Abstract
Pemetrexed (PMTX) is an anti-folate drug as methotrexate. The purpose of this study was to assess the efficacy of PMTX on collagen-induced arthritis (CIA). Forty Wistar albino rats were randomized into four groups. Arthritis was induced by intradermal injection of chicken type II collagen combined with incomplete Freund’s adjuvant. Animals were sacrificed at the 15th day after the onset of arthritis. Tumor necrosis factor alpha (TNF-α), interleukin (IL)-17, and malondialdehyde (MDA) levels were increased, and superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) activities and the expressions of nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1) were decreased in the arthritis group. In the PMTX-treated (0.2 and 1 mg/kg/week i.p.) groups, the levels of TNF-α, IL-17, and MDA were decreased; the activities of SOD, CAT, and GPx and the expressions of Nrf2 and HO-1 were restored, and perisynovial inflammation and cartilage–bone destruction were decreased. PMTX has anti-arthritic potential in the CIA model and may be a therapeutic agent for rheumatoid arthritis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that causes morbidity and mortality [1]. The treatment of RA is difficult because of its uncertain etiopathogenesis and variable clinical findings. Currently, pro-inflammatory cytokines are accepted playing a key role in the pathogenesis of RA [2]. They directly build pannus formation and lead to joint damage. Moreover, free radicals released from inflammatory cells enhance the joint damage. In addition to the increase of oxidants, anti-oxidants are decreased in patients with RA. Joint erosions occur within the first six monthly period of this disease [3].
Pemetrexed (PMTX) is an anti-folate drug as methotrexate (MTX). PMTX inhibits several folate-dependent enzymes including mainly thymidylate synthase, more effectively than MTX. Because of these features, PMTX has been included into multiple target folic acid antagonists. In addition, PMTX versus MTX is better substrate for folylpolyglutamate synthase that increases potency and half-life of folic acid analogs [4]. Positive results have been obtained by using PMTX in oncological diseases that MTX is not effective [5, 6].
Collagen-induced arthritis (CIA) is an experimental arthritis model that is widely used in the studies on treatment methods [7]. The CIA model has been used as a model of experimental arthritis since 1977 [7]. CIA is a well-defined animal model of human RA. Injection of type II collagen leads to the development of severe polyarticular arthritis in mammals and rodents. This model which is associated with the immune system causes erosion of both bone and cartilage leading to severe loss of joint function. The mechanism of arthritis formation has not clearly defined. However, it is known that both B and T cells play a role in the pathogenesis of arthritis as in human RA [8].
The purpose of the present study is to explore the efficacy of PMTX treatment in a CIA model.
MATERIALS AND METHODS
Animals
Female albino Wistar rats obtained from the Firat University Research Center, Elazig, Turkey, were used in the study. This study was conducted on 40 female albino Wistar rats, 8–10 weeks old, weighing 200–250 g. They were housed four per cage at a temperature of 22 ± 2 °C and humidity of 55 ± 5 % with 12-h light/dark cycle under a controlled environment. Rats were fed a standard pellet diet and water ad libitum. The study was approved by the Animal Experiments Ethics Committee of Firat University.
Experimental Design
The rats were randomized into four groups (n = 10 in each group). Group I as control group, group II as arthritic sham group, group III as lower-dose PMTX group, and group IV as higher-dose PMTX group were assigned. Type 2 collagen (Sigma-Aldrich, St. Louis, USA) obtained from chicken sternum was diluted with 0.1 M acetic acid (1 mg/ml). The collagen solution was emulsified with an equal amount of incomplete Freund’s adjuvant (Difco Laboratories, Detroit, USA). The resulting solution was administered to group II, III, and IV rats with intradermal injections into the tail dorsal (100 μg/rat) and back paws (50 μg to each paw and total 200 μg to each rat). Seven days after the first administration, booster injection (100 μg to each rat) was given through the tail dorsal. Following the collagen injection, each rat was assessed on a daily basis for the development of arthritis and the clinical scoring of arthritis. Clinical scoring of arthritis was performed on each back paws on a scale between 0 and 4 points as described previously [9]. Group III and group IV rats were given intraperitoneal doses of 0.2 mg/kg/week and 1 mg/kg/week PMTX (Alimta, Eli Lilly, Istanbul, Turkey), respectively, at the 14th, 21st, and 28th days after onset of arthritis.
Sample Collection
All rats were sacrificed by decapitation on day 29 at the 15th day after the onset of arthritis. Blood samples were collected, and the back paws were amputated from the knee down for further histopathological analysis. The blood samples were centrifuged at 3,000 rpm for 10 min, and the harvested sera were kept at −20 °C until the day of analysis. One back paw was fixed with 10 % formalin solution and embedded in paraffin for histopathological examination, and the other back paw was stored immediately at −80 °C for Western blot analysis.
Histopathological Evaluations
Tissue samples fixed in formalin solution were decalcified with 10 % nitric acid (30 days) to prepare paraffin blocks. Cross sections taken from the blocks were stained with hematoxylin and eosin (H&E). Then, they were examined by a specialist pathologist under ×40, ×100, ×200, and ×400 magnifications in a light microscope to assess inflammatory cell infiltration, pannus formation, and bone destruction around the joint. The samples were scored on a scale between 0 and 4 points for histopathological scoring as described previously (Tables 1 and 2) [10, 11].
Biochemical Analysis
Serum TNF-α (Invitrogen, Camarillo, CA, USA) and interleukin (IL)-17 (Uscn Life Science Inc., China) levels were studied using the relevant commercial kits according to the enzyme-linked immunosorbent assay (ELISA) method. Serum superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) activities were analyzed using appropriate commercial kits (Cayman Chemical Company, Ann Arbor, MI, USA) in accordance with ELISA method. Serum malondialdehyde (MDA) levels were measured with a high-performance liquid chromatography device (Shimadzu, Japan).
Western Blot Analysis
Joint tissue samples were analyzed for the expression of nuclear factor erythroid 2-related factor-2 (Nrf2) and heme oxygenase-1 (HO-1) using the Western blot technique. In all groups, the hind paws were excised rapidly from sacrificed rats and then quickly frozen at −80 °C. Small pieces of the paw joints in each group of animals were pooled together for Western blot analysis. Homogenates were prepared in ice-cold lysis buffer containing 50 mM Tris–HCl (pH, 8.0), 150 mM NaCl, 5 mM EDTA, 1 % Triton X-100, 0.26 % sodium deoxycholate, 50 mM sodium fluoride, 10 mM β-glycerophosphate, 0.1 mM sodium orthovanadate, 10 μg/ml leupeptin, and 50 μg/ml phenylmethylsulfonyl fluoride (PMSF) and incubated on ice for 40 min [12]. Eighty microliters of 10 % Nonidet P-40 (NP-40) solution was added to the homogenates, and the mixture was then centrifuged for 2 min at 14,000g at 4 °C for removing the cellular debris and isolating total protein. Concentration of the protein was determined according to the procedure described by Lowry et al. [13] using a protein assay kit supplied by Sigma (St. Louis, MO, USA). Sodium dodecyl sulfate–polyacrylamide gel electrophoresis sample buffer containing 2 % β-mercaptoethanol was added to the supernatant. Equal amounts of protein (50 μg) were electrophoresed and subsequently transferred to nitrocellulose membranes (Schleicher and Schuell Inc., Keene, NH, USA). Nitrocellulose blots were washed twice for 5 min each in phosphate-buffered saline (PBS) and blocked with 1 % bovine serum albumin in PBS for 1 h prior to application of the primary antibody. The antibody against Nrf2 and HO-1 was purchased from Abcam Inc. (Abcam, Cambridge, UK). Primary antibody was diluted (1:1000) in the same buffer containing 0.05 % Tween-20. The nitrocellulose membrane was incubated overnight at 4 °C with protein antibody. The blots were washed and incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (Abcam, Cambridge, UK). Specific binding was detected using diaminobenzidine and H2O2 as substrates. Protein loading was controlled using a monoclonal mouse antibody against β-actin antibody (A5316; Sigma). Blots were performed at least three times to confirm the reproducibility of the results. Bands were analyzed densitometrically using an image analysis system (Image J; National Institute of Health, Bethesda, USA).
Statistical Analysis
Statistical evaluations were performed using the SPSS package program, version 21. Data were presented as mean ± standard deviation. Kruskal–Wallis one-way analysis of variance was used for comparisons among the groups, and the Mann–Whitney U test was used for dual comparisons. Differences in continuous values (clinical scoring of arthritis on 14th and 29th days) were assessed using the Wilcoxon rank-sum test. A p value of <0.05 was considered to be significant.
RESULTS
Clinical Scoring of Arthritis
Arthritis was clinically developed in all rats of the groups II, III, and IV at 12 to 13 days after the injection of collagen. The 29th day scores were decreased in the groups III and IV compared to the own 14th day score (p < 0.05 for both), while it was increased in the group II (p < 0.05). Moreover, the mean arthritis scores on the 29th day in the groups III and IV were lower than those in the group II (p < 0.01 and p < 0.001, respectively) (Fig. 1).
Assessments of daily arthritis score in the all study groups. Multiplication sign the mean 14th day clinical arthritis scores were higher in the groups II, III, and IV than in the group I (p < 0.01 for all). Plus sign the mean 29th day score of group II was higher than that of groups III and IV (p < 0.01 and p < 0.001, respectively). Asterisk the 29th day scores were decreased in the groups III and IV compared to the own 14th day score (p < 0.05 for both).
Histopathological Evaluations
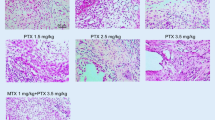
The histopathological scoring of joint tissue samples showed a significant decrease in inflammation and destruction scores of groups III and IV when compared with group II (Table 3). Destruction score in the group IV was lower than in the group III (p < 0.01), but inflammation scores were similar in the groups III and IV (p > 0.05) (Fig. 2).
Histopathological sections of joints in study groups (H&E ×40). Normal perisynovial tissue and cartilage–bone appearance in the group I (a). Obvious perisynovial inflammation and destruction of cartilage–bone in the group II (b). Decreased perisynovial inflammation and synovial hyperplasia in group III (c) and group IV (d).
Serum Pro-inflammatory Cytokine Levels
Serum TNF-α, and IL-17 levels in the group II were higher than in the group I (p < 0.01 for both). They were similar in the groups III and IV (p > 0.05). However, their levels were lower in the groups III and IV than in the group II (Table 3). The mean serum TNF-α levels of groups III and IV were similar in the group I (p > 0.05 for both), while serum IL-17 levels were higher in the groups III and IV than in the group I (p < 0.01 and p < 0.01, respectively) (Table 3).
Serum Malondialdehyde Levels
Serum MDA levels were higher in the group II than in the group I (p < 0.01). On the other hand, serum MDA levels were lower in the groups III and IV than in the group II (p < 0.01 for both) (Table 3). No significant difference in MDA levels was observed between groups III and IV (p > 0.05).
Serum Anti-oxidants
SOD, CAT, and GPx activities were lower in the group II than in the group I (p < 0.01 for all). However, they were higher in the group III than in the group II (p < 0.01, p < 0.05, and p < 0.05, respectively). SOD and GPx activities were higher in the group IV than in the group II (p < 0.01 for all). However, there was no significant difference between groups II and IV in terms of the CAT activities (p > 0.05) (Table 3).
Western Blot Analysis
The Nrf2 and HO-1 expressions were lower in the group II when compared to the group I (p < 0.05 for both). Nrf2 and HO-1 expressions were higher in the group III than in the group II (p < 0.05). Moreover, Nrf2 and HO-1 expressions were higher in the group IV than in the group II (p < 0.01 and p < 0.05, respectively) (Fig. 3).
Western blot analysis and densitometric quantifications of Nrf2 (a) and HO-1 (b). Representative blots, repeated at least three times (n = 4), are shown. Actin was included to ensure equal protein loading. The densitometric quantifications were normalized to actin densities for each sample and expressed as mean ± SD. Different letters (a–b, a–c, and b–c) indicate group mean differences (p < 0.05). Nrf2 nuclear factor erythroid 2-related factor-2, HO-1 heme oxygenase-1.
DISCUSSION
In the present study, synovial inflammatory cell infiltrations; cartilage–bone destruction; and the serum levels of TNF-α, IL-17, MDA, and anti-oxidant enzyme activities (SOD, CAT, and GPx) were investigated in an experimental model of arthritis CIA. Moreover, the effectiveness of PMTX, an anti-folate drug in the treatment of experimental arthritis, was evaluated. PMTX decreased the inflammatory cell infiltrations; cartilage–bone destruction; and serum TNF-α, IL-17, and MDA levels, while it restored SOD, CAT, and GPx anti-oxidant enzyme activities. The therapeutic potential of PMTX in CIA model was observed in the present study.
RA is a chronic systemic inflammatory disease that can cause morbidity and mortality [1, 14]. Recent studies show that 50 % of patients with RA became unable to work in the first ten yearly periods [15]. RA severely impacts on the quality of life, and patients with RA have high rate of work disability. Consequently, the economic burden of RA is substantial. The main purpose of RA treatment should be the minimizing loss of function.
There are many studies about efficacy of alternative biologic therapy agents developed against disease-modifying anti-rheumatic drugs (DMARDs). In a study about radiographic progression, it has been shown that there is no difference between the treatments of conventional DMARDs and biological therapy agents. Cost effectiveness analysis shows that biological agents are not superior to conventional DMARDs [16]. Moreover, biological agents consist of several concerns. For instance, the risk of bacterial infection has been increased in patients treated with these agents [17]. Therefore, cost-effective new agents comprising less toxicity are needed to enhance the treatment options of RA [18].
Nowadays, MTX is the most commonly used DMARD [19]. Several mechanisms are proposed for the activity of MTX. These include anti-inflammatory, anti-proliferative, and immunosuppressive effects [20]. PMTX is an anti-folate drug as MTX. While MTX inhibits only dihydrofolate reductase, PMTX inhibits multiple targets. Its effects caused by disrupting several folate-dependent metabolic pathways essential for cell replication through inhibiting thymidylate synthase, glycinamide ribonucleotide transferase, and, to a lesser extent, at 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase and dihydrofolate reductase enzymes [21]. In our study, arthritis scores were reduced in mice treated with PMTX. This preliminary study documents the anti-arthritic effect of PMTX but cannot suggest that PMTX is superior to MTX. For instance, oral bioavailability of PMTX has not been evaluated in human yet. However, the oral absorption of PMTX has been documented to be low in mice.
IL-17 plays an important role in the pathogenesis of RA. IL-17 is a pro-inflammatory cytokine that leads to bone destruction by RANKL activation. In RA patients, high levels of IL-17 have been determined in both peripheral blood and synovial fluid [22]. Miranda-Carus et al. [23] have showed that treatment with MTX reduced IL-17 levels in an experimental arthritis model. TNF-α is an important cytokine, which plays critical role in the pathogenesis of inflammation and proliferation in RA. TNF-α is released from monocytes, and it is determined at high levels in the synovial fluid, tissues, and serum of RA patients [24]. Arthritis has been shown to be suppressed by the treatment methods targeting to TNF-α. Seitz et al. [25] have showed that MTX treatment reduced TNF-α levels in RA patients. In our study, IL-17 and TNF-α levels increased in the arthritis group and decreased in the PMTX-treated groups.
Oxidative stress plays a critical role in the pathogenesis of RA [26]. In RA patients, radicals released from inflammatory cells in the synovial fluid such as superoxide and hydrogen peroxide cause joint damage and pannus formation [27]. SOD, CAT, and GPx are the enzymes that play a role in defense system against free radicals. Thus, they act anti-oxidant actions. The activities of these anti-oxidant enzymes have been shown to be decreased in patients with RA [28–31]. On the other hand, Salvenimi et al. [32] have showed that SOD treatment reduces the cartilage and bone destruction in experimental arthritis. In our study, SOD, CAT, and GPx activities are decreased in experimental arthritis, while PMTX treatment restored their activities.
Free radicals damage protein, lipid, and DNA structure [33]. They generate lipid peroxidation reacting with membrane lipids. Lipid peroxidation damages membrane structure and other cell components by producing reactive aldehyde. Thus, it causes many diseases and tissue damage [34]. MDA, which is an oxidative stress marker, is a product of lipid peroxidation [35]. In previous studies on RA patients, high levels of MDA have been determined in both synovial fluid and sera [36]. On the other hand, Bauerova et al. [37] have showed that MDA levels reduced with MTX treatment in experimental arthritis model. Similarly, MDA levels were determined to be increased in arthritis group when compared with control group in our study. Moreover, treatment with PMTX decreased MDA levels.
Nrf2, a redox-sensitive transcription factor, binds to anti-oxidant response elements (ARE) encoding many phase II detoxifying or anti-oxidant enzymes and related stress-responsive proteins including glutathione S-transferase, GPx, and HO-1. Thus, Nrf2 regulates redox status and plays key roles in cellular defense by enhancing the removal of reactive oxygen species [38]. It has been documented that Nrf2-knockout mice had more severe cartilage injuries and more oxidative damage, and the expression of Nrf2 target genes was enhanced in Nrf2 wild-type, but not in knockout, mice during arthritis [39, 40]. These results [39, 40] support a protective role of Nrf2 against joint inflammation. In the present study, Nrf2 and HO-1 expressions were decreased in the arthritis group, while PMTX treatment increased their expressions. It may be concluded that anti-inflammatory effects of PMTX lead to the restoration of oxidative stress. At the end of the treatment, anti-oxidant potentials of PMTX may contribute to its anti-arthritic effects.
The present study has several limitations. Firstly, our study does not comprise a MTX comparison group. It would be better to examine similar and different actions of MTX and PMTX.
In conclusion, PMTX inhibits several pathways that play a significant role for the pathogenesis of RA, in CIA model. These results suggest that PMTX may be a novel candidate DMARD as alternative to MTX. However, further and more detailed studies are needed for use of PMTX on human RA as an alternative treatment agent.
References
Hobbs, K.F., and M.D. Cohen. 2012. Rheumatoid arthritis disease measurement: A new old idea. Rheumatology (Oxford) 51: vi21–27.
Schett, G., and E. Gravallese. 2012. Bone erosion in rheumatoid arthritis: Mechanisms, diagnosis and treatment. Nature Reviews. Rheumatology 8(11): 656–664.
Breedveld, F.C., M.H. Weisman, A.F. Kavanaugh, S.B. Cohen, K. Pavelka, R. van Vollenhoven, et al. 2006. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis and Rheumatism 54(1): 26–37.
Shih, C., V.J. Chen, L.S. Gossett, S.B. Gates, W.C. MacKellar, L.L. Habeck, et al. 1997. LY231514, a pyrrolo[2,3-d]pyrimidine-based antifolate that inhibits multiple folate-requiring enzymes. Cancer Research 57(6): 1116–1123.
Vogelzang, N.J., J.J. Rusthoven, J. Symanowski, C. Denham, E. Kaukel, P. Ruffie, et al. 2003. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. Journal of Clinical Oncology 21(14): 2636–2644.
Cohen, M.H., R. Justice, and R. Pazdur. 2009. Approval summary: Pemetrexed in the initial treatment of advanced/metastatic non-small cell lung cancer. The Oncologist 14(9): 930–935.
Williams, R.O. 2004. Collagen-induced arthritis as a model for rheumatoid arthritis. Methods in Molecular Medicine 98: 207–216.
Lawson, B.R., S.M. Belkowski, J.F. Whitesides, P. Davis, and J.W. Lawson. 2007. Immunomodulation of murine collagen-induced arthritis by N, N-dimethylglycine and a preparation of Perna canaliculus. BMC Complementary and Alternative Medicine 7: 20.
Trentham, D.E., A.S. Townes, and A.H. Kang. 1977. Autoimmunity to type II collagen an experimental model of arthritis. Journal of Experimental Medicine 146(3): 857–868.
Larsson, P., S. Kleinau, R. Holmdahl, and L. Klareskog. 1990. Homologous type II collagen-induced arthritis in rats. Characterization of the disease and demonstration of clinically distinct forms of arthritis in two strains of rats after immunization with the same collagen preparation. Arthritis and Rheumatism 33(5): 693–701.
Barsante, M.M., E. Roffe, C.M. Yokoro, W.L. Tafuri, S.G. Souza, V. Pinho, et al. 2005. Anti-inflammatory and analgesic effects of atorvastatin in a rat model of adjuvant-induced arthritis. European Journal of Pharmacology 516(3): 282–289.
Choi, J., B.J. Yoon, Y.N. Han, K.T. Lee, J. Ha, H.J. Jung, et al. 2003. Antirheumatoid arthritis effect of Rhus verniciflua and of the active component, sulfuretin. Planta Medica 69(10): 899–904.
Lowry, O.H., N.J. Rosebrough, A.L. Farr, and R.J. Randall. 1951. Protein measurement with the folin phenol reagent. Journal of Biological Chemistry 193: 165–175.
Sokka, T. 2003. Work disability in early rheumatoid arthritis. Clinical and Experimental Rheumatology 21(5 Suppl 31): S71–74.
Young, A., G. Koduri, M. Batley, E. Kulinskaya, A. Gough, S. Norton, et al. 2007. Mortality in rheumatoid arthritis. Increased in the early course of disease, in ischaemic heart disease and in pulmonary fibrosis. Rheumatology (Oxford) 46(2): 350–357.
Finckh, A., N. Bansback, C.A. Marra, A.H. Anis, K. Michaud, S. Lubin, et al. 2009. Treatment of very early rheumatoid arthritis with symptomatic therapy, disease-modifying antirheumatic drugs, or biologic agents: A cost-effectiveness analysis. Annals of Internal Medicine 151(9): 612–621.
Nam, J.L., K.L. Winthrop, R.F. van Vollenhoven, K. Pavelka, G. Valesini, E.M. Hensor, et al. 2010. Current evidence for the management of rheumatoid arthritis with biological disease-modifying antirheumatic drugs: A systematic literature review informing the EULAR recommendations for the management of RA. Annals of the Rheumatic Diseases 69(6): 976–986.
Lu, L.J., C.D. Bao, M. Dai, J.L. Teng, W. Fan, F. Du, et al. 2009. Multicenter, randomized, double-blind, controlled trial of treatment of active rheumatoid arthritis with T-614 compared with methotrexate. Arthritis and Rheumatism 61(7): 979–987.
Fan, P.T., and K.H. Leong. 2007. The use of biological agents in the treatment of rheumatoid arthritis. Annals of the Academy of Medicine, Singapore 36(2): 128–134.
Xinqiang, S., L. Fei, L. Nan, L. Yuan, Y. Fang, X. Hong, et al. 2010. Therapeutic efficacy of experimental rheumatoid arthritis with low-dose methotrexate by increasing partially CD4+CD25+ Treg cells and inducing Th1 to Th2 shift in both cells and cytokines. Biomedicine and Pharmacotherapy 64(7): 463–471.
Chattopadhyay, S., R.G. Moran, and I.D. Goldman. 2007. Pemetrexed: Biochemical and cellular pharmacology, mechanisms, and clinical applications. Molecular Cancer Therapeutics 6(2): 404–417.
Niimoto, T., T. Nakasa, M. Ishikawa, A. Okuhara, B. Izumi, M. Deie, et al. 2010. MicroRNA-146a expresses in interleukin-17 producing T cells in rheumatoid arthritis patients. BMC Musculoskeletal Disorders 11: 209.
Miranda-Carus, M.E., A. Balsa, M. Benito-Miguel, C. Perez de Ayala, and E. Martin-Mola. 2004. IL-15 and the initiation of cell contact-dependent synovial fibroblast-T lymphocyte cross-talk in rheumatoid arthritis: Effect of methotrexate. Journal of Immunology 173(2): 1463–1476.
Manicourt, D.H., R. Triki, K. Fukuda, J.P. Devogelaer, C. Nagant de Deuxchaisnes, and E.J. Thonar. 1993. Levels of circulating tumor necrosis factor alpha and interleukin-6 in patients with rheumatoid arthritis. Relationship to serum levels of hyaluronan and antigenic keratan sulfate. Arthritis and Rheumatism 36(4): 490–499.
Seitz, M., M. Zwicker, and P.M. Villiger. 2003. Pretreatment cytokine profiles of peripheral blood mononuclear cells and serum from patients with rheumatoid arthritis in different American college of rheumatology response groups to methotrexate. Journal of Rheumatology 30(1): 28–35.
Hitchon, C.A., and H.S. El-Gabalawy. 2004. Oxidation in rheumatoid arthritis. Arthritis Research and Therapy 6(6): 265–278.
Halliwell, B., J.R. Hoult, and D.R. Blake. 1988. Oxidants, inflammation, and anti-inflammatory drugs. FASEB Journal 2(13): 2867–2873.
Imadaya, A., K. Terasawa, H. Tosa, M. Okamoto, and K. Toriizuka. 1988. Erythrocyte antioxidant enzymes are reduced in patients with rheumatoid arthritis. Journal of Rheumatology 15(11): 1628–1631.
Nivsarkar, M. 2000. Improvement in circulating superoxide dismutase levels: Role of nonsteroidal anti-inflammatory drugs in rheumatoid arthritis. Biochemical and Biophysical Research Communications 270(3): 714–716.
Rasool, M., and P. Varalakshmi. 2007. Protective effect of Withania somnifera root powder in relation to lipid peroxidation, antioxidant status, glycoproteins and bone collagen on adjuvant-induced arthritis in rats. Fundamental and Clinical Pharmacology 21(2): 157–164.
Kerimova, A.A., M. Atalay, E.Y. Yusifov, S.P. Kuprin, and T.M. Kerimov. 2000. Antioxidant enzymes; possible mechanism of gold compound treatment in rheumatoid arthritis. Pathophysiology 7(3): 209–213.
Salvemini, D., E. Mazzon, L. Dugo, I. Serraino, A. De Sarro, A.P. Caputi, et al. 2001. Amelioration of joint disease in a rat model of collagen-induced arthritis by M40403, a superoxide dismutase mimetic. Arthritis and Rheumatism 44(12): 2909–2921.
Dalle-Donne, I., R. Rossi, D. Giustarini, A. Milzani, and R. Colombo. 2003. Protein carbonyl groups as biomarkers of oxidative stress. Clinica Chimica Acta 329(1–2): 23–38.
Girotti, A.W. 1998. Lipid hydroperoxide generation, turnover, and effector action in biological systems. Journal of Lipid Research 39(8): 1529–1542.
Garg, N., R. Singh, J. Dixit, A. Jain, and V. Tewari. 2006. Levels of lipid peroxides and antioxidants in smokers and nonsmokers. Journal of Periodontal Research 41(5): 405–410.
Kamanli, A., M. Naziroglu, N. Aydilek, and C. Hacievliyagil. 2004. Plasma lipid peroxidation and antioxidant levels in patients with rheumatoid arthritis. Cell Biochemistry and Function 22(1): 53–57.
Bauerova, K., E. Paulovicova, D. Mihalova, F. Drafi, M. Strosova, C. Mascia, et al. 2010. Combined methotrexate and coenzyme Q(1)(0) therapy in adjuvant-induced arthritis evaluated using parameters of inflammation and oxidative stress. Acta Biochimica Polonica 57(3): 347–354.
Hayes, J.D., and A.T. Dinkova-Kostova. 2014. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends in Biochemical Sciences 39(4): 199–218.
Wruck, C.J., A. Fragoulis, A. Gurzynski, L.O. Brandenburg, Y.W. Kan, K. Chan, et al. 2011. Role of oxidative stress in rheumatoid arthritis: Insights from the Nrf2-knockout mice. Annals of the Rheumatic Diseases 70: 844–850.
Maicas, N., M.L. Ferrándiz, R. Brines, L. Ibáñez, A. Cuadrado, M.I. Koenders, W.B. van den Berg, and M.J. Alcaraz. 2011. Deficiency of Nrf2 accelerates the effector phase of arthritis and aggravates joint disease. Antioxidants and Redox Signaling 15(4): 889–901.
Conflict of Interests
None.
Ethics Approval
Approval was obtained from the Ethics Committee of Firat University.
Provenance and Peer Review
Not commissioned; externally peer reviewed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karatas, A., Koca, S.S., Ozgen, M. et al. Pemetrexed Ameliorates Experimental Arthritis in Rats. Inflammation 38, 9–15 (2015). https://doi.org/10.1007/s10753-014-0002-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-014-0002-3