Abstract
Rheumatoid arthritis is the most common arthritis and is mainly characterized by symmetric polyarticular joint disorders. Eleutheroside E (EE), a principal active constituent of Acanthopanax senticosus, is reported to have anti-inflammatory effect by inhibiting NF-κB activities. However, the effects of EE on rheumatoid arthritis (RA) severity are largely unknown. The purpose of this study was to indicate whether EE could ameliorate arthritis and reduce inflammatory cytokine release in collagen-induced arthritis (CIA) mice. The results showed that EE attenuated the severity of arthritis by reducing the mean arthritis score and arthritis incidence. EE also significantly decreased the inflammatory cell infiltration, pannus formation, cartilage damage, and bone erosion of CIA mice. Furthermore, EE caused a marked decrease of the production of TNF-α and IL-6 in vivo and in vitro. These observations identify a novel function of EE that results in inhibition of cytokine release, highlighting EE was a potential therapeutic agent for RA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Rheumatoid arthritis (RA) is characterized by chronic and progressive inflammation of the synovial joints leading to destruction of cartilage and articular bone [1]. Inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) produced by inflammatory cells, play key roles in the pathogenesis of RA [2]. Overproduction of IL-6 has been found in the synovial fluid and blood of RA patients, and IL-6 levels correlate with disease activity [3, 4]. IL-6 can promote synovitis by inducing pannus proliferation, resulting in infiltration of inflammatory cells and synovial hyperplasia [5]. In terms of joint erosion, IL-6 causes bone resorption by inducing osteoclast formation and cartilage degeneration in synovial cells and chondrocytes [6, 7]. Therefore, IL-6 is a pleiotropic cytokine and a suitable target in the treatment of RA.
TNF-α is now recognized as mediating a wide variety of effector functions relevant to the pathogenesis of RA, including endothelial cell activation and chemokine amplification, leading to leukocyte accumulation [8], osteoclast and chondrocyte activation, promoting articular destruction [9]. Many experiments suggested that TNF-α had a key role in the inflammation of the synovial joint seen in RA. Therefore, IL-6 and TNF-α could be therapeutic targets of RA. Currently, protein-based biological agents targeting to block IL-6 and TNF-α such as MRA, infliximab, etanercept, and adalimumab are recognized as effective treatments for RA.
Although protein-based biological agents provide new insight to treat RA, some side effects were reported during the clinical trial recently. Infliximab may reactivate of latent Mycobacterium tuberculosis [10], increase risk of other infections, cause injection/infusion site reactions, induce autoantibodies and autoimmune diseases [11, 12], and worsen severe heart failure. The toxic effects of MRA remain under investigation, particularly with respect to cardiovascular safety. Additionally, protein-based biological agents remain expensive, and the use of them requires subcutaneous or intravenous administration. The challenge for the future is to develop novel cheaper therapies using small-molecule technology that can be given orally. Thus, small molecule immune modulators extracted from Traditional Chinese Medicine (TCM) will provide new hopes to treat RA in future.
Acanthopanax senticosus (Rupr. et Maxim) Harms is a medicinal plant and belongs to the Araliaceae family, which is widely distributed in the Changbai Mountains of Jilin Province, P.R. China. A. senticosus has long been used as a traditional medicine to treat rheumatoid arthritis, tumors, diabetes, and hypertension and exhibit immunomodulating activities in vivo [13–15]. Our group has previously reported that eleutheroside E (EE) was an important active constituent from A. senticosus [16]. In continuation of our previous study and development of novel small molecule drugs to treat RA, we testified the inhibitory effect of EE on macrophage activations and elucidated the beneficial effect of EE on collagen-induced arthritis (CIA) mice for the first time in this article. EE was found to significantly inhibit cultured macrophages to produce TNF-α and IL-6 in a dose- and time-dependent manner. Considering the role of TNF-α and IL-6 in the pathogenesis of RA, we investigated the therapeutic effect of EE on bone destruction in collagen-induced arthritis mice model. The results of this study confirmed the anti-inflammatory effect of EE in CIA mice, including reducing swelling of the ankle and arthritis score, ameliorating bone destruction, and decreasing inflammatory cytokines release.
MATERIALS AND METHODS
Reagents, Cell Line, and Cell Culture
Eleutheroside E (with its purity above 98 %) was purchased from Sigma-Aldrich (Louis, MO, USA). Bovine collagen type II, complete Freund's adjuvant, and incomplete Freund's adjuvant were purchased from Chondrex (Redmond, WA, USA). THP-1 cell line was obtained from the Institute of Hematology of Soochow University. RPMI-1640, penicillin, streptomycin, and fetal bovine serum were purchased from Gibco Life Technologies (Rockville, MD, USA). THP-1 cells were cultured in RPMI-1640 with 10 % fetal bovine serum, 1,000 U/ml penicillin, and 10 mg/ml streptomycin at 37 °C in a humidified atmosphere of 5 % CO2 in air.
CIA Induction
Male DBA/1 J mice (8 weeks old) were purchased from Shanghai SLAC Laboratory Animal Co. Ltd (Shanghai, China). Mice were randomly divided into five groups each with six animals. CIA was induced by subcutaneous injection with a 200 μl emulsion which contained 100 μg of bovine collagen type II and 100 μl of CFA (5 mg/ml BCG) at the back of the mouse. On day 21, 100 μl of booster emulsion containing 50 μg of bovine collagen type II and 50 μl IFA was injected subcutaneously at the base of the tail.
Assessment of Arthritis
Mice were examined for signs of joint inflammation and scored as follows: 0, no change; 1, significant swelling and redness of one digit; 2, mild swelling and erythema of the limb or swelling of more than two digits; 3, marked swelling and erythema of the limb; and 4, maximal swelling and redness of the limb and later, ankylosis. The average macroscopic score was expressed as a cumulative value for all paws, with a maximum possible score of 16.
Administration of Eleutheroside E
EE and tripterygium glycosides (TG, used as a positive control) were dissolved in 1 % sodium carboxymethylcellulose (CMC-Na), respectively. Both the compound and vehicle were administered by oral gavage daily. CIA mice received EE (15, 30, 60 mg/kg orally) or TG (15 mg/kg orally) or vehicle (orally) daily, starting from 21 days after first immunization for 3 weeks. The control group was injected with vehicle alone.
Histological Assessment
Mice were sacrificed and the hind paws (including the paw and ankle) were fixed in 10 % neutral formalin, decalcified by immersing in 10 % EDTA solution for 2 days, and embedded in paraffin. Tissue sections (5 μm) were stained with hematoxylin and eosin (H&E). The treatment and clinical disease activity score of each sample was not disclosed to the trained observer who scored the histology slides. Sections were scored on microscopic alterations such as the infiltration of the synovium and periarticular tissue with neutrophils and mononuclear cells (inflammation), marginal zone pannus (pannus), bone resorption (bone erosion), and cartilage damage (proteoglycan loss, chondrocyte death, and collagen matrix destruction). The mean overall score was based on forepaw, knee, and joint parameters. All were scored on a 0–5 scale: 0 = no disease, 1 = minimal, 2 = mild, 3 = moderate, 4 = marked, 5 = severe. A mean score for each mouse was determined for each parameter, and these were averaged to determine group means.
Measurements of Cytokines
Sera were obtained from anesthetized animals by retroorbital puncture at the end of the study. The levels of TNF-α, IL-6, IFN-γ, and IL-17A from sera were measured by ELISA (HuShang Biological Technology Co.Ltd, China) according to the manufacturer's instructions. The levels of IL-2, IL-6, IL-8, IL-10, TNF-α, and IFN-γ in culture supernatant from THP-1 cells were measured by FCM using a CBA cytokine kit (BD Cytometric Bead Array, USA) according to the manufacturer's instructions.
Measurements of Anti-collagen Type II IgG
Serum levels of anti-collagen type II IgG (anti-CII IgG) was measured by indirect ELISA. Bovine collagen type II (10 μg/ml) was coated on 96-well polystyrene microtiter plates (Yangzi Company, Jiangshu, China) and incubated overnight at 4 °C in 0.01 mol/L PBS (1 mmol/L KH2PO4, 10 mmol/L Na2HPO4, 137 mmol/L NaCl, 2.7 mmol/L KCl, pH 7.4). After washing for three times with washing buffer (PBST, 0.01 mol/l PBS, 0.05 % Tween 20), 200 μl per well of blocking buffer was added (0.01 mol/L PBS, 5 % skim milk) followed by incubation at 37 °C for 2 h. The sera were diluted at 1:32,000 with sample buffer (0.01 M PBST, 5 % skim milk), added to wells in duplicate and incubated at 37 °C for 1 h. Afterwards, plates were washed three times followed by the addition of 100 μl per well of goat anti-mouse IgG/HRP (Abgent) at 1:2,000 dilution and incubation at 37 °C for 1 h. After washing three times, the substrate solution of TMB (Amresco) was added and incubated at room temperature for 10 min for color development which was stopped with 50 μl per well of 2 M sulfuric acid. The optical density (OD) of the color in each well of plates was determined at 450 nm on an automated ELISA plate reader. The results were expressed as A450 ± SD.
Radiographic Evaluations
Plain radiographs of paws were obtained using a mammographic imager (Siemens Medical Solutions, Germany). The joint destruction and bone erosion were scored on a scale of 0–5 in a blind manner: 0, no damage; 1, minor bone destruction observed in one enlightened spot; 2, moderate changes, with two to four spots in one area; 3, marked changes with two to four spots in more areas; 4, severe erosions of the joint; and 5, complete destruction of the joints.
Micro-computed Tomography (micro-CT) Analysis
Mice used in the micro-CT analyses were killed on day 56. The hind paws (from the tip of the toes to the mid of the tibia) were placed in phosphate-buffered saline containing 0.1 % sodium azide and scanned using a SkyScan micro-CT apparatus, operating at a resolution of 9 μm voxel size. Three-dimensional reconstructions were performed using NRECON software, and the images were further processed with CT-analyzer software. The projection images were reconstructed into three-dimensional images using NRECON software and CT-analyzer (both from SkyScan).
Statistics
Data were presented as the mean ± SEM. Statistical analyses were performed using SPSS 16.0. Statistical comparisons were performed using one-way analysis of variance or the Mann–Whitney U test. The differences between groups were determined using Student's unpaired t test. P < 0.05 was considered significant.
RESULTS
EE Treatment Attenuated the Severity of Arthritis in CIA Mice
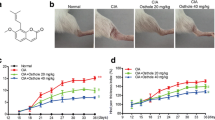
To investigate the effect of EE on arthritis, the CIA model in DBA/1 mice was used. Oral administration of EE once a day started from day 21 to day 42 after primary immunization. TG was used as a positive control. As shown in Fig. 1a, the severe swelling, erythema, and joint rigidity were developed in the hind paws of vehicle-treated CIA mice. In contrast, the incidence and severity of CIA were significantly decreased in mice treated with EE and TG compared to vehicle-treated mice. As shown in Fig. 1b and c, compared to vehicle-treated CIA mice, 15 mg/kg TG treatment and 30 and 60 mg/kg EE treatment obviously decreased the arthritis scores and body weight loss in CIA mice (P < 0.01). Noticeably, the arthritis scores and body weight loss of mice treated with 15 mg/kg EE were lower than those of vehicle-treated mice, but the statistical difference was not significant (P > 0.05). Further, hind paw swelling was measured to analyze the beneficial effects of EE in CIA mice, and the results were consistent with the arthritis scores. As shown in Fig. 1d, the hind paw thickness in CIA mice was significantly decreased from day 42 to day 56 after 30 and 60 mg/kg EE treatment, but 15 mg/kg EE treatment did not show the therapeutic effects in CIA mice. The results indicated that EE treatment (30–60 mg/kg) was effective to attenuate the severity of arthritis in CIA mice.
Effect of EE on arthritis scores, body weight and hind paw thickness of CIA mice. Mice were orally administered EE (15, 30, and 60 mg/kg, respectively), or TG or vehicle for 3 weeks from 21st day after first immunization. TG served as a positive control. a Representative photographs of joint swelling of the hind paws in mice treated with EE, TG, or vehicle in CIA; b cumulative arthritis scores in mice treated with EE, TG, or vehicle during the progression of CIA; c changes of body weight in mice treated with EE, TG, or vehicle during the progression of CIA; d the changes of hind paw thickness in mice treated with EE, TG, or vehicle during the progression of CIA. Data were presented as mean ± SEM (n = 6); **P < 0.01, ***P < 0.001 as compared to vehicle-treated mice. Similar results were observed in three separated experiments.
EE Treatment Reduced Bone Destruction in CIA Mice
In order to observe the bone changes of hind paws after EE treatment, radiographs of hind paws by mammography and micro-CT were taken. The representative mammography radiographs of hind paws in vehicle-treated mice showed typical changes of arthritis in CIA mice, including articular destruction, joint displacement, and irregular bony proliferation cloaking the entire ankle region (Fig. 2a). After treating with EE (30–60 mg/kg) and TG (15 mg/kg) for 3 weeks, the mice showed markedly less bone destruction in hind paws from the radiographic findings (Fig. 2a) and mean radiographic scores (Fig. 2c) (P < 0.01). Noticeably, CIA mice treated with 15 mg/kg EE did not show significant differences compared with vehicle-treated mice (P > 0.05), which was consistent with the results of arthritis scores and hind paw thickness. To further elucidate the effects of EE on periarticular bone integrity, micro-CT analysis was performed on CIA mice treated with EE, TG, and vehicle. As shown in Fig. 2b, obvious joint destruction in vehicle-treated mice, and EE (30–60 mg/kg) or TG (15 mg/kg) treatment markedly prevented bone erosions at the ankle joints. The results could suggest that EE treatment (30–60 mg/kg) was an effective therapeutic approach to reduced joint destruction in CIA mice.
Reduced bone destruction assessed by radiology in EE-treated CIA mice. a Representative mammography radiographs of the hind paws of CIA mice on day 56. b Representative micro-CT images of the hind paws of CIA mice on day 56 are shown. c Radiological scores were determined as described in the “Materials and methods” section. Data were presented as mean ± SEM (n = 6); **P < 0.01, ***P < 0.001 as compared to vehicle-treated mice. Similar results were observed in three separated experiments.
EE Treatment Suppressed the Histopathological Changes in the Synovial Tissues of CIA Mice
At the end of the experiment, the ankle and knee joints of CIA mice were evaluated histologically. The severity of synovial inflammatory cell infiltration, formation of pannus, cartilage damage, and bone erosion was scored semiquantitatively. The representative photographs of H&E staining (Fig. 3a) showed marked infiltration of inflammatory cells, pannus information, cartilage damage, and bone erosion in the joints of vehicle-treated CIA mice. However, the infiltration of inflammatory cells and formation of pannus in CIA mice were significantly reduced after treating with EE (30–60 mg/kg) and TG (15 mg/kg). Additionally, cartilage damage and bone erosion in the CIA mice were also significantly inhibited by TG or EE (30–60 mg/kg) treatment (Fig. 3b, P < 0.001). Unsurprisingly, CIA mice treated with 15 mg/kg EE did not show significant differences compared to vehicle-treated mice (P > 0.05). In accordance with the clinical and radiographic observation, EE dose-dependently inhibited the histological severity scores. These observations indicated that EE exerts preventive action against arthritic diseases in vivo.
EE inhibits the histological changes in the joints of CIA mice. Paraffin sections of knee joints were stained with hematoxylin and eosin, and joint histology was evaluated. a Representative photographs of sections from vehicle-, TG-, and EE-treated CIA mice, respectively. Arrow indicates the site of cartilage damage, asterisk indicates the site of bone erosion, and circle indicates the site of synovium inflammation and pannus formation. b Pathological severity scores of inflammatory cell infiltration, pannus extension, cartilage damage, and bone erosion in the joints of CIA mice. Pathological changes were scored on a 0–5 scale. Data were presented as mean ± SEM (n = 6); ***P < 0.001 as compared to vehicle-treated mice. Similar results were observed in three separated experiments.
EE Treatment Decreased the Level of Anti-collagen II Antibody
Since autoantibodies to collagen II have been widely reported in adult and juvenile rheumatoid arthritis [17], the level of anti-collagen II antibody in sera of CIA mice was detected after collagen II immunization for 56 days. As shown in Fig. 4, the antibody level in vehicle-treated CIA mice was high, and EE (30–60 mg/kg) and TG (15 mg/kg) treatment were effective to reduce serum anti-collagen II antibody with statistical significance (P < 0.01). Surprisingly, anti-collagen II antibody in mice treated with 15 mg/kg EE was significant lower than those in vehicle-treated mice (P < 0.001). The results could suggest that the production of anti-collagen II antibody in CIA mice was effectively inhibited by EE in low dosage (15 mg/kg).
Effects of EE on levels of anti-collagen II antibody in vivo. The level of anti-collagen II IgG antibody in the serum was evaluated at study termination (day 56) by ELISA. Data were presented as mean ± SEM (n = 6); **P < 0.01, ***P < 0.001 as compared to vehicle-treated mice. Similar results were observed in three separated experiments.
EE Treatment Inhibited the Production of TNF-a, IL-6, and IL-23 In Vivo
CIA is characterized by the marked expression of inflammatory cytokines. To ascertain whether EE inhibited the cytokine releases, vehicle-, TG-, and EE-treated mice with CIA were bled on day 56 after primary immunization. TNF-α, IL-6, IL-17A, and IL-23 levels in sera were measured by ELISA. The EE-treated mice showed significant lower levels of TNF-α, IL-6, and IL-23 in sera than those from vehicle-treated mice (P < 0.05) (Fig. 5). Compared to naive DBA/1 mice, vehicle-treated mice showed higher level of TNF-α, IL-6, and IL-23 in sera. IL-17A was undetectable in the sera from those mice. The results suggested that EE might have a therapeutic effect on arthritis severity by inhibiting the production of inflammatory cytokines.
EE Inhibited the Production of TNF-α and IL-6 In Vitro
To confirm whether EE can inhibit the production of inflammatory cytokines, a human monocytic leukemia cell line, THP-1, was used to evaluate. THP-1 cells were induced to differentiate into macrophage-like cells by adding PMA for 24 h. Then, EE (20–160 μmol/L) were added to co-cultured for another 48 h. The level of IL-2, IL-6, IL-8, IL-10, TNF-α, and IFN-γ in the supernatants were measured by flow cytometry. As demonstrated in Fig. 6a, PMA could stimulate THP-1 cells to produce inflammatory cytokines, mainly IL-6 and TNF-α, while IL-2, IL-8, IL-10, and IFN-γ were not detected. However, incubation with EE revealed a partial inhibition of TNF-α release from THP-1 cells at a concentration of 20–40 μmol/L and a nearly complete inhibition at concentration of 80 μmol/L and higher (Fig. 6b); besides, nearly 80 % IL-6 release by THP-1 cells was inhibited at concentration of 160 μmol/L (Fig. 6c). The levels of TNF-α in supernatants from different time points (EE was added for 2–48 h) showed that 160 μmol/L EE could significantly inhibit TNF-α release early at 2 h, and a nearly complete inhibition at 48 h (Fig. 6d). To determine whether the inhibitory effect was a result of the apoptosis or death of THP-1, the cell growth inhibition was measured by MTT assay. As shown in Fig. 6e, 20–160 μmol/L EE did not cause cell death or inhibition of cell proliferation. The results suggested that EE could significantly inhibit THP-1 cells to release TNF-α and IL-6 quickly and efficiently at a lower concentration.
TNF-α and IL-6 release from THP-1 cells was reduced by EE incubation. a CBA assay was performed to monitor the levels of six different inflammatory cytokines in the culture supernatants. PMA could stimulate THP-1 cells to produce high level of TNF-α and IL-6, and EE incubation could markedly reduce the release of TNF-α and IL-6. TNF-α (b) and IL-6 (c) were significantly inhibited by incubating with different concentrations of EE (20–160 μmol/L); (d) EE (160 μmol/L) could markedly inhibit the release of TNF-α at different time points (0–48 h); e different concentrations of EE (20–160 μmol/L) did not stimulate or inhibit the proliferation of THP-1 cells. Data were presented as mean ± SEM; *P < 0.05, **P < 0.01 as compared to baseline. Similar results were observed in three separated experiments.
DISCUSSION
RA is a chronic and systemic autoimmune disease which is characterized by multi-joint synovitis and articular damage [18]. CIA has become one of the most widely used models to screen new drugs to treat rheumatoid diseases because it has been proven to share various immunological and pathological features with human RA [19]. Therefore, CIA mice were used to demonstrate the therapeutic effect of EE on arthritis severity in the current study.
EE, the principal active constituent of A. senticosus, is known to have anti-inflammatory effect by suppressing the gene expression of inflammatory proteins through inhibiting NF-κB and AP-1 binding activities [20–22]. Consistent with the results, EE significantly inhibits cytokine releases of activated whole blood cultures such as IL-4, IL-5, IL-6, IL-12, and IL-13 with high concentration [15]. In addition, EE has also been reported to alleviate behavioral alterations induced by sleep deprivation [23] and to alleviate diabetes by improved hepatic glucose metabolism in obese type 2 diabetic mice [24]. However, as far as we know, the effects of EE on RA severity have not yet been studied. To explore this, we have used the CIA mice to elucidate the beneficial effect of EE on RA.
In our study, CIA was developed in most mice at about 3 weeks following the primary immunization. Red and swelling in the paws, formation of pannus, cartilage damage and bone erosion, and joint deformation were observed in vehicle-treated mice. EE could attenuate the severity of arthritis by reducing the mean arthritis score as well as arthritis incidence with a dose-dependent manner. Besides, pathological and radiological results indicated that the inflammatory cell infiltration, pannus formation, cartilage damage, and bone destruction of CIA mice were improved significantly by EE treatment. These results suggest that EE effectively prevents the arthritis progression and decreases severity of arthritis in CIA mice. Furthermore, EE treatment caused a marked decrease in the expression of TNF-α, IL-6 in vivo and in vitro.
Inflammatory cytokines play critical roles in RA. TNF-α can mediate a wide variety of effector functions relevant to the pathogenesis of RA. Initial results of TNF blocking antibodies in an open-label study showed significant improvements in measures of disease activity, including reduction in swelling and tenderness in joints and a reduced acute phase response, accompanying with decreased erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) [25]. Visual analogue measures of the general health of patients also improved. This study was followed by a double-blind placebo-controlled trial showed efficacy in individuals with active RA [26]. Subsequently, longer term studies have also shown a reduction in joint damage by a reduced number of erosions and less joint-space narrowing [27]. IL-6, a B cell regulatory factor, is likely the primary driver of the hepatic acute phase response in RA. Excess production of IL-6 was found in the synovial fluid and blood of RA patients and correlates with the disease activity and joint destruction [4]. IL-6 promotes synovitis by inducing neovascularisation via pannus proliferation, resulting in infiltration of inflammatory cells and synovial hyperplasia [28]. In terms of joint erosion, IL-6 causes bone resorption by inducing osteoclast formation [6] and cartilage degeneration by producing matrix metalloproteinases (MMPs) in synovial cells and chondrocytes [7]. IL-6 deletion protects DBA/1 mice from CIA, and neutralization of IL-6 using antibodies specific for either the cytokine or the α-chain of its receptor (IL-6R) ameliorates disease [29]. IL-6 inhibitor, a humanized mAb specific for IL-6R, was shown to suppress disease activity and erosive progression in patients with RA that is resistant to traditional DMARDs [30]. Therefore, according to those experiment data, we hypothesized that EE might ameliorate arthritis severity in collagen-induced arthritis mice model by suppressing inflammatory cytokine release.
In addition to the function of inflammatory cytokine in the pathogenesis of RA, the cellular and humoral response to collagen type II results in the development of synovial hyperplasia, infiltration of mononuclear cells and cartilage degradation were also seen [31]. Anti-collagen II antibodies have been found in serum and synovial fluid and eluted from cartilage explants of RA patients. Studies have demonstrated that the transfer of anti-collagen II antibodies can induce disease in naive mice [32]. The anti-collagen II antibodies form immune complexes, activating complement, which results in the infiltration of activated neutrophils and macrophages and the production of inflammatory cytokines. Anti-collagen antibodies have been shown to appear early after immunization with type II collagen, prior to joint swelling [33]. In our previous study, we found that anti-collagen II antibodies had close correlations with arthritis scores and hind paw thickness in CIA mice (data not shown). Consistent with the results, EE treatment could significantly reduce CII antibodies production and relieve the severity of RA in CIA mice in this study.
In our previous study, it was found that both prophylactic and therapeutic treatment with EE (30 mg/mL) could significantly reduce arthritis severity in CIA mice, suggesting that EE could be used as therapeutic and prophylactic agent to treat RA. The common phenomenon in China is that patients do not seek medical help until they experience signs and symptoms of RA. Therefore, the therapeutic effect of EE may be used to better meet the patients’ needs. Besides, in this study, we found treatment with as little as 30 mg/kg/day EE was able to significantly decrease all arthritic parameters measured: clinical arthritis scores, disease incidence, histopathology, anti-collagen antibodies, and cytokine production. However, 15 mg/kg EE treatment did not show the same effect, suggesting that doses of 30 mg/kg/day may be required to reduce disease in CIA mice. Considering the possible toxicity of EE, we have tested the toxic effect of EE in vitro and in vivo. EE (160 μmol/L) was added to co-culture with human PBMC, murine splenocytes, and THP-1 cells for 48 h, respectively. The cell proliferation was detected by MTT and the results showed that EE (160 μmol/L) was not toxic to primary cells and transformed cell line. We also tested the toxicity of EE (30 mg/kg) to immune cells in vivo. The body weight and the weight of the livers, spleens, thymuses, and kidneys of CIA mice have measured after EE (30 mg/kg/day) treatment for 3 weeks. The ratios of liver weight to body weight, spleen weight to body weight, thymus weight to body weight, and kidney weight to body weight did not change obviously compared to naive DBA/1 J mice. Therefore, 30 mg/kg EE is quite safe for mice.
CONCLUSION
In this study, we tested and verified the therapeutic effect of EE, a compound isolated from A. senticosus, in CIA mice. The results showed an overall reduction in arthritis scores, hind paw thickness, inflammation, bone erosion, and cartilage destruction in the joints of CIA mice treated with EE. In addition, EE could be taken orally and be less expensive than biologic agents. Oral formulation and low cost would be significant added values to develop novel drugs.
References
Weissmann, G. 2006. The pathogenesis of rheumatoid arthritis. Bulletin of the NYU Hospital for Joint Diseases 6: 12–15.
Feldmann, M., and S.R. Maini. 2008. Role of cytokines in rheumatoid arthritis: an education in pathophysiology and therapeutics. Immunological Reviews 223: 7–19.
Sack, U., R.W. Kinne, T. Marx, P. Heppt, S. Bender, and F. Emmrich. 1993. Interleukin-6 in synovial-fluid is closely associated with chronic synovitis in rheumatoid arthritis. Rheumatology International 13: 45–51.
Madhok, R., A. Crilly, J. Watson, and H.A. Capell. 1993. Serum interleukin-6 levels in rheumatoid arthritis-correlations with clinical and laboratory indexes of disease activity. Annals Rheumatic Diseases 52: 232–234.
Hashizume, M., and M. Mihara. 2011. The roles of interleukin-6 in the pathogenesis of rheumatoid arthritis. Arthritis. doi:10.1155/2011/765624.
Palmqvist, P., E. Persson, H.H. Conaway, and U.H. Lerner. 2002. IL-6, leukemia inhibitory factor, and oncostatin M stimulate bone resorption and regulate the expression of receptor activator of NF-kappa B ligand, osteoprotegerin, and receptor activator of NF-kappa B in mouse calvariae. Journal of Immunology 169: 3353–3362.
Ohta, S., K. Imai, K. Yamashita, T. Matsumoto, I. Azumano, and Y. Okada. 1998. Expression of matrix metalloproteinase 7 (matrilysin) in human osteoarthritic cartilage. Laboratory Investigation 78: 79–87.
Feldmann, M., and R.N. Maini. 2001. Anti-TNFα therapy or rheumatoid arthritis: what have we learned? Annual Review Immunology 19: 163–196.
Dixon, W.G., and D.P. Symmons. 2007. What effects might anti-TNF alpha treatment be expected to have on cardiovascular morbidity and mortalityin rheumatoid arthritis? A review of the role of TNF alpha in cardiovascular pathophysiology. Annals of the Rheumatic Disease 66: 1132–1136.
Gomez-Reino, J.J., L. Carmona, V.R. Valverde, E.M. Mola, and M.D. Montero. 2003. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis and Rheumatism 48: 2122–2127.
Charles, P.J., R.J. Smeenk, J. De Jong, M. Feldmann, and R.N. Maini. 2000. Assessment of antibodies to double-stranded DNA induced in rheumatoid arthritis patients following treatment with infliximab, a monoclonal antibody to tumor necrosis factor alpha: findings in open-label and randomized placebo-controlled trials. Arthritis and Rheumatism 43: 2383–2390.
Shakoor, N., M. Michalska, C.A. Harris, and J.A. Block. 2002. Drug-induced systemic lupus erythematosus associated with etanercept therapy. Lancet 359: 579–580.
Nishibe, S., H. Kinoshita, H. Takeda, and G. Okano. 1990. Phenolic compounds from stem bark of Acanthopanax senticosus and their pharmacological effect in chronic swimming stressed rats. Chemical & Pharmaceutical Bulletin 38: 1763–1765.
Yu, C.Y., S.H. Kim, J.D. Lim, M.J. Kim, and I.M. Chung. 2003. Intraspecific relationship analysis by DNA markers and in vitro cytotoxic and antioxidant activity in Eleutherococcus senticosus. Toxicology In Vitro 17: 229–236.
Schmolz, M.W., F. Sacher, and B. Aicher. 2001. The synthesis of Rantes, G-CSF, IL-4, IL-5, IL-6, IL-12 and IL-13 in human whole-blood cultures is modulated by an extract from Eleutherococcus senticosus L. roots. Phytotherapy Research 15: 268–270.
Yan, Z.W., J.P. Liu, D. Lu, R. Narlawar, P. Groundwater, and P.Y. Li. 2013. Two new ceramides from the fruit pulp of Acanthopanax senticosus (Rupr.et Maxim) Harms. Natural Product Research. doi:10.1080/14786419/2013/856908.
Clague, R.B., K. Morgan, T.I. Reynolds, and H.J. Williams. 1994. The prevalence of serum IgG antibodies to type ii collagen in American patients with rheumatoid arthritis. British Journal of Rheumatology 33: 336–338.
Weyand, C.M. 2000. New insights into the pathogenesis of rheumatoid arthritis. Rheumatology 39: 3–8.
Brand, D.D., A.H. Kang, and E.F. Rosloniec. 2003. Immunopathogenesis of collagen arthritis. Springer Semin Immunopathology 25: 3–18.
Tokiwa, T., T. Yamazaki, and S. Sakurai. 2006. Anti-inflammatory effect of eleutheroside E from Acanthopanax senticosus. Foods and Food Ingredients Journal of Japan 211: 576–582.
Yamazaki, T., T. Matsumura, T. Tsukiyama, and T. Tokiwa. 2006. Anti-inflammatory effects of eleutheroside E from Acanthopanax senticosus. Tissue Culture Research Communications 25: 137–145.
Yamazaki, T., S. Shimosaka, H. Sasaki, T. Matsumura, T. Tukiyama, and T. Tokiwa. 2007. (+)-Syringaresinol-di-O-beta-D-glucoside, a phenolic compound from Acanthopanax senticosus Harms, suppresses proinflammatory mediators in SW982 human synovial sarcoma cells by inhibiting activating protein-1 and/or nuclear factor-kappaB activities. Toxicology In Vitro 21: 1530–1537.
Huang, L.Z., L. Wei, H.F. Zhao, B.K. Huang, K. Rahman, and L.P. Qin. 2011. The effect of eleutheroside E on behavioral alterations in murine sleep deprivation stress model. European Journal Pharmacology 658: 150–155.
Ahn, J., M.Y. Um, H. Lee, C.H. Jung, S.H. Heo, and T.Y. Ha. 2013. Eleutheroside E, an active component of Eleutherococcus senticosus, ameliorates insulin resistance in type 2 diabetic db/db mice. Evidence Based Complementary and Alternative Medicine. doi:10.1155/2013/934183.
Elliott, M.J., R.N. Maini, M. Feldmann, A. Long-Fox, P. Charles, H. Bijl, and J.N. Woody. 1994. Repeated therapy with monoclonal antibody to tumour necrosis factor alpha (cA2) in patients with rheumatoid arthritis. Lancet 344: 1125–1127.
Elliott, M.J., R.N. Maini, M. Feldmann, J.R. Kalden, C. Antoni, J.S. Smolen, B. Leeb, F.C. Breedveld, J.D. Macfarlane, H. Bijl, et al. 1994. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet 344: 1105–1110.
Lipsky, P.E., D.M. van der Heijde, E.W. St Clair, D.E. Furst, F.C. Breedveld, J.R. Kalden, J.S. Smolen, M. Weisman, P. Emery, M. Feldmann, G.R. Harriman, and R.N. Maini. 2000. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. The New England Journal of Medicine 343: 1594–1602.
Maruotti, N., F.P. Cantatore, E. Crivellato, A. Vacca, and D. Ribatti. 2006. Angiogenesis in rheumatoid arthritis. Histology of Histopathology 21: 557–566.
Alonzi, T., E. Fattori, D. Lazzaro, P. Costa, L. Probert, G. Kollias, F. De Benedetti, V. Poli, and G. Ciliberto. 1998. Interleukin 6 is required for the development of collagen-induced arthritis. Journal of Experimental Medicine 187: 461–468.
Smolen, J.S., A. Beaulieu, A. Rubbert-Roth, C. Ramos-Remus, J. Rovensky, E. Alecock, T. Woodworth, and R. Alten. 2008. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 371: 987–997.
Brand, D.D., K.A. Latham, and E.F. Rosloniec. 2007. Collagen-induced arthritis. Nature Protocols 2: 1269–1275.
Rowley, M.J., K.S. Nandakumar, and R. Holmdahl. 2008. The role of collagen antibodies in mediating arthritis. Modern Rheumatology 18: 429–441.
Kuhn, K.A., L. Kulik, B. Tomooka, K.J. Braschler, W.P. Arend, W.H. Robinson, and V.M. Holers. 2006. Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. Journal of Clinical Investigation 116: 961–973.
Acknowledgments
This work was supported by the grants from the National Natural Science Foundation of China (No. 81202348 and No. 81202394) and Jiangsu Province National Natural Science Funds (No. BK2012171) and the Natural Science Research Project of Higher Education of Jiangsu (No. 12KJD310006), the Applied Basic Research Programs of Suzhou Sci-tech Bureau (No. SYS201219), and the Medical Research Projects of Health Department of Jiangsu Province (No. Z201304).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Chunyan He and Xiaohui Chen contributed equally to this work.
Rights and permissions
About this article
Cite this article
He, C., Chen, X., Zhao, C. et al. Eleutheroside E Ameliorates Arthritis Severity in Collagen-Induced Arthritis Mice Model by Suppressing Inflammatory Cytokine Release. Inflammation 37, 1533–1543 (2014). https://doi.org/10.1007/s10753-014-9880-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-014-9880-7