Abstract
Objective
To compare the therapeutic efficacy of paclitaxel (PTX) alone to its combination with methotrexate (MTX) on rheumatoid arthritis.
Methods
A collagen-induced arthritis (CIA) rat model was established by induction of type II collagen. Rats were divided into blank control group, CIA model group, MTX group 1 mg/kg, PTX 1.5 mg/kg, PTX 2.5 mg/kg, PTX 3.5 mg/kg, and MTX 1 mg/kg + PTX 3.5 mg/kg, with 10 rats per group. The inflammation of the ankle joint was analyzed by H&E staining and interleukin (IL)-1β and IL‑6 expression was detected by immunohistochemistry. TUNEL assay was performed to detect synovial tissue cell apoptosis after administration of PTX and MTX either alone or in combination. TLR4 and p‑NF-κBp65 protein expression in synovial tissue and the changes of serum IL‑1β, IL‑6, IL‑12, MMP‑3, and TNFα protein factors were detected by western blot and ELISA, respectively.
Results
PTX and MTX improved histopathological changes in CIA rats. Besides, the apoptosis rate of synovial tissue cells in the PTX 3.5 mg/kg group was more than that of the PTX + MTX group. Immunohistochemistry and western blot results indicated that PTX and MTX reduce the expression rate of IL‑6 and IL‑1β and downregulate TLR4 and p‑NF-κBp65 protein expression. Furthermore, TLR4 and p‑NF-κBp65 reduced the concentration of MMP‑3, IL‑12, IL‑6, IL1‑β, and TNFα.
Conclusion
Both PTX and MTX exert significant suppression on rheumatoid arthritis, and the combined effect of the two drugs is weaker than that of PTX alone. Moreover, intraperitoneal injection of PTX 3.5 mg/kg every other day was the optimal dose observed in this study.
Zusammenfassung
Ziel
Ziel der Studie war, die therapeutische Wirksamkeit von Paclitaxel (PTX) allein und in Kombination mit Methotrexat (MTX) bei rheumatoider Arthritis zu vergleichen.
Methoden
Im Rattenmodell wurde eine kollageninduzierte Arthritis (CIA) durch Verabreichung von Typ-II-Kollagen induziert. Die Ratten wurden eingeteilt in eine leere Kontrollgruppe, eine CIA-Modell-Gruppe, eine MTX-Gruppe mit 1 mg/kg, eine PTX-Gruppe mit 1,5 mg/kg, eine PTX-Gruppe mit 2,5 mg/kg, eine PTX-Gruppe mit 3,5 mg/kg und eine Gruppe mit MTX 1 mg/kg + PTX 3,5 mg/kg, dabei waren 10 Ratten in jeder Gruppe. Die Inflammation des Sprunggelenks wurde in der Hämatoxilin-Eosin(HE)-Färbung sowie anhand der Expression von Interleukin(IL)-1β und IL‑6 in der immunhistochemischen Untersuchung ausgewertet. Mit einem TUNEL-Assay („TdT mediated-dUTP nick-end labeling“) wurde die Apoptose von Zellen im Synovialgewebe nach Gabe von PTX und MTX allein oder in Kombination untersucht. Die TLR4- und p‑NF-κBp65-Protein-Expression im Synovialgewebe und die Veränderungen von IL‑1β, IL‑6, IL‑12, Matrixmetalloproteinase(MMP)-3 und Tumornekrosefaktor(TNF)-α-Protein-Faktoren im Serum wurden mittels Westernblot bzw. ELISA („enzyme linked immunosorbent assay“) ermittelt.
Ergebnisse
PTX und MTX führten zur Verbesserung der histopathologischen Veränderungen bei CIA-Ratten. Außerdem war die Apoptoserate der Zellen des Synovialgewebes in der Gruppe mit PTX 3,5 mg/kg höher als in der Gruppe PTX + MTX. Immunhistochemie und Westernblot zeigten, dass PTX und MTX die Expressionsrate von IL‑6 und IL‑1β vermindern sowie die TLR4- und p‑NF-κBp65-Protein-Expression downregulieren. Darüber hinaus reduzierten TLR4 und p‑NF-κBp65 die Konzentration von MMP‑3, IL‑12, IL‑6, IL1‑β und TNFα.
Schlussfolgerung
Sowohl PTX als auch MTX bewirken eine signifikante Suppression der rheumatoiden Arthritis, dabei ist die kombinierte Wirkung der beiden Substanzen schwächer als die von PTX allein. Außerdem erwies sich die intraperitoneale Injektion von PTX 3,5 mg/kg jeden zweiten Tag als optimale Dosis in dieser Studie.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a common autoimmune disease characterized by chronic inflammatory lesions of joint tissues, which mainly affects small joints of the hands and feet, as well as other organs or tissues such as the lungs, heart, and nervous system [1]. The primary pathological changes of RA are erosion of cartilage and bone tissues, joint synovial cell infiltration, and synovial sputum formation [2]. Its pathogenesis may be closely related to the autoimmune response of genetically susceptible individuals caused by persistent infection of hemolytic Streptococcus or other bacterial metabolites and viruses [3]. Despite high incidence of RA, its etiology and pathogenesis are still poorly understood.
Several studies have shown that autoimmune response and inflammatory factors have a close relationship to the pathogenesis of RA [4, 5]. As important cytokines in inflammation, pro-inflammatory cytokines including tumor necrosis factor α (TNFα), matrix metalloproteinase (MMP)-3, and interleukin (IL)-12, IL‑6, and IL‑1β are considered to be the most significant mediators involved in the pathogenesis of RA [6]. Roy et al. have indicated that TLR4 is expressed on the surface of all immune cells except NK cells and B and T lymphocytes, and contributes greatly to the pathogen-specific recognition and activation of the natural immune system during the invasion of pathogenic microorganisms [7]. Besides, TLR4 has also been reported to mediate the expression of inflammatory factors in pancreatic tissue and is expressed on the membranes of macrophages and fibroblasts, which are the most important cells in the synovial tissue of RA patients [8]. Nuclear factor-κB (NF-κB) is a class of proteins that specifically bind to and promote transcription at the κB site of various gene promoters [9]. Regulation of NF-κB activation can effectively control the expression of inflammatory cytokines and participate in acute inflammatory reactions and chronic inflammatory diseases, including RA [10]. Therefore, the TLR4/NF-κB signaling pathway plays an essential role in RA.
Methotrexate (MTX) can reduce the rate of erythrocyte sedimentation and improve bone erosion, thus delaying the progression of RA [11]. MTX has become the basic drug for an RA combined regimen in clinical practice [12, 13]. Paclitaxel (PTX) extracted from the bark of Taxus cuspidata has anti-tumor activity against lung, gastric, and nasopharyngeal carcinomas [14]. PTX can not only block the mitotic cycle of cells and inhibit proliferation, but also affect a range of critical cellular immune and inflammatory activities [15]. Zhao et al. have pointed out that PTX can alleviate the degree of joint swelling in collagen-induced arthritis (CIA) rats [16]. PTX and MTX are both potential drugs for the treatment of arthritis. Nevertheless, the comparison of therapeutic efficacy and the mechanism of PTX alone or in combination with MTX on arthritis has not been studied.
In this study, a CIA rat model was established by induction of type II collagen. The therapeutic effects of PTX alone or in combination with MTX on RA were analyzed by observing pathological sections of ankle joint. The mechanism of MTX and PTX in treating RA was further studied by detecting TLR4 and p‑NF-κ-Bp65 protein expression and the contents of MMP‑3, IL‑12, IL‑6, IL‑1β, and TNFα.
Materials and methods
Animals
Female Sprague Dawley (SD) rats weighing 120–150 g were purchased from Shanghai Slack Laboratory Animal Co. Ltd. (Shanghai, China, license number: SCXX [Shanghai] 2017-0005). All animal experiments were conducted in accordance with international ethical guidelines and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Construction of the CIA rat model
A total of 70 SD rats were initially divided into a blank control group (n = 10) and a model group (n = 60). Type II collagen immunizing emulsion was prepared. Briefly, the type II collagen (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 0.1 mol/L glacial acetic acid to prepare a 2 mg/mL type II collagen solution, and then mixed with an equal volume of Freund’s complete adjuvant (Sigma-Aldrich, St. Louis, MO, USA) to prepare an emulsion. The concentration of type II collagen in the emulsion was 1 mg/mL. Then, three inoculation sites were chosen on the central axis of the back of rats in the model group, and 100 μL immunological emulsion was inoculated into each inoculation site. Meanwhile, 200 μL emulsion was injected into the root of the rat tail. After 7 days, the same dose of emulsion was inoculated at the same site to enhance the immunization. Fourteen days later, the CIA rat model with red limbs was successfully constructed.
Grouping and treatment
Successfully modeled CIA rats were randomly subdivided into a model group, an MTX group (1.5 mg/kg), a PTX low-dose group (1.5 mg/kg), a PTX medium-dose group (2.5 mg/kg), a PTX high-dose group (3.5 mg/kg), and an MTX + PTX group (MTX 1 mg/kg, PTX 3.5 mg/kg), with 10 rats in each group. The rats in the blank group and the CIA model group were routinely raised. Both MTX (M111239–500 mg, Aladdin Chemistry Co. Ltd., Shanghai, China) and PTX (P106868–10 mg, Aladdin Chemistry Co. Ltd., Shanghai, China) were administered intraperitoneally from day 1 (once a week for MTX, once every other day for PTX). The administration of the drug was terminated after 4 weeks of continuous administration in each of the above groups.
Enzyme-linked immunosorbent assay
Blood samples were taken from the tail vein of the rats in each group and centrifuged at 1500 r/min for 10 min at 4 °C. The concentrations of MMP‑3, IL‑12, IL‑6, IL‑1β, and TNFα in serum were determined using an ELISA kit according to the manufacturer’s instructions (Beijing Chenglin Biotechnology, Co. Ltd., Beijing, China).
Hematoxylin and eosin staining
Rats were sacrificed after being anesthetized with sodium pentobarbital (60 mg/kg) until muscular flaccidity. Complete ankle joint of the right hind limb was collected. Paraffin-embedded tissues were cut into 4‑μm sections, then deparaffinized in xylene and rehydrated with gradient ethanol. Then sections were stained with hematoxylin and eosin (Beyotime, Beijing, China). Results were observed under a light microscope.
Immunohistochemical staining
The freshly obtained tissues were fixed with 4% paraformaldehyde solution and embedded in a copper mold. Then tissues were sliced and routinely dewaxed into water for immunohistochemical analysis. Five fields with the strongest positive expression were selected from each slide, and the ratio of IL‑6- and IL‑1β-positive cells was calculated by a true color multi-functional cell image analysis management system (Image-Pro Plus software, Media Cybernetics, Silver Spring, Rockville, MD, USA).
Western blot
Synovial tissues of the symptomatic left hind limb joint were placed in lysis buffer (Beyotime, Shanghai, China) to collect supernatant. Proteins were measured by a BCA protein quantification kit (Thermo Scientific, Shanghai, China). Protein samples were separated by SDS polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA, USA). The membrane was blocked with 5% skimmed milk for 1 h at room temperature, and incubated with primary antibodies (TLR4, 1: 200; P‑NFκBp65, 1: 200, Abcam, Cambridge, UK) at 4 °C overnight. After three washes in PBST, the membrane was incubated with the secondary antibody at room temperature for 90 min. Finally, immunoreactive bands were detected by the ECL kit (Zsbio, Ltd., Beijing, China). It was scanned by a UVP analyzer (Thermo Scientific, Shanghai, China) with β‑actin as an internal control to determine the relative expression of the target protein.
TdT-mediated dUTP nick-end labeling (TUNEL) assay
Cultured synovial tissue cells were dewaxed by xylene, followed by rehydration in graded alcohols. Then cells were incubated with proteinase K working solution at 37 °C for 20 min. After washing with PBS three times, TUNEL mixture solution (11684817910, Roche Ltd., Lewes, East Sussex, U.K.) was added in each group and the slides were incubated at 37 °C for 60 min in the dark. Notably, the blank group was only added 50 μL luciferin-labeled dUTP solution, and experimental groups were added 50 μL TdT +450 μL luciferin-labeled dUTP solution. Converter-POD 50 μL was added for incubation in dark at 37 °C for 30 min. Subsequently, the slides were washed three times, stained with hematoxylin, and sealed with neutral gum. Three samples were selected from each group, and the images were observed under a fluorescence microscope (Olympus, Tokyo, Japan) to calculate the apoptosis rate. Apoptosis rate = number of positive cells/total number of cells, and the average value was taken to represent the apoptosis rate of each sample.
Statistical analysis
All data were analyzed by SPSS 19.0 statistical software (IBM Corp., Armonk, NY, USA), and the measurement information was described by x ± stanard deviation. Statistical analysis was performed by one-way analysis of variance (ANOVA) and post-hoc test by the Dunnett method. The difference was considered statistically significant at P < 0.05.
Results
Ankle histopathological changes
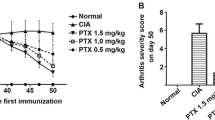
To compare the therapeutic effects of PTX and MTX on RA, CIA rats were treated with PTX alone or in combination with MTX. The pathological condition of the right ankle joint of CIA rats was detected by H&E staining. Compared with the blank group, the typical CIA model group exhibited an incomplete synovial membrane infiltrated with numerous inflammatory cells, as well as tissue edema, structural disorder, increased vascular dilatation, and partial vascular congestion. In contrast, the histopathological damage of the five treatment groups was significantly improved (Fig. 1). Among them, PTX 3.5 mg/kg showed a relatively intact synovial structure, occasional inflammatory cell infiltration, no edema or congestion, and the number of blood vessels was at normal group level. These data indicated that both PTX and MTX improved histopathology of CIA, PTX 3.5 mg/kg having the most significant effect.
PTX and MTX induced cell apoptosis
TUNEL assay was performed to determine the apoptotic effect of PTX and MTX either alone or in combination on synovial tissue cells. Cell apoptosis of each experimental group was markedly lower than that of the control group. In addition, the apoptosis rate of synovial tissue cells in the PTX group was more than that of the PTX and MTX combined group, with no statistical difference (Fig. 2).
Paclitaxel (PTX) and methotrexate (MTX) induced cell apoptosis. TdT mediated-dUTP Nick-End Labeling (TUNEL) assay was performed to determine the apoptotic effect of PTX and MTX either alone or in combination on synovial tissue cells (magnification ×200; scale bar: 100 μm). **P < 0.01, ***P < 0.001 compared with blank group
PTX and MTX downregulated IL‑6 and IL‑1β expression
There were almost no positive cells in the blank group, while the rate of IL‑6- and IL‑1β-positive cells in the CIA model group was 65% and 59%, respectively, which were apparently higher than that in the blank group (Figs. 3 and 4). Compared with the model group, the number of IL‑6- and IL‑1β-positive cells in the MTX 1 mg/kg, PTX 1 mg/kg, PTX 1.5 mg/kg, PTX 3.5 mg/kg, and MTX + PTX groups were all decreased to varying degrees, accompanied by weakened positive signal intensity (42%, 58%, 51%, 23%, and 47% for IL‑6; 32%, 48%, 39%, 14%, 35% for IL‑1β, respectively), with the lowest in the PTX 3.5 mg/kg group. These findings indicated that PTX and MTX could reduce the expression rate of IL‑6- and IL‑1β-positive cells.
Paclitaxel (PTX) and methotrexate (MTX) reduced the rate of IL‑6-positive cells. IL‑6-positive cell rate in collagen-induced arthritis (CIA) rats was detected by immunohistochemistry (magnification ×400; scale bar: 50 μm). Immunohistochemistry quantification of IL‑6-positive cells. **P < 0.01 compared with blank group; #P < 0.05, ##P < 0.01 compared with model group
Paclitaxel (PTX) and methotrexate (MTX) lowered the rate of IL‑1β-positive cells. The rate of IL‑1β-positive cells in collagen-induced arthritis (CIA) rats was examined by immunohistochemistry (magnification ×400; scale bar: 50 μm). Immunohistochemistry quantification of IL‑1β-positive cells. ***P < 0.001 compared with blank group; #P < 0.05, ###P < 0.001 compared with model group
PTX and MTX inhibited the expression of TLR4 and p‑NF-κBp65 proteins
Western blot was conducted to confirm the impact of PTX and MTX on TLR4 and p‑NF-κBp65 protein expression. As shown in Fig. 5, the expression levels of TLR4 and p‑NF-κBp65 proteins in the CIA model group were significantly higher than those of the blank group (P < 0.001). Compared with the model group, TLR4 and p‑NF-κBp65 protein expression in the treatment groups was downregulated to different degrees, with the most significant effect in the PTX 3.5 mg/kg group (P < 0.001).
Paclitaxel (PTX) and methotrexate (MTX) inhibited TLR4 and phosphorylated (p)-κBp65 protein expression. a The expression levels of TLR4 and p‑NF-κBp65 proteins in the synovial tissue of left hind limb joints of collagen-induced arthritis (CIA) rats were detected by western blot. Quantifications of (b) TLR4 and (c) p‑NF-κBp65 protein expression. ***P < 0.001 compared with blank group; #P < 0.05, ##P < 0.01, ###P < 0.001 compared with model group
TLR4 and p‑NF-κBp65 reduced the concentration of MMP-3, IL‑12, IL‑6, IL1-β, and TNFα
To further examine the influence of TLR4 and p‑NF-κBp65 on downstream inflammatory factors, the concentrations of MMP‑3, IL‑12, IL‑6, IL‑β, and TNF‑α were detected by ELISA. In line with the results, the MMP‑3, IL‑12, IL‑6, IL‑1β, and TNFα concentrations in the model group were significantly higher than those in the blank group (P < 0.001; Fig. 6). Compared with the model group, the concentrations of MMP‑3, IL‑12, IL‑6, IL‑β, and TNFα in the treatment groups were decreased to diverse degrees, with PTX 3.5 mg/kg showing the most obvious inhibitory effect.
TLR4 and phosphorylated (p)-NF-κBp65 reduced the concentrations of MMP‑3, IL‑12, IL‑6, IL1‑β, and TNFα. Detection of (a) MMP‑3, (b) IL‑12, (c) IL‑6, (d) IL‑1β, and (e) TNFα concentrations in serum of collagen-induced arthritis (CIA) rat tail vein by enzyme-linked immunosorbent assay (ELISA). Paclitaxel (PTX), methotrexate (MTX) ***P < 0.001 compared with blank group; #P < 0.05, ##P < 0.01, ###P < 0.001 compared with model group
Discussion
In this study, we compared the therapeutic effects of PTX alone or in combination with MTX on RA and found that the combination of PTX and MTX was less potent than PTX 3.5 mg/kg alone. Moreover, PTX and MTX could lower the concentrations of MMP‑3, IL‑12, IL‑6, IL‑β, and TNF‑α by inhibiting TLR4 and p‑NF-κBp65 protein expression, thereby achieving the purpose of treating RA.
Currently, the major therapeutic drugs for RA are non-steroidal anti-inflammatory drugs, anti-rheumatic drugs, glucocorticoids, and biologics, which can alleviate the disease, but cannot prevent development of the disease and are accompanied by toxicity [17]. As an internationally recognized arthritis model with many similar immunological and pathological features to human RNA, CIA has become an ideal model to study the pathogenesis of RA and to screen therapeutic drugs [18]. Hence, a CIA rat model was established in this study to verify the therapeutic efficacy and related mechanisms of PTX and MTX on RA.
Previous studies have revealed the anti-cancer function of PTX in various tumors including breast and ovarian cancers. Yoshifumi et al. also found that intraperitoneal injection of PTX (6, 12, 24, and 48 mg/kg) suppressed cell proliferation and exhibited cytotoxicity toward PC-3 cells in a time-dependent manner. Among them, PTX 12 mg/kg did not result in severe leukopenia or liver damage, but still had similar anti-cancer efficacy to high-dose PTX. For cancer patients undergoing chemotherapy, PTX 135–175 mg/m2 is recommended [19]. With the exception of anti-tumor activity, recent research has identified the therapeutic impact of low-dose PTX on non-cancer human diseases such as renal and liver fibrosis and skin disorders [20,21,22]. Xu et al. suggested that PTX (1.0 and 1.5 mg/kg) could lower arthritis scores and alleviate CIA in a dose-dependent manner [23]. Besides, prophylaxis with PTX before CIA onset can completely prevent CIA, and PTX treatment after CIA onset can relieve clinical symptoms, including redness and fever. Tony et al. believed that low-dose MTX could improve the main symptoms, signs, and inflammatory exudation of RA patients [24]. These findings suggested that PTX and MTX play therapeutic roles in RA treatment. Interestingly, a study conducted by Zhao confirmed the superior therapeutic effect of PTX 2.5 mg/kg over MTX 1 mg/kg in the treatment of CIA rats [16]. However, the impact of PTX alone or in combination with MTX on RA remains unclear. Thus, different doses of PTX alone or in combination with MTX were adopted in CIA rats to compare their therapeutic effects on RA. As expected, our results demonstrated that both MTX and PTX improved the pathological features of RA and downregulated the expression levels of inflammatory factors IL‑6 and IL‑1β. Surprisingly, the effect after engagement of MTX and PTX combined therapy was weaker than that after PTX 3.5 mg/kg administration. This result might be related to the fact that MTX mainly acted on the S phase of the cell cycle and decreased G0/G1 to S phase transition in cells, while PTX mainly acted on the G2/M phase of the cell cycle [25, 26]; the combination of MTX and PTX inhibited the proliferation of inflammatory cells and excessively interfered with the mitosis of normal cells, resulting in drug-induced tissue damage. Taken together, the combination of PTX and MTX was less effective than intraperitoneal injection of PTX 3.5 mg/kg every other day, which was the most appropriate dose observed in this study.
The autoimmune response is closely associated with the pathogenesis of RA [4, 5]. It has been found that TLR4 is an important protein molecule involved in non-specific immunity and also a bridge connecting specific and non-specific immunity [27]. Intracellular signal transduction initiated by TLR4 can activate NF-κB, which is involved in the amplification of inflammatory responses by regulating the expression of inflammatory cytokines [28]. NF-κB activation is characterized by inhibition of IKBα phosphorylation and degradation. After activation, NF-κB enters nucleus and triggers the expression of specific genes and a variety of inflammatory cytokines [34]. Therefore, we speculated that the therapeutic function of PTX and MTX on RA by may be related to the expression of TLR4 and p‑NF-κBp65. To verify this idea, western blot was performed to evaluate TLR4 and p‑NF-κBp65 expression in synovial tissues of the left hind limb joints of CIA rats. Results showed that PTX and MTX dramatically inhibited TLR4 and p‑NF-κBp65 protein expression.
Numerous studies have shown that IL‑12, IL‑6, and IL1‑β can improve vascular permeability and promote angiogenesis of synovial tissues and the formation of chronic inflammation of the synovium [29, 30]. TNFα facilitates the secretion of inflammatory transmitters by synovial cells and stimulates the dilation of blood vessels, thereby enhancing the inflammatory response [31]. MMP‑3 is a major mediator involved in arthritis, contributing to the formation of fibroblasts and participation in the activation of glial proteases, leading to inflammation [32]. Zhou et al. found that inhibition of IL‑1β and TNFα expression could achieve the purpose of anti-articular inflammation, indicating that inflammatory factors are indispensable in the development of RA [33]. In this study, PTX and MTX downregulated the expression of the inflammatory factors MMP‑3, IL‑12, IL‑6, IL‑1β, and TNFα in serum. Luo et al. [34] put forward that TRL4 in peripheral blood of patients with pneumonia was positively correlated with downstream inflammatory cytokines, such as TNFα, IL‑1, IL‑6, and IL‑10. Similarly, our results showed that inhibition of TLR4 and p‑NF-κBp65 proteins expression reduced MMP‑3, IL‑12, IL‑6, IL1‑β, and TNFα levels, indicating that TLR4 and p‑NF-κBp65 expression was positively correlated with the expression of inflammatory factors in serum.
A main limitation of this study was the lack of parameters such as joint infiltration, edematous tissue, structure disorder, and quality of synovial membrane. Further research on these issues is required.
In summary, PTX and MTX have therapeutic effects on RA by downregulating TLR4 and p‑NF-κBp65 protein expression in synovial tissue of CIA model, thereby reducing the concentrations of inflammatory factors in serum. Moreover, PTX alone is superior to PTX in combination with MTX, and intraperitoneal injection of PTX 3.5 mg/kg every other day is the most optimal dose observed in this study.
References
Cleutjens F, Boonen A, van Onna MGB (2019) Geriatric syndromes in patients with rheumatoid arthritis: a literature overview. Clin Exp Rheumatol 37:496–501
Zhang YL, Ouyang GL (2009) Research advances of mechanism of sinomenine in treating rheumatoid arthritis. J Chin Integr Med 7:775–778
Heng HS, Lim M, Absoud M et al (2014) Outcome of children with acetylcholine receptor (AChR) antibody positive juvenile myasthenia gravis following thymectomy. Neuromuscul Disord 24:25–30
Ren ST, Yu-Sheng LI (2015) Clinical significance of th1/th2 imbalance for inflammatory lesions of rheumatoid arthritis. Label Immunoass Clin Med 9:864–866
Wang J (2014) Research progress of sinomenine to treat rheumatoid arthritis. China Med Her 11:161–163
Fan T, Zhong F, Liu R et al (2018) siRNA-mediated c‑Rel knockdown ameliorates collagen-induced arthritis in mice. Int Immunopharmacol 56:9–17
Roy A, Srivastava M, Saqib U et al (2016) Potenzial therapeutic targets for inflammation in toll-like receptor 4 (TLR4)-mediated signaling pathways. Int Immunopharmacol 40:79–89
Marie L, Guillaume C, Charlotte P et al (2015) Macrophage activation syndrome with acute hepatitis E during tocilizumab treatment for rheumatoid arthritis. Joint Bone Spine 82:278–279
Yao RBR, Cai HC (2006) Research progress of the correlation between nuclear factor-κappa B and rheumatoid arthritis. J Med Postgrad 19:267–270
Khan MA, Ahmed RS, Chandra N et al (2019) In vivo, extract from withania somnifera root ameliorates arthritis via regulation of key immune mediators of inflammation in experimental model of arthritis. Antiinflamm Antiallergy Agents Med Chem 18:55–70
Castañeda S, Loza E, Daudén E, Carmona L (2016) Consensus statement on the management of comorbidity in patients with rheumatoid arthritis and psoriasis. J Rheumatol 43:990
Gao JS, Xie X, Hong WU et al (2006) Methotrexate combined with lefluomide for a 15-month treatment of severe rheumatoid arthritis. Chin J New Drugs Clin Rem 25:766–769
Ye LI (2007) Clinical observation on methotrexate combined with tripterygium wilfordii polyglycoside in treating rheumatoid arthritis. Mod Med Health 21:895–6
Zhao Y, Mu X, Du G (2016) Microtubule-stabilizing agents: new drug discovery and cancer therapy. Pharmacol Ther 162:134–143
Leung JC, Cassimeris L (2019) Reorganization of paclitaxel-stabilized microtubule arrays at mitotic entry: roles of depolymerizing kinesins and severing proteins. Cancer Biol Ther 20:1337–1347
Zhao Y, Chang ZF, Li R et al (2016) Paclitaxel suppresses collagen-induced arthritis: a reevaluation. Am J Transl Res 8:5044
Zhou S, Zou H, Chen G, Huang G (2019) Synthesis and biological activities of chemical drugs for the treatment of rheumatoid arthritis. Top Curr Chem 377:28
Shen J, Shang Q, Tam LS (2016) Targeting inflammation in the prevention of cardiovascular disease in patients with inflammatory arthritis. Transl Res 167:138–151
Qi N, Li F, Li X et al (2016) Combination use of paclitaxel and avastin enhances treatment effect for the NSCLC patients with malignant pleural effusion. Medicine (Baltimore) 95:e5392
Mirzapoiazova T, Kolosova IA, Moreno L et al (2007) Suppression of endotoxin-induced inflammation by taxol. Eur Respir J 30:429–435
Sun L, Zhang D, Liu F et al (2011) Low-dose paclitaxel ameliorates fibrosis in the remnant kidney model by down-regulating miR-192. J Pathol 225:364–377
Liu X, Zhu S, Wang T et al (2005) Paclitaxel modulates TGFbeta signaling in scleroderma skin grafts in immunodeficient mice. PLoS Med 2:e354
Xu J, Feng Z, Chen S et al (2019) Taxol alleviates collagen-induced arthritis in mice by inhibiting the formation of microvessels. Clin Rheumatol 38:19–27
Tony HP, Roll P, Mei HE et al (2015) Combination of B cell biomarkers as independent predictors of response in patients with rheumatoid arthritis treated with rituximab. Clin Exp Rheumatol 33:887
Liu QH, Teng Y, Wang H et al (2004) Effects of methotrexate(MTX)on cell proliferation and cell cycling of synovial fibroblasts in patients with rheumatoid arthritis. China J Mod Med 14:8–10
Guo H, Zhang X, Dong H et al (2017) Effect of LBP combined with paclitaxel on proliferation and apoptosis of human ovarian cancer cell HO-8910PM. J Ningxia Med Univ 39:631–635
Ya-Ying MI, Yang LL, Sun LP (2013) Expression and significance of HMGB1 and TLR2/TLR4 in rheumatoid arthritis. Basic Clin Med 33:476–479
Zhixian T, Zongren Z, Ziyou L et al (2017) A vitro experiment study of role of TLR4/NF-κB signal pathway in pathogenesis of brain injury during deep hypothermia circulatory arrest. J Pract Med 33:3344–3347
Clavel G, Bessis N, Lemeiter D et al (2007) Angiogenesis markers (VEGF, soluble receptor of VEGF and angiopoietin-1) in very early arthritis and their association with inflammation and joint destruction. Clin Immunol 124:158–164
Vuolteenaho K, Moilanen T, Hämäläinen M, Moilanen E (2003) Regulation of nitric oxide production in osteoarthritic and rheumatoid cartilageRole of endogenous IL‑1 inhibitors. Acta Rheumatol Scand 32:19–24
Yu-Bin W, An-Min C, Feng-Jin G, Yu-Jun X (2007) Expression and significance of TNF‑α,IL‑6,TGF‑β and IL‑9 in osteoarthritis. Shandong Med J 47:11–12
Wei X, Wang D, Yong C, Fang W (2009) The correlation between SERUM MMP‑3 AND IL‑6,TNF‑Α,HS-CRP on early osteoarthritis. J Qinghai Med Coll 30:183–185
Zhou W, Zhang Y, Shen WG et al (2009) Effect of thalidomide combined with methotrexate on the inflammation and cytokines of rats with collagen type II induced arthritis. Chin J Clin Pharmacol Ther 14:167–170
Luo J, Li Q, Li L (2017) Toll- like receptor 4 and downstream mediators of inflammation detection in the clinical application of hospital acquired pneumonia. J Med Res 46:138–141
Acknowledgements
This study was supported by Science and Technology Project of Liuzhou Guangxi Province (no. 2015J030522).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Z. Sheng, J. Zeng, W. Huang, L. Li, B. Li, C. Lv, and F. Yan declare that they have no competing interests.
All animal experiments were conducted in accordance with international ethical guidelines and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Additional information
Redaktion
U. Müller-Ladner, Bad Nauheim
U. Lange, Bad Nauheim
Rights and permissions
About this article
Cite this article
Sheng, Z., Zeng, J., Huang, W. et al. Comparison of therapeutic efficacy and mechanism of paclitaxel alone or in combination with methotrexate in a collagen-induced arthritis rat model. Z Rheumatol 81, 164–173 (2022). https://doi.org/10.1007/s00393-020-00940-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00393-020-00940-x