad Prism 8.0 (La Jolla, CA, USA). Multiple group comparisons were determined by a one-way analysis of variance, and the data were expressed as means ± standard errors of the mean (SEM) and considered statistically significant when p values < 0.05.
RESULTS
Administration of MSCs Reduced Airway Inflammation in Chronic Asthma Mice
To determine whether MSCs have an effect on chronic allergic airway inflammation, a chronic asthma mouse model was established via repeated OVA challenges. As shown in H&E staining, MSCs injection did not cause a large inflammatory cell infiltration in the PBS/PBS/MSCs group, suggesting that the MSCs injection had no inflammatory response because of itself. Simultaneously, H&E staining demonstrated that prominent inflammatory cell infiltration in the lung of OVA/OVA/PBS group mice compared with PBS/PBS/PBS group, while these inflammatory cells were significantly decreased by MSCs treatment (Fig. 1b, d). Goblet cell hyperplasia was assessed with PAS-positive cells. The number of PAS-positive cells was higher in the OVA/OVA/PBS group, whereas MSCs administration resulted in a significant decrease in the number of PAS-positive cells (Fig. 1c, e). Next, the inflammatory cells and cytokine levels in BALF and serum of each group were examined. In BALF, the number of total inflammatory cells and differential subsets were significantly increased in the OVA/OVA/PBS group. Then, the MSCs injection reduced the number of inflammatory cells, such as eosinophils, lymphocytes, and macrophages recruited to the lungs in BALF (Fig. 2a). Because Th2 cells play an essential role in allergic asthma, we subsequently observed Th2 cytokine expression in serum and BALF in the different groups. As shown in Fig. 2b–g, IL-4, IL-5, and IL-13 levels in BALF and serum of the OVA/OVA/PBS group mice was substantially enhanced than that in PBS/PBS/PBS group mice. However, MSCs treatment significantly reversed the enhanced expression of IL-4, IL-5, and IL-13 in BALF and serum. These data suggest that MSCs are able to inhibit the inflammatory cells infiltration, lower the Th2 cytokine levels, and alleviate pathology observed in chronic allergic asthma mice.
MSCs treatment reduced airway inflammatory cells and cytokine in chronic asthma mice. a The number of total cells and differential cell counts in BALF. b–d The levels of IL-4, IL-5, and IL-13 in BALF. e–g The levels of IL-4, IL-5, and IL-13 in serum. The data represent the means ± standard errors of the mean (SEM). * represents versus the PBS/PBS/PBS group. *p < 0.5, **p < 0.01, ***p < 0.001. # represents versus the OVA/OVA/PBS group. #p < 0.5, ##p < 0.01, ###p < 0.001.
Administration of MSCs Inhibited Airway Remodeling in Chronic Asthma Mice
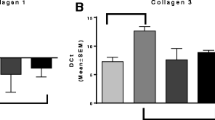
First, based on the analysis of Masson staining, we confirmed that repeated challenges with OVA exhibited increased ECM deposition in the subepithelial region. This increase in ECM deposition was reduced by MSCs treatment (Fig. 3a, b). Next, the expression of collagen was detected in the peribronchial lung tissue by real-time PCR and immunohistochemistry. Both the PCR and immunohistochemistry results indicated that the expression of collagen I and collagen III in the lung tissue was markedly elevated in the OVA/OVA/PBS group than in the PBS/PBS/PBS group mice; however, collagen deposition and its expression significantly decreased with MSCs treatment (Fig. 3c–g). The above results suggest that MSCs treatment can prevent collagen synthesis and ECM deposition following repeated OVA challenges.
MSCs treatment prevented airway remodeling in chronic asthma mice. a, b Representative Masson trichrome staining images and semi-quantitative analysis of lung sections in each group (200×). Scale bar: 50 μm. c, d The mRNA expression of collagen I and collagen III in each group. e–g Immunohistochemistry was performed to evaluate collagen I and collagen III expression in the lung tissue and semi-quantitative analysis (200×). Scale bar: 50 μm. The data represent the means ± standard errors of the mean (SEM). * represents versus the PBS/PBS/PBS group. *p < 0.5, **p < 0.01, ***p < 0.001. # represents versus the OVA/OVA/PBS group. #p < 0.5, ##p < 0.01, ###p < 0.001.
MSCs Administration Inhibits the TGF-β1-Smad2/Smad3 Signaling Pathway in Chronic Asthma Mice
To further validate the effect of MSCs treatment on the regulation of airway remodeling, we tested the expression of the TGF-β1-Smad2/Smad3 signaling pathway. The expression of TGF-β1 in lung tissue was measured by real-time PCR and western blotting. The OVA/OVA/PBS group showed a significant increase in TGF-β1 mRNA and protein levels, whereas MSCs treatment decreased TGF-β1 levels (Fig. 4a–c). The expression of p-Smad2/Smad3 and Smad2/Smad3 was measured by western blotting. Our data showed that the p-Smad2/Smad3 protein level was increased in the OVA-induced mice; however, the increased expression of the p-Smad2/Smad3 was reversed by MSCs treatment (Fig. 4b, d). Therefore, it is indicated that MSCs treatment regulates chronic airway remodeling by weakening the TGF-β1-Smad2/Smad3 signaling in the OVA-induced chronic asthma mice.
MSCs treatment decreased the levels of the TGF-β1/Smad2/Smad3 pathway in chronic asthma mice. a The mRNA expression of TGF-β1 was measured by real-time PCR in lung tissue. b Western blot analysis of TGF-β1, p-Smad2/Smad3, and Smad2/3 expression in the lungs from each group. c, d Quantification of TGF-β1, p-Smad2/Smad3, and Smad2/3 expression. The data represent the means ± standard errors of the mean (SEM). * represents versus the PBS/PBS/PBS group. *p < 0.5, **p < 0.01, ***p < 0.001. # represents versus the OVA/OVA/PBS group. #p < 0.5, ##p < 0.01, ###p < 0.001.
MSCs Suppress OVA-Induced M2 Polarization of Macrophages in the Lung
We further evaluated the effect of MSCs on macrophage polarization in the chronic asthma mouse model. Compared with the PBS//PBS/PBS group, the mRNA expression of CD68, a marker for macrophages, was significantly increased in the OVA/OVA/PBS group but declined after treatment with MSCs. In particular, the mRNA expression of CD206, a marker for M2 macrophages, was significantly increased in the OVA/OVA/PBS group but declined after treatment with MSCs (Fig. 5a, b). The expression of CD206 was also verified by immunofluorescence, and the result was consistent with PCR results (Fig. 5c). Moreover, the mRNA expression of arginase-1 (Arg1), another marker of M2 macrophages, was significantly higher in the OVA-induced asthma model than that of the control group, and the MSCs also markedly inhibited the over-expression (Fig. 5d). However, it had no statistically significant effect on the expression of the M1 macrophage marker, inducible nitric oxide synthase (iNOS) (Fig. 5e). Similarly, the expression of macrophage cytokine TNF-α, IL-6, and IL-1β observed a statistical decrease in the lung tissue after MSCs treatment (Fig. 5f–h). These data verified that chronic allergic airway remodeling mouse model is dominated by macrophage M2 polarization, and MSCs exert immunoregulatory effects on the polarization of M2 macrophages.
MSCs treatment inhibited M2 macrophages polarization in chronic asthma mice. a, b The mRNA expressions of CD68 and CD206 were studied by real-time PCR. c Representative immunofluorescence images of CD206 (400×). Scale bar: 20 μm. d, e The mRNA expressions of Arg1 and iNOS were determined by real-time PCR. f–h Relative mRNA expression levels of IL-1β, TNF-α, and IL-6 in lung tissue of each group. The data represent the means ± standard errors of the mean (SEM). * represents versus the PBS/PBS/PBS group. *p < 0.5, **p < 0.01, ***p < 0.001. # represents versus the OVA/OVA/PBS group. #p < 0.5, ##p < 0.01, ###p < 0.001.
MSCs Administration Inhibits the NF-κB p65 Nuclear Translocation, ER Stress, and Oxidative Stress in Lung Tissue
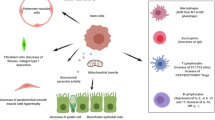
Previous studies have found that NF-κB signaling pathways, ER stress, and oxidative stress are involved in macrophage polarization. In our study, we used immunofluorescence to investigate the effect of MSCs on NF-κB p65 nuclear translocation in the lung tissues. The nuclear level of NF-κB p65 was markedly elevated in the OVA/OVA/PBS group than in the PBS/PBS/PBS group mice; however, the nuclear level of NF-κB p65 was remarkably suppressed by the administration with MSCs (Fig. 6a, b). Previous studies have found that oxidative stress and ER stress aggravate airway inflammation involved in the pathogenesis of asthma. Western blot analysis was used to examine the expression of ER stress and oxidative stress marker proteins. The OVA/OVA/PBS group showed a significant increase in ATF4, CHOP, and GRP78 protein levels, whereas MSCs treatment decreased ATF4, CHOP, and GRP78 levels (Fig. 7a–d). Results also showed that OVA treatment markedly inhibited Nrf2 and HO-1 expression, while Nrf2 and HO-1 expression levels were significantly increased after MSCs administration (Fig. 7e–g). The above data indicated that MSCs may regulate macrophage M2 polarization by affecting NF-κB signaling pathways, ER stress, and Nrf2/HO-1 signaling pathways in chronic allergic airway remodeling model (Fig. 8).
MSCs treatment inhibited NF-κB pathway in chronic asthma mice. a, b Representative immunofluorescence images of NF-κB p65 (400×). Scale bar: 20 μm. The data represent the means ± standard errors of the mean (SEM). * represents versus the PBS/PBS/PBS group. *p < 0.5, **p < 0.01, ***p < 0.001. # represents versus the OVA/OVA/PBS group. #p < 0.5, ##p < 0.01, ###p < 0.001.
MSCs treatment inhibited ER stress and oxidative stress in chronic asthma mice. a The protein expressions of ER-stress related marker GRP78, CHOP, and ATF4 were studied by western blot. b–d Quantification of GRP78, CHOP, and ATF4 expression in lung tissue of each group. e The protein expressions of Nrf2 and HO-1 were studied by western blot. f–g Quantification of Nrf2 and HO-1 expression in lung tissue of each group. The data represent the means ± standard errors of the mean (SEM). * represents versus the PBS/PBS/PBS group. *p < 0.5, **p < 0.01, ***p < 0.001. # represents versus the OVA/OVA/PBS group. #p < 0.5, ##p < 0.01, ###p < 0.001.
MSCs treatment prevents OVA-induced chronic asthma mice airway remodeling by suppressing macrophage M2 polarization via ER stress, oxidative stress, and NF-κB pathway (created with BioRender.com).
DISCUSSION
Currently, inhaled long-acting β2-adrenergic agonists and glucocorticoids are the traditional treatments for asthma to relieve symptoms, expand broncho-constriction, and inhibit airway inflammation [22]. However, these methods have a low effect on airway remodeling, and due to long-term use, some patients still have low compliance or adverse side effects. Therefore, as a new method to treat asthma, cell therapy is receiving more attention.
This study assessed whether MSCs treatment can improve chronic allergic airway remodeling and the detailed mechanisms. Based on some reports, green fluorescent protein (GFP)-transfected iPSC-MSCs were observed in the lung at 1 h, 4 h, 1 day, and even 4 days after intratracheal transplantation in OVA-treated mice [23]. GFP-iPSC-MSCs were present in the lungs on day 1 and day 7 after tail vein transplantation of OVA-treated mice, but not on day 10 [15]. Therefore, whether the MSCs with short survival time have a long-term effect on chronic allergic airway inflammation and remodeling is one of the key points observed in this study. Studies have shown that BM-MSCs are more effective than AD-MSCs in improving lung mechanics and lung remodeling [24, 25]. Therefore, BM-MSCs were studied in this experiment. Our study used an OVA-sensitized and challenged BALB/c mice asthma animal model, which is now considered the gold standard for this type of research. In order to better simulate chronic airway remodeling, our study extended the modeling time to 84 days. Our experimental results agreement with previous showed that BM-MSCs treatment could also attenuate 84 days-repeated OVA-induced chronic asthma mice inflammatory cell infiltration, goblet cell hyperplasia, the level of Th2 cytokines, subepithelial collagen deposition, and the expression of collagen I and collagen III in the lung tissue.
MSCs are multipotent stem cells with their self-renewal and differentiation abilities. In recent years, in addition to their direct differentiation potential, researchers have shown great interest in the immunomodulatory function of MSCs. Macrophages are vital inflammatory cells in the pathogenesis of asthma. The two phenotypes of macrophages play different roles in lung inflammatory injury and tissue repair. Our study explored the phenotype of macrophages involved in OVA-induced allergic airway inflammation and remodeling. We found that the expression of M2 macrophage phenotype markers, such as CD206 and Arg1, was significantly increased in chronic allergic asthma mice. In contrast, the expression of M1 macrophage phenotype markers, such as iNOS, had no statistically significant change. It suggested that M2 macrophages are the key cells in chronic allergic airway inflammation and remodeling mouse model. And our study found that the expression of M2 polarization markers, CD206 and Arg1, was markedly reduced in OVA-induced mice after MSCs treatment. This result is inconsistent with previous research showed that MSCs can regulate a pro-inflammatory M1 phenotype toward anti-inflammatory M2 types capable of regulating immune responses to alleviate airway inflammation in asthma mice model [26]. The reason may be related to the fact that OVA-sensitized and challenged allergic airway inflammation mainly induces Th2 cell inflammatory response. Studies have shown that M1 macrophages are mainly involved in non-allergic airway inflammation associated with the Th1/Th17 inflammatory response. However, M2 macrophages regulate eosinophilic infiltration in Th2 cell inflammation, thereby participating in allergic airway inflammation [27]. Another reason may be related to chronic inflammation, as studies have shown that in chronic injuries, inflammatory mediators can transform M1 macrophages into M2 macrophages, which are responsible for ECM deposition. M2 macrophage phenotype includes several subtypes. IL-4 or IL-13 could induce M2a macrophages with high expression of CD206, Arg1, resist in-like molecule-α, and primary histocompatibility complex class II involved in the Th2 immune response. M2b macrophages could be induced by the immune complex, which produces anti- and pro-inflammatory cytokines such as IL-10, IL-1β, IL-6, and TNF-α. M2c macrophages produce IL-10 and TGF-β, which are associated with tissue remodeling and matrix deposition [28]. Although several studies have shown that M2 macrophages are essential for alleviating airway inflammation responses, excessive M2 macrophages may promote goblet cell hyperplasia and collagen deposition, leading to airway remodeling. A study by Guo et al. showed that inhibiting polarization of M2 macrophage by Schisandra prevents the development of idiopathic pulmonary fibrosis [29]. Another study also demonstrated that microcystin-LR ameliorates pulmonary fibrosis by suppressing M2 macrophage polarization [30]. This experiment revealed that MSCs could inhibit airway remodeling in chronic allergic asthma by inhibiting M2 macrophage activation.
TGF-β1 is a pro-fibrotic factor that plays a vital role in airway remodeling. Smads are vital downstream signal transduction molecules of the TGF-β superfamily. Previous studies have shown that the TGF-β1/Smad pathway leads to collagen synthesis and extracellular matrix imbalance, contributing to airway remodeling [31, 32]. In addition, studies have also shown that TGF-β1/Smad pathways also mediate macrophage M2 polarization reprogram [33]. In general, when TGF-β1 is activated, TGF-β1 binds to the cell’s TGF-β receptor type II (TβRII). Then, TβR II recruits and phosphorylates TGF-β receptor type I (TβRI) and phosphorylates Smad2/3, subsequently translocating the activated Smad complex into the nucleus and regulating other transcriptional factors [34]. However, the TGF-β dependent M2 phenotype reprogramming was controlled by Smad7. Smad7 can inhibit Smad2/3 phosphorylation by binding with TβRI or degrading Smad2/3 by proteasomes [35]. In our study, we observed a decreased expression of TGF-β1 and p-Smad2/3 in OVA-induced mice after MSCs treatment. These results show that MSCs inhibited chronic allergic airway remodeling by regulating the TGF-β1/Smad2/3 signaling pathway. This may also be one of the key mechanisms by which MSCs inhibit the polarization of M2 macrophages in OVA-induced chronic asthma mice model.
CHOP and GRP-78 are important markers of ER stress. Recently, studies have shown that abnormal expression of CHOP has been reported in asthmatic patients and allergic asthma animal models. ER stress has been shown to be activated by PERK, which leads to ATF-4 upregulation through eIF2a. Wang et al. findings showed that loss of CHOP protects mice from OVA-induced allergic airway remodeling by inhibiting M2 macrophages polarization in the asthma. The mechanism may be related to regulating macrophage M2 programming by modulating IL-4 positive feedback loops [36]. Our study investigated the effects of MSCs on ER stress in chronic asthma mice model. The results showed that the expression of CHOP, GRP-78, and ATF4 protein was significantly increased in the OVA-induced chronic allergic asthma mice model, while MSCs strongly decreased the ER stress in this study, which is consistent with the role of MSCs in other models [37, 38]. Nrf2 is released from the KEAP1-Nrf2 complex in response to stress signals, then translocated to the nucleus, binds to the target gene promoter’s antioxidant response element (AREs), activates transcription of downstream genes, and thus exerts physiological functions. Nrf2 is a transcription factor that regulates the cell protection gene HO-1 [39]. The important role of Nrf2 in pulmonary fibrosis has been reported in previous studies. Wu et al. revealed that 4-OI inhibits M2 macrophage activation and M2-related TGF-β1 production by activating Nrf2 in bleomycin-induced pulmonary fibrosis model [40]. In the present study, we also found that MSCs strongly activated Nrf2/HO-1 pathway in chronic asthma mice. Thus, we hypothesized that MSCs might regulate macrophage M2 polarization by dual inhibition ER stress and oxidative stress in chronic allergic airway remodeling.
The therapeutic advantages of MSCs may be related to their paracrine functions, such as the release of exosomes. MSC-derived exosomes are therapeutic membrane-derived vesicles through their bioactive components, such as various RNAs, lipids, and proteins [41]. MSCs-derived exosomes have been observed to show beneficial effects similar to MSCs in mouse models of asthma. Dehnavi et al. reported that sublingual administration of OVA-loaded MSC-derived exosomes inhibited airway allergic inflammation and improved immunomodulatory responses [42, 43]. The research by Shan et al. suggested that MSC-derived exosomes suppress airway remodeling in asthmatic mice through the miR-188/JARID2/Wnt/β-catenin axis [44]. In the future, we will explore the role and mechanism of MSC-derived exosomes in chronic allergic airway remodeling.
One of the limitations of this study is the lack of further in vitro studies to better reveal the regulatory effect of MSCs on macrophage M2 polarization. In addition, the use of inhibitors or activators of endoplasmic reticulum stress pathways and oxidative stress pathways may also help to confirm the conclusions of this in vivo study.
CONCLUSION
MSCs, as a new cell therapy, was able to prevent airway remodeling in mouse model of chronic asthma. In addition, MSCs attenuated chronic allergic airway remodeling, which is related to the immunomodulatory function of inhibiting M2 macrophage polarization. Mechanistically, this may be associated with the dual inhibition of ER stress and oxidative stress by MSCs, which regulates the polarization of M2 macrophages in chronic allergic airway remodeling process. This discovery enriches our knowledge of the therapeutic effect of MSCs on chronic allergic airway remodeling, which provides a new theoretical basis for the future clinical application of MSCs.
DATA AVAILABILITY
No datasets were generated or analysed during the current study.
References
Mims, J.W. 2015. Asthma: Definitions and pathophysiology. International Forum of Allergy & Rhinology 5 (Suppl 1): S2–S6. https://doi.org/10.1002/alr.21609.
Saradna, A., D.C. Do, S. Kumar, Q.L. Fu, and P. Gao. 2018. Macrophage polarization and allergic asthma. Translational Research 191: 1–14. https://doi.org/10.1016/j.trsl.2017.09.002.
Mattoo, H., D. Bangari, S. Cummings, Z. Humulock, D. Habiel, E. Xu, N. Pate, R. Resnick, V. Savova, G. Qian, C. Beil, E. Rao, F. Nestle, P. Bryce, and A. Subramaniam. 2023. Molecular features and stages of pulmonary fibrosis driven by type 2 inflammation. American Journal of Respiratory Cell and Molecular Biology 69: 404–421. https://doi.org/10.1165/rcmb.2022-0301OC.
Hong, S., Y. Lu, S. Chen, C. Hsu, Y. Lu, C. Wang, and K. Huang. 2023. Targeting pathogenic macrophages by the application of SHP-1 agonists reduces inflammation and alleviates pulmonary fibrosis. Cell Death & Disease 14: 352. https://doi.org/10.1038/s41419-023-05876-z.
Lee, H., J. Hur, J. Kang, C. Rhee, and S. Lee. 2021. MicroRNA-21 inhibition suppresses alveolar M2 macrophages in an ovalbumin-induced allergic asthma mice model. Allergy, Asthma & Immunology Research 13: 312–329. https://doi.org/10.4168/aair.2021.13.2.312.
Liu, L., Y. Qin, Z. Cai, Y. Tian, X. Liu, J. Li, and P. Zhao. 2021. Corrigendum to: Effective-components combination improves airway remodeling in COPD rats by suppressing M2 macrophage polarization via the inhibition of mTORC2 activity. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology 92: 153976. https://doi.org/10.1016/j.phymed.2021.153759.
Asgari Taei, A., P. Khodabakhsh, S. Nasoohi, M. Farahmandfar, and L. Dargahi. 2022. Paracrine effects of mesenchymal stem cells in ischemic stroke: opportunities and challenges. Molecular Neurobiology 59: 6281–6306. https://doi.org/10.1007/s12035-022-02967-4.
Shi, Y., Y. Wang, Q. Li, K. Liu, J. Hou, C. Shao, and Y. Wang. 2018. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nature Reviews Nephrology 14: 493–507. https://doi.org/10.1038/s41581-018-0023-5.
Wang, Y., B. Huang, T. Jin, D. Ocansey, J. Jiang, and F. Mao. 2022. Intestinal fibrosis in inflammatory bowel disease and the prospects of mesenchymal stem cell therapy. Frontiers in Immunology 13: 835005. https://doi.org/10.3389/fimmu.2022.835005.
Wang, M., N. Zhao, C. Wang, Z. Jin, and L. Zhang. 2023. Immunomodulatory properties of mesenchymal stem cells: A potential therapeutic strategy for allergic rhinitis. Allergy 78: 1425–1440. https://doi.org/10.1111/all.15729.
Abreu, S., L. Alves, L. Carvalho, D. Xisto, N. Blanco, L. Castro, P. Olsen, and J. Lapa E Silva, M. Morales, M. Lopes-Pacheco, D. Weiss, P. Rocco,. 2023. Serum from patients with asthma potentiates macrophage phagocytosis and human mesenchymal stromal cell therapy in experimental allergic asthma. Cytotherapy 25: 967–976. https://doi.org/10.1016/j.jcyt.2023.05.014.
Gholami, M., K. Ghorban, M. Sadeghi, M. Dadmanesh, N. Rouzbahani, and S. Dehnavi. 2023. Mesenchymal stem cells and allergic airway inflammation; a therapeutic approach to induce immunoregulatory responses. International Immunopharmacology 120: 110367. https://doi.org/10.1016/j.intimp.2023.110367.
Moroncini, G., C. Paolini, F. Orlando, C. Capelli, A. Grieco, C. Tonnini, S. Agarbati, E. Mondini, S. Saccomanno, G. Goteri, S. Svegliati Baroni, M. Provinciali, M. Introna, N. Del Papa, and A. Gabrielli. 2018. Mesenchymal stromal cells from human umbilical cord prevent the development of lung fibrosis in immunocompetent mice. PloS ONE 13: e01960481. https://doi.org/10.1371/journal.pone.0196048.
Dong, L., Y. Wang, T. Zheng, Y. Pu, Y. Ma, X. Qi, W. Zhang, F. Xue, Z. Shan, J. Liu, X. Wang, and C. Mao. 2021. Hypoxic hUCMSC-derived extracellular vesicles attenuate allergic airway inflammation and airway remodeling in chronic asthma mice. Stem Cell Research & Therapy 12: 4. https://doi.org/10.1186/s13287-020-02072-0.
Zhong, H., X.L. Fan, S.B. Fang, Y.D. Lin, W. Wen, and Q.L. Fu. 2019. Human pluripotent stem cell-derived mesenchymal stem cells prevent chronic allergic airway inflammation via TGF-β1-Smad2/Smad3 signaling pathway in mice. Molecular Immunology 109: 51–57. https://doi.org/10.1016/j.molimm.2019.02.017.
Pathinayake, P., D. Waters, K. Nichol, A. Brown, A. Reid, A. Hsu, J. Horvat, L. Wood, K. Baines, J. Simpson, P. Gibson, P. Hansbro, and P. Wark. 2022. Endoplasmic reticulum-unfolded protein response signalling is altered in severe eosinophilic and neutrophilic asthma. Thorax 77: 443–451. https://doi.org/10.1136/thoraxjnl-2020-215979.
Michaeloudes, C., H. Abubakar-Waziri, R. Lakhdar, K. Raby, P. Dixey, I. Adcock, S. Mumby, P. Bhavsar, and K. Chung. 2022. Molecular mechanisms of oxidative stress in asthma. Molecular Aspects of Medicine 85: 101026. https://doi.org/10.1016/j.mam.2021.101026.
Shi, B., Y. Hao, W. Li, H. Dong, M. Xu, and P. Gao. 2022. TIPE2 may target the Nrf2/HO-1 pathway to inhibit M1 macrophage-related neutrophilic inflammation in asthma. Frontiers in Immunology 13: 883885. https://doi.org/10.3389/fimmu.2022.883885.
Ning, H., H. Chen, J. Deng, C. Xiao, M. Xu, L. Shan, C. Yang, and Z. Zhang. 2021. Exosomes secreted by FNDC5-BMMSCs protect myocardial infarction by anti-inflammation and macrophage polarization via NF-κB signaling pathway and Nrf2/HO-1 axis. Stem Cell Research & Therapy 12: 519. https://doi.org/10.1186/s13287-021-02591-4.
Gu, W., R. Cui, T. Ding, X. Li, J. Peng, W. Xu, F. Han, and X. Guo. 2017. Simvastatin alleviates airway inflammation and remodelling through up-regulation of autophagy in mouse models of asthma. Respirology (Carlton, Vic.) 22: 533–541. https://doi.org/10.1111/resp.12926.
Sun, Y.Q., M.X. Deng, J. He, Q.X. Zeng, W. Wen, D.S. Wong, H.F. Tse, G. Xu, Q. Lian, J. Shi, and Q.L. Fu. 2012. Human pluripotent stem cell-derived mesenchymal stem cells prevent allergic airway inflammation in mice. Stem Cells (Dayton, Ohio) 30: 2692–2699. https://doi.org/10.1002/stem.1241.
Reddy, A.P., and M.R. Gupta. 2014. Management of asthma: The current US and European guidelines. Advances in Experimental Medicine and Biology 795: 81–103. https://doi.org/10.1007/978-1-4614-8603-9_6.
Yao, Y., X. Fan, D. Jiang, Y. Zhang, X. Li, Z. Xu, S. Fang, S. Chiu, H. Tse, Q. Lian, and Q. Fu. 2018. Connexin 43-mediated mitochondrial transfer of iPSC-MSCs alleviates asthma inflammation. Stem Cell Reports 11: 1120–1135. https://doi.org/10.1016/j.stemcr.2018.09.012.
Abreu, S.C., M.A. Antunes, D.G. Xisto, F.F. Cruz, V.C. Branco, E. Bandeira, J. Zola Kitoko, A.F. de Araújo, L. Dellatorre-Texeira, P.C. Olsen, D.J. Weiss, B.L. Diaz, M.M. Morales, and P.R.M. Rocco. 2017. Bone marrow, adipose, and lung tissue-derived murine mesenchymal stromal cells release different mediators and differentially affect airway and lung parenchyma in experimental asthma. Stem Cells Translational Medicine 6: 1557–1567. https://doi.org/10.1002/sctm.16-0398.
Choi, J., J. Hur, S. Jeon, C. Jung, and C. Rhee. 2022. Effects of human adipose tissue- and bone marrow-derived mesenchymal stem cells on airway inflammation and remodeling in a murine model of chronic asthma. Scientific Reports 12: 12032. https://doi.org/10.1038/s41598-022-16165-8.
Cui, Z., Y. Feng, D. Li, T. Li, P. Gao, and T. Xu. 2020. Activation of aryl hydrocarbon receptor (AhR) in mesenchymal stem cells modulates macrophage polarization in asthma. Journal of Immunotoxicology 17: 21–30. https://doi.org/10.1080/1547691x.2019.1706671.
Zhu, X., J. Cui, L. Yi, J. Qin, W. Tulake, F. Teng, W. Tang, Y. Wei, and J. Dong. 2020. The role of T cells and macrophages in asthma pathogenesis: A new perspective on mutual crosstalk. Mediators of Inflammation 2020: 7835284. https://doi.org/10.1155/2020/7835284.
Mantovani, A., A. Sica, S. Sozzani, P. Allavena, A. Vecchi, and M. Locati. 2004. The chemokine system in diverse forms of macrophage activation and polarization. Trends in Immunology 25: 677–686. https://doi.org/10.1016/j.it.2004.09.015.
Guo, Z., S. Li, N. Zhang, Q. Kang, and H. Zhai. 2020. Schisandra inhibit bleomycin-induced idiopathic pulmonary fibrosis in rats via suppressing M2 macrophage polarization. BioMed Research International 2020: 5137349. https://doi.org/10.1155/2020/5137349.
Wang, J., L. Xu, Z. Xiang, Y. Ren, X. Zheng, Q. Zhao, Q. Zhou, Y. Zhou, L. Xu, and Y. Wang. 2020. Microcystin-LR ameliorates pulmonary fibrosis via modulating CD206(+) M2-like macrophage polarization. Cell Death & Disease 11: 136. https://doi.org/10.1038/s41419-020-2329-z.
Lee, H.Y., I.K. Kim, H.K. Yoon, S.S. Kwon, C.K. Rhee, and S.Y. Lee. 2017. Inhibitory effects of resveratrol on airway remodeling by transforming growth factor-β/Smad signaling pathway in chronic asthma model. Allergy, Asthma & Immunology Research 9: 25–34. https://doi.org/10.4168/aair.2017.9.1.25.
Chen, M., Z. Lv, L. Huang, W. Zhang, X. Lin, J. Shi, W. Zhang, R. Liang, and S. Jiang. 2015. Triptolide inhibits TGF-β1-induced cell proliferation in rat airway smooth muscle cells by suppressing Smad signaling. Experimental Cell Research 331: 362–368. https://doi.org/10.1016/j.yexcr.2014.10.016.
Bansod, S., N. Doijad, and C. Godugu. 2020. Berberine attenuates severity of chronic pancreatitis and fibrosis via AMPK-mediated inhibition of TGF-β1/Smad signaling and M2 polarization. Toxicology and Applied Pharmacology 403: 115162. https://doi.org/10.1016/j.taap.2020.115162.
Roach, K.M., C. Feghali-Bostwick, H. Wulff, Y. Amrani, and P. Bradding. 2015. Human lung myofibroblast TGFβ1-dependent Smad2/3 signalling is Ca(2+)-dependent and regulated by KCa3.1 K(+) channels. Fibrogenesis & Tissue Repair 8: 5. https://doi.org/10.1186/s13069-015-0022-0.
Malyshev, I., and Y. Malyshev. 2015. Current concept and update of the macrophage plasticity concept: Intracellular mechanisms of reprogramming and M3 macrophage “switch” phenotype. BioMed Research International 2015: 341308. https://doi.org/10.1155/2015/341308.
Wang, Y., J. Zhu, L. Zhang, Z. Zhang, L. He, Y. Mou, Y. Deng, Y. Cao, P. Yang, Y. Su, J. Zhao, S. Zhang, Q. Yu, J. Hu, Z. Chen, Q. Ning, X. Xiang, Y. Xu, C. Wang, and W. Xiong. 2017. Role of C/EBP homologous protein and endoplasmic reticulum stress in asthma exacerbation by regulating the IL-4/signal transducer and activator of transcription 6/transcription factor EC/IL-4 receptor α positive feedback loop in M2 macrophages. The Journal of Allergy and Clinical Immunology 140: 1550–1561.e1558. https://doi.org/10.1016/j.jaci.2017.01.024.
Chen, J., J. Chen, Y. Cheng, Y. Fu, H. Zhao, M. Tang, H. Zhao, N. Lin, X. Shi, Y. Lei, S. Wang, L. Huang, W. Wu, and J. Tan. 2020. Mesenchymal stem cell-derived exosomes protect beta cells against hypoxia-induced apoptosis via miR-21 by alleviating ER stress and inhibiting p38 MAPK phosphorylation. Stem Cell Research & Therapy 11: 97. https://doi.org/10.1186/s13287-020-01610-0.
Liao, Z., R. Luo, G. Li, Y. Song, S. Zhan, K. Zhao, W. Hua, Y. Zhang, X. Wu, and C. Yang. 2019. Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo. Theranostics 9: 4084–4100. https://doi.org/10.7150/thno.33638.
Ahmed, S., L. Luo, A. Namani, X. Wang, and X. Tang. 1863. Nrf2 signaling pathway: Pivotal roles in inflammation, biochimica et biophysica acta. Molecular Basis of Disease 2017: 585–597. https://doi.org/10.1016/j.bbadis.2016.11.005.
Wu, Y., Y. Zhang, F. Jiang, S. He, Y. Zhang, D. Chen, Y. Tong, Y. Nie, and Q. Pang. 2023. 4-OI ameliorates bleomycin-induced pulmonary fibrosis by activating Nrf2 and suppressing macrophage-mediated epithelial-mesenchymal transition. Inflammation Research 72: 1133–1145. https://doi.org/10.1007/s00011-023-01733-z.
Toh, W., R. Lai, B. Zhang, and S. Lim. 2018. MSC exosome works through a protein-based mechanism of action. Biochemical Society Transactions 46: 843–853. https://doi.org/10.1042/bst20180079.
Asadirad, A., A. Ghadiri, A. Amari, M. Ghasemi Dehcheshmeh, M. Sadeghi, and S. Dehnavi. 2023. Sublingual prophylactic administration of OVA-loaded MSC-derived exosomes to prevent allergic sensitization. International Immunopharmacology 120: 110405. https://doi.org/10.1016/j.intimp.2023.110405.
Dehnavi, S., A. Khodadadi, A. Asadirad, and A. Ghadiri. 2023. Immune response modulation by allergen loaded into mesenchymal stem cell-derived exosomes as an effective carrier through sublingual immunotherapy. Immunobiology 228: 152361. https://doi.org/10.1016/j.imbio.2023.152361.
Shan, L., S. Liu, Q. Zhang, Q. Zhou, and Y. Shang. 2022. Human bone marrow-mesenchymal stem cell-derived exosomal microRNA-188 reduces bronchial smooth muscle cell proliferation in asthma through suppressing the JARID2/Wnt/β-catenin axis. Cell Cycle (Georgetown, Tex.) 21: 352–367. https://doi.org/10.1080/15384101.2021.2020432.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81770023).
Author information
Authors and Affiliations
Contributions
Dr. Haiyang Yu and Guiyin Zhu contributed to the study conduct and drafted the manuscript. Qiangqiang Qin contributed to data analysis. Dr. Xueting Wang provided language help. Dr. Wen Gu and Dr. Xuejun Guo contributed to the study design and guide. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors have no conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, H., Zhu, G., Qin, Q. et al. Mesenchymal Stromal Cell Therapy Alleviates Ovalbumin-Induced Chronic Airway Remodeling by Suppressing M2 Macrophage Polarization. Inflammation 47, 1298–1312 (2024). https://doi.org/10.1007/s10753-024-01977-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-024-01977-9