Abstract
Exosomes, 60–90-nm-sized vesicles, are produced by a large number of cell types, including tumor cells, neurons, astrocytes, hemocytes, intestinal epithelial cells, and so on. Dendritic cell (DC), the most potent professional antigen-presenting cell in the immune system, produces exosomes in the course of maturation. Mature DCs produce exosomes with the ability to elicit potent immunoactivation, resulting in tumor eradication and bacterial or virus elimination. Given the notion that exosomes are stable and easy to be modified artificially, autologous mature DC-derived exosomes have been vaccinated into patients with malignant diseases. In clinical trials utilizing exosomes as therapeutic approaches, researchers observed considerable curative effect with little side effect. However, immature or suppressive DC-derived exosomes harbor anti-inflammatory properties distinct from mature DC-derived exosomes. In murine models of autoimmune disease and transplantation, immature DC-derived exosomes reduced T cell-dependent immunoactivation, relieved clinical manifestation of autoimmune disease, and prolonged survival time of transplantation. Although the exact mechanism of how immature DC-derived exosomes function in vivo is still unclear, and there are no clinical trials regarding application of exosome vaccine into patients with autoimmune disease, we will analyze the promise of immature DC-derived exosomes as a subcellular vaccine in autoimmunity in this review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Dendritic cell (DC) is the most potent professional antigen-presenting cell (APC) in the immune system. DCs are derived from bone marrow cells and differentiate into several subsets located in different peripheral tissues, such as the skin, intestine, spleen, lymph nodes, and liver [1–5]. Under different maturation states, DCs function inversely in the immune system, that is, immune stimulation and immune suppression [6, 7]. Mature DCs express high levels of major histocompatibility complex class II (MHC II) and costimulatory molecules, which elicit immune stimulation by inducing naive T cells to transform into T helper (Th)1 and/or Th17 subcytes [8, 9], while immature DCs harbor low levels of MHC II and costimulatory molecules, which reduce immune activation by directing T cells to transform into Th2 and Treg cells or causing T cell apoptosis [10, 11]. The state of DC maturation is in charge of the direction of immune responses. Hence, many groups attempt to eradicate immune disorders by regulating DC maturation state. Mature DCs pulsed with relevant antigen have been applied for treatment of tumor and pathogen infectious diseases from animal models to clinical trials [12–17]. Moreover, immature DCs and DCs modified to be suppressive have been investigated in suppressing onset and progression of murine models of autoimmune disease and transplant rejection, which revealed certain curative effect [18–21]. However, due to the unstable characteristics of DC in vitro and in vivo, DC vaccine is not completely satisfactory as therapeutic vehicle for autoimmunity.
The discovery of exosomes has brought new advances in therapeutic approaches for autoimmunity. Exosomes have been extensively studied in recent years. Exosomes are 60–90 nm in size, cup-shaped vesicles produced virtually by all live cells, including tumor cells, neurons, astrocytes, hemocytes, intestinal epithelial cells, and so on [22–26]. DCs produce exosomes in the course of maturation. When not encountering adequate inflammatory stimuli, DCs remain immature with abundant MHC II-positive lysosomes inside the cells, where MHC II molecules are destined to degrade [27]. The immature DCs are much more effective in internalization of antigen than presentation of MHC II-antigen complexes on the plasma membrane. When encountering adequate inflammatory stimuli, such as lipopolysaccharide stimulation, DCs become mature and express major histocompatibility complex (MHC)-peptide complexes and costimulatory molecules in the plasma membrane, migrating to T cell areas of lymphoid organs where DCs provide the first and second signals for T cell expansion and differentiation [28]. In an alternative way, especially during interacting with cognate T cells, DCs generate multivesicular bodies with MHC-peptide complexes and CD9 carrying luminal vesicles, which release exosomes by fusion with the plasma membrane [29, 30].
In recent years, DC-derived exosomes have gained much attention in immunological researches because exosomes resemble the biology of cells that they are derived from [31]. As a large number of exosomes are produced during DC maturation, there are at least two phenotypes of DC-derived exosomes: mature DC-derived exosomes and immature DC-derived exosomes. In murine models, mature DC-derived exosomes elicit immune activation, resulting in tumor eradication and bacterial or virus elimination [32–34]. Given the fact that exosomes are stable during purification and will not go through phenotypical changes in vivo [35], autologous DC-derived exosomes were vaccinated into patients with malignant diseases in phase I and II clinical trials, which gained much curative effect with little side effect [36, 37]. On the other side, some experiments demonstrated that exosomes from immature or suppressive DCs exhibited the capacity to induce immune tolerance in murine models of transplantation and autoimmune disease [38–45]. The role of DC-derived exosomes in autoimmunity has begun to be unraveled; so in this review, we attempt to summarize the present knowledge of DC-derived exosomes in autoimmunity and analyze the superiority of applying immature or suppressive DC-derived exosomes in autoimmune disease.
MOLECULAR CHARACTERISTICS OF IMMATURE DC-DERIVED EXOSOMES
In order to unravel the molecular basis of exosome-induced immune tolerance in vivo, we searched the molecular composition of exosomes from immature or suppressive DCs of genetic modification in published literatures. We conclude that immature or suppressive DC-derived exosomes have three main molecular compositions: protein, lipid, and genetic composition.
Protein Composition
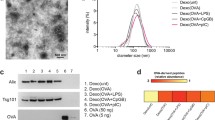
Numerous studies have evidenced that there are some proteins commonly found in exosomes. That is, (1) cytoskeleton and cytoskeleton-binding proteins (tubulin, actin, cofilin, profilin I, and elongation factor-1a) [46]; (2) membrane-associated proteins involved in intracellular transportation (annexins I, II, IV, V, and VII; small GTPase family members; or related proteins rab7, rab11, rap1B, and rab GDP dissociation inhibitor); (3) cytosolic proteins involved in signal transduction (Gi2a, syntenin, and 14-3-3) or protein translation (elongation factor-1a and elongation initiation factor-4A) [47, 48]; (4) Tsg101 and Alix, components of the conserved machinery which select ubiquinated cargo proteins for sorting to intraluminal vesicles [49].
In addition to commonly found proteins, immature dendritic cell-derived exosomes harbor unique proteins which are responsible for their specific functions including glycosylphosphatidylinositol-anchored proteins CD55 and CD59 (protection for immature DC-derived exosomes as they shield exosomes from attack of the complement system) [50]; MFG-E8/lactadherin, Mac-1α/β, CD9, and tetraspan protein family (CD63, CD81, and CD82) (cell targeting); syntenin Gi2α and 14-3-3 (signal transduction); galectin-3 (lectin binding); MHC II, MHC class I (MHC I), CD86, CD80, and CD40 (antigen presentation and T cell stimulation); hsc73 and hsp84 (antigen peptide binding); gag, annexins (I, II, IV, V, and VII), rabGD1, rap1B, and rab7 (membrane fusion); intercellular adhesion molecule-1 (ICAM-1)/CD54, CD11b, CD11c, and CD58 (adhesion) [51, 52]; Alix, TPx, 14-3-3, and galectin-3 (apoptosis); and Lamp 2 (a lysosomal marker protein) [53, 54]. Among these proteins, molecules for cell targeting (MFG-E8/lactadherin, Mac-1α/β, CD9, and tetraspan protein family), adhesion (intercellular adhesion molecule-1 (ICAM-1)/CD54, CD11b, CD11c, and CD58), and membrane fusion (gag; annexins I, II, IV, V, and VII; rabGD1; rap1B; and rab7) are indispensable candidates in the vaccine delivery system because these molecules can address exosomes to target cells in immune system. Molecules for antigen peptide binding (hsc73 and hsp84) and T cell stimulation (MHC II, MHC I, CD86, CD80, and CD40) are ingredients of the vaccine of immature DC-derived exosomes against autoimmune disease as they facilitate binding of specific peptides and influence of T cell action [55]. In murine models of autoimmune arthritis, MHC II (although with a low level) and FasL were required for the suppressive effect of exosomes from IL-4 and FasL genetically modified DCs [42, 43], suggesting that molecules involved in antigen presentation or apoptosis pathway might be required for exosome-induced immune tolerance.
Immature and mature DC-derived exosomes share most categories of proteins. The major reason for their distinguished functions lies in the contents of proteins involved in immune regulation. For example, immature DC-derived exosomes bear low levels of MHC class II, CD86, and ICAM-1 and undetected level of CD40 and CD80 in the membrane, which account for the suppressive function of those exosomes in immune responses [56], while mature DC-derived exosomes bear higher levels of MHC class II and costimulatory molecules which facilitate strong T cell stimulation. Besides, immature DC-derived exosomes contain much higher MFG-E8 than mature DC-derived exosomes [57, 58], which implies that immature DC-derived exosomes are much more efficient in cell targeting than mature DC-derived exosomes (Fig. 1).
Representative model for comparison of protein characteristic between immature and mature DC-derived exosomes. The left part of the graph represents immature DC-derived exosomes with low levels of MHC II, CD80, CD86, CD40, and ICAM-1 as well as high level of MFG-E8. The right part of the graph is a representative of mature DC-derived exosomes with high levels of MHC II, CD80, CD86, CD40, and ICAM-1 as well as low level of MFG-E8. The middle part of the graph is a representative of the common protein composition—which is the same in category and contents—between immature and mature DC-derived exosomes.
Lipid Composition
As mentioned above, proteins are pivotal components for biology and function of exosomes, while the role of lipid in exosomes remains unclear. Studies suggest that lipids are the skeleton component of exosomal membrane. If the integrity of membrane is disrupted, the suppressive effects of immature DC-derived exosomes also disappear [32]. Exosomes have a specific phospholipid composition which is different from the plasma membrane of DCs. The proportion of sphingomyelin in exosomes is twice as high as that in DCs, whereas phosphatidylcholine in exosomes is much lower [59, 60]. In addition, the molar ratio of diglycerides/phospholipids in exosomes from immature DCs is decreased to half of the original value. Laulagnier et al. [60] demonstrated that phospholipase D2 was enriched in immature DC-derived exosomes and suggested that phospholipid D carried by those exosomes could be involved in putative signaling transduction.
Genetic Composition
MicroRNA is a noncoding single-strand RNA of approximately 22 nucleotides in length that regulates gene expression by direct degradation of the targeted mRNAs or posttranscriptional silence of protein translation [61]. MicroRNAs play key roles in cell biology and function [62]. Recent evidence has pointed out that exosomes, as well as some lipid based vesicles, carry profiles of microRNA inside the vesicles which participate in cell–cell communication [63, 64]. MicroRNAs are believed to be encapsulated inside exosomes for indirect evidence that exosomal microRNAs are resistant to digestion of high ribonuclease activity in body fluids, and exosomal microRNAs are stable even after proteinase K treatment [65]. The transfer of microRNAs by exosomes is functional, supported by evidence that exosomal microRNAs regulate gene expression and activity of the recipient cells [64–67]. This new way of genetic material dissemination has renewed our understanding on how genetic material spread between cells and organism, which might have diagnostic potential in a variety of diseases [68, 69].
DCs under different maturation states secrete exosomes with distinct sets of microRNAs [65]. Bone marrow immature DC-derived exosomes encapsulate 144 microRNAs, among which 139 microRNAs are shared by bone marrow mature DC-derived exosomes [65]. The exact role for microRNAs in exosomes remains unclear; it is thought that exosomes act as vehicles for transporting microRNAs among immune cells, which regulate mRNA and protein expression in recipient cells [64–66]. Although there is no confirmative evidence about whether exosomal carriers of microRNAs affect cells in the microenvironment or at a distance, the mechanism of microRNA transfer in exosomes enlightens and guides us to modify exosomes not just in the contents of protein but also in the category and quantity of specific microRNAs, through which method a new therapeutic application might arise.
SUPPRESSIVE FUNCTION OF IMMATURE DC-DERIVED EXOSOMES
Both in vivo and in vitro mechanisms for suppressive action of immature DC-derived exosomes have been investigated in animal models of autoimmune disease and transplantation. Until now, researchers have generated immature bone marrow DCs and suppressive DCs either by genetic modification or cytokine treatment [40, 42–45, 70]. In murine models of delayed type hypersensitivity (DTH), exosomes from immature or suppressive DCs exhibited regulatory effects on reduction of inflammation in joints and alleviation of animal suffering of autoimmune arthritis. The explored mechanism ranges from the phenotype and action of exosomes from DCs and the in vivo trafficking and interaction between exosomes and immune cells.
In Vitro Assay
DC-derived exosomes are internalized and processed by other DCs [71]. During this process, immature DC-derived exosomes yield their luminal contents into the recipient cells to render them suppressive. The suppressive efficacy of exosomes depends to a major extent on the state of their parental cells. Until now, unmodified immature DCs or DCs modified with IL-10, FasL, IL-4, indoleamine 2,3-dioxygenase (IDO), CTLA-4, and TGF-β have been used as the parental DCs for generation of suppressive exosomes for the purpose of correcting aberrant immunoactivation.
The in vitro function of bone marrow DC-derived exosomes was classically tested in mixed lymphocyte response (MLR). Exosomes from unmodified immature DCs harbored partial anti-inflammatory properties, and exosomes from IL-10-modified immature DCs showed a greater suppressive effect of T cell proliferation than exosomes from unmodified DCs, but lesser suppressive effect than their parental DCs [43]. Keeping in view that the coculture system of DCs and T cells could not avoid the presence of DC-derived exosomes, this result indicates that exosomes could suppress T cells directly or influence T cells indirectly via regulation of DCs. The suppressive effect of exosomes is required for intact exosomal membrane, which is supported by the evidence that, after cycles of freeze-thaw or sonication treatment, the intact membrane of exosomes is disrupted, and the suppressive effect disappears too [43]. Using flow cytometry and western blot, researchers investigated the molecular expression of suppressive exosomes from DCs. CD80, CD86, CD11c, and MHC II molecules were found in exosomes from immature and suppressive DCs regardless of how DCs were prepared. Unexpectedly, exosomes from FasL-modified DCs and IL-4-modified DCs both showed the existence of FasL in their membrane. This implies that Fas-FasL pathway might participate in T cell suppression mediated by exosomes from Th2 cytokine and proapoptotic molecule-modified DCs. Later, the same group supplemented that the suppressive effect of exosomes was MHC II dependent, FasL partial dependent, and antigen specific. More recently, it was found that exosomes from transforming growth factor beta 1 (TGF-β1)-modified DC downregulated Th17 responses and induced CD4+FOXP3+Treg cells in vitro [45]. In the rat model of intestinal transplantation, immature DC-derived exosomes reduced the level of IFN-γ and increased the level of IL-10 secretion by T cells in the presence of fresh DCs in an MLR experiment [44].
In Vivo Assay
Theoretically, exosomes exert their suppressive effect by fusing and internalizing with immune cells. Thus, it is important to know the circulation route and cell types that exosomes interact with in vivo. Seon et al. demonstrated that exosomes from IL-4-treated bone marrow DCs were associated with CD11c+ cells in the dermis after intradermal injection. Moreover, CD11c+ cells with exosomes inclusion were detected in the draining lymph node of the treated side, but not the untreated side. It is hard to explain the clinical findings that intradermal injection of exosomes not only reduced paw swelling in the injected side but also in the contralateral side [40]. In addition, there was no detection of exosomes in the liver and spleen, either [40]. When exosomes were injected systemically, they were found internalized by F4/80+ splenic macrophages and F4/80− cells in the spleen, hepatic F4/80+ Kupffer cells and a few CD11c+ DCs in liver [40] (Fig. 2). In order to find the functional target for exosome action, an adoptive trafficking experiment was carried out. Results showed that it was the CD11c+ cells transfer rather than CD3+ cells transfer that inhibited the DTH responses [40]. Taken together, exosomes are internalized by APCs in the draining lymph nodes and spleen, thus conveying tolerant signals to APCs, which take part in regulation of immune responses.
Schematic model for immature DC-derived exosomes trafficking in vivo after intradermal and intravenous injection. After intradermal injection, exosomes were first associated with CD11c+ cells in the dermis. Several hours later, exosomes and CD11c+ cells complexes were found in the draining lymph nodes of injected site, but not the contralateral side. Surprisingly, no exosomes were detected in the spleen and liver. After intravenous injection, exosomes were circulating in the blood system and internalized by F4/80+ macrophages and F4/80− cells in the spleen, as well as F4/80+ Kupffer cells and a few CD11c + dendritic cells in the liver.
PROGRESS OF DC-DERIVED EXOSOME VACCINE IN VARIOUS DISEASES
The discovery of exosomes opened up a new way for us to produce a satisfactory vaccine. Taking into account the in vivo and in vitro findings, exosomes are considered to have the following characteristics: (1) exosomes are stable in vivo. They do not go through phenotypic changes in the presence of cytokines and immune cells [72]. (2) Exosomes are easy to preserve in vitro. They can be sterilized through microinfiltration. Even if exosomes are contaminated by microbes, they will not respond to produce anti-inflammatory molecules against contamination [73]. (3) The side effects of exosomes are tolerable. In clinical trials of nonsmall cell lung cancer (NSCLC), patients underwent the skin DTH test with exosome injection before the therapy trial of NSCLC [37]. Results showed that two out of nine patients had 5 mm of induration and erythema on their skin, and one patient had 6 mm of induration and erythema 48 h later. After the formal experiment, one patient had flu-like symptoms; eight patients had indurations, erythema, and swelling in the injection site, and one other patient had peripheral edema in the arm, which indicated that the side effects of exosome vaccine were less and tolerable. (4) Exosomes can be loaded with peptide indirectly by feeding DCs with antigens and then collecting the supernatant for isolation of exosomes [74]. Peptide loaded in this way guarantees its association with MHC molecules, which plays a pivotal role in the interaction between DCs and T cells. The limitation for this method lies in the type of antigen which must be recognized and processed by DCs. Another way to load protein onto exosomes is exosome display technology [75]. This technique allows for appending any soluble antigen onto exosomes. Therefore, it can be utilized to modify exosomes with additional functions, such as adding adhesion molecules for targeting exosomes to some certain locations and cells.
So far, exosomes from DCs have been applied in tumor immunotherapy. In a murine model of adenocarcinoma, vaccination of exosomes from DCs pulsed with tumor antigen primed cytotoxic T lymphocytes eradication and inhibition of established tumors [32]. In a murine model of melanoma, injection of exosomes from DCs pulsed with relevant tumor antigen increased IL-15Ra-dependent cell activation and NKG2D-dependent natural killer cell proliferation [76]. The addition of cyclophosphamide along with exosomes enhanced both CD4+ and CD8+ T cell activation [77]. Based on experimental results of animal models, two phase I clinical trials have been carried out in 15 melanoma [36, 76] and 13 NSCLC patients [37], respectively, using large-scale clinical grade DC-derived exosomes. Although detailed mechanisms have not been thoroughly elucidated, and long-term effects have not been observed, vaccine of DC-derived exosomes exhibited considerable curative effects and less side effects compared with DC vaccine.
Application of immature DC-derived exosomes for regulation of immune disorders has been studied extensively in several models of autoimmune disease and transplantation. Under expectation, injection of immature DC-derived exosomes prolonged survival time of cardiac and intestinal transplantation [41, 44]. Vaccination of exosomes from IL-4, IL-10, FasL, and indoleamine 2,3-dioxygenase-modified DC also reduced the clinical manifestation of mice with rheumatoid arthritis [40, 42, 43, 70]. In vivo administration of exosomes from TGF-β1-modified DCs reduced disease activity and incidence of intestinal bleeding in the murine model of inflammatory bowel disease (IBD) [45]. Thus far, no clinical trial regarding the therapeutic efficacy of immature or suppressive DC-derived exosomes was conducted in autoimmune disease.
PERSPECTIVES
Doctors and researchers all over the world have been struggling for decades to find a better way for treatment and prevention of autoimmune disease. The optimal way for treatment of autoimmune disease is to blunt excessive antigen-specific immunoactivation without interfering with normal immune responses. The discovery of exosomes, especially after the application of DC-derived exosomes into tumor patients in clinical trials, enlightens us to apply exosomes in autoimmune disease. Until now, results from animal models of rheumatoid arthritis revealed that local (footpad or periarticular injection) and systemic (intravenous injection) administration exosomes of immature DC or immature DC modified to express IL-4/IL-10/FasL/IDO could reduce the incidence of rheumatoid arthritis as well as prevent the progression of joint destruction. In another animal model of autoimmune disease, IBD, administration of TGF-β1-modified immature DC-derived exosomes relieved the disease severity and reduced adverse clinical manifestations. No severe side effects have been observed. Besides, immature DC-derived exosomes could significantly prolong the survival time of transplant and cardiac transplantation. These observed results are encouraging. However, there is a long way to go before applying exosomes in patients of autoimmune disease. First, the exact mechanism for immune tolerance induced by immature DC-derived exosomes is still not clear. Second, for distinct autoimmune disease, the way to generate most efficient DCs for production of suppressive exosomes remains to be elucidated. In addition, the method of generating clinical grade purified exosomes with antigen specificity still needs to be improved. Third, the route (intravenous or intradermal) and dose for exosomes in autoimmune disease need further exploration. After solving basic and clinical problems, exosome vaccine may be applied in autoimmune diseases in the future.
References
Rossi, M., and J.W. Young. 2005. Human dendritic cells: potent antigen-presenting cells at the crossroads of innate and adaptive immunity. Journal of Immunology 175: 1373–1381.
Romani, N., B.E. Clausen, and P. Stoitzner. 2010. Langerhans cells and more: langerin-expressing dendritic cell subsets in the skin. Immunological Reviews 234: 120–141.
Thomson, A.W., D.A. Geller, C. Gandhi, N. Murase, A.J. Demetris, and D. Beer-Stolz. 2011. Hepatic antigen-presenting cells and regulation of liver transplant outcome. Immunologic Research 50: 2221–2227.
Segura, E., J. Valladeau-Guilemond, M.H. Donnadieu, X. Sastre-Garau, V. Soumelis, and S. Amigorena. 2012. Characterization of resident and migratory dendritic cells in human lymph nodes. Journal of Experimental Medicine 209: 653–660.
Tan, J.K., and H.C. O’Neill. 2007. Concise review: dendritic cell development in the context of the spleen microenvironment. Stem Cells 25: 2139–2145.
Zanoni, I., and F. Granucci. 2011. The regulatory role of dendritic cells in the induction and maintenance of T-cell tolerance. Autoimmunity 4: 23–32.
Morva, A., S. Lemoine, A. Achour, J.O. Pers, P. Youinou, and C. Jamin. 2012. Maturation and function of human dendritic cells are regulated by B lymphocytes. Blood 119: 106–114.
Prado, C., F. Contreras, H. González, P. Díaz, D. Elgueta, M. Barrientos, A.A. Herrada, Á. Lladser, S. Bernales, and R. Pacheco. 2012. Stimulation of dopamine receptor D5 expressed on dendritic cells potentiates Th17-mediated immunity. Journal of Immunology 188: 3062–3070.
Wang, Z., A. Sokolovska, R. Seymour, J.P. Sundberg, and H. Hogenesch. 2012. SHARPIN is essential for cytokine production, NF-κB signaling, and induction of Th1 differentiation by dendritic cells. PLoS One 7: e31809.
Yang, H., Y. Zhang, M. Wu, J. Li, W. Zhou, G. Li, X. Li, B. Xiao, and P. Christadoss. 2010. Suppression of ongoing experimental autoimmune myasthenia gravis by transfer of RelB-silenced bone marrow dendritic cells is associated with a change from a T helper Th17/Th1 to a Th2 and Foxp3+ regulatory T cell profile. Inflammation Research 59: 197–205.
Wakkach, A., N. Fournier, V. Brun, J.P. Breittmayer, F. Cottrez, and H. Groux. 2003. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity 18: 605–617.
Zhang, X., J.R. Gordon, and J. Xiang. 2002. Advances in dendritic cell-based vaccine of cancer. Cancer Biotherapy and Radiopharmaceuticals 17: 601–619.
Markowicz, S., Z.I. Nowecki, P. Rutkowski, A.W. Lipkowski, M. Biernacka, A. Jakubowska-Mucka, T. Switaj, A. Misicka, H. Skurzak, H. Polowniak-Pracka, and J. Walewski. 2012. Adjuvant vaccination with melanoma antigen-pulsed dendritic cells in stage III melanoma patients. Medical Oncology. doi:10.1007/s12032-012-0168-1.
Decker, W.K., and A. Safdar. 2010. Dendritic cell vaccines for the immunocompromised patient: prevention of influenza virus infection. Expert Review of Vaccines 9: 721–730.
Ren, W.N., C.K. Chang, H.H. Fan, F. Guo, Y.N. Ren, J. Yang, J. Guo, and X. Li. 2011. A combination of exosomes carrying TSA derived from HLA-A2-positive human white buffy coat and polyl: C for use as a subcellular antitumor vaccine. Journal of Immunoassay and Immunochemistry 32: 207–218.
Chaput, N., N.E. Schartz, F. André, J. Taïeb, S. Novault, P. Bonnaventure, N. Aubert, J. Bernard, F. Lemonnier, M. Merad, G. Adema, M. Adams, M. Ferrantini, A.F. Carpentier, B. Escudier, T. Tursz, E. Angevin, and L. Zitvogel. 2004. Exosomes as potent cell-free peptide-based vaccine. II. Exosomes in CpG adjuvants efficiently prime naive Tc1 lymphocytes leading to tumor rejection. Journal of Immunology 172: 2137–2146.
André, F., N. Chaput, N.E. Schartz, C. Flament, N. Aubert, J. Bernard, F. Lemonnier, G. Raposo, B. Escudier, D.H. Hsu, T. Tursz, S. Amigorena, E. Angevin, and L. Zitvogel. 2004. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. Journal of Immunology 172: 2126–2136.
Cools, N., V.F. Van Tendeloo, E.L. Smits, M. Lenjou, G. Nijs, D.R. Van Bockstaele, Z.N. Berneman, and P. Ponsaerts. 2008. Immunosuppression induced by immature dendritic cells is mediated by TGF-beta/IL-10 double-positive CD4+ regulatory T cells. Journal of Cellular and Molecular Medicine 12: 690–700.
Oh, K., Y.S. Kim, and D.S. Lee. 2011. Maturation-resistant dendritic cells ameliorate experimental autoimmune uveoretinitis. Immune Network 11: 399–405.
Sun, X., Z.J. Gong, Z.W. Wang, T. Li, J.Y. Zhang, H.C. Sun, S. Liu, L. Huang, C. Huang, and Z.H. Peng. 2012. IDO-competent-DCs induced by IFN-γ attenuate acute rejection in rat liver transplantation. Journal of Clinical Immunology 32: 837–847.
Ezzelarab, M., and A.W. Thomson. 2011. Tolerogenic dendritic cells and their role in transplantation. Seminars in Immunology 23: 252–263.
Keller, S., M.P. Sanderson, A. Stoeck, and P. Altevogt. 2006. Exosomes: from biogenesis and secretion to biological function. Immunology Letters 107: 102–108.
Yang, C., S.H. Kim, N.R. Bianco, and P.D. Robbins. 2011. Tumor-derived exosomes confer antigen-specific immunosuppression in a murine delayed-type hypersensitivity model. PLoS One 6: e22517.
Lachenal, G., K. Pernet-Gallay, M. Chivet, F.J. Hemming, A. Belly, G. Bodon, B. Blot, G. Haase, Y. Goldberg, and R. Sadoul. 2011. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Molecular and Cellular Neuroscience 46: 409–418.
Mallegol, J., G. van Niel, and M. Heyman. 2005. Phenotypic and functional characterization of intestinal epithelial exosomes. Blood Cells Molecules and Diseases 35: 11–16.
Guescini, M., S. Genedani, V. Stocchi, and L.F. Agnati. 2010. Astrocytes and glioblastoma cells release exosomes carrying mtDNA. Journal of Neural Transmission 117: 1–4.
Pierre, P., S.J. Turley, E. Gatti, M. Hull, J. Meltzer, A. Mirza, K. Inaba, R.M. Steinman, and I. Mellman. 1997. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature 388: 787–792.
Mellman, I., S.J. Turley, and R.M. Steinman. 1998. Antigen processing for amateurs and professionals. Trends in Cell Biology 8: 231–237.
Tamai, K., N. Tanaka, T. Nakano, E. Kakazu, Y. Kondo, J. Inoue, M. Shiina, K. Fukushima, T. Hoshino, K. Sano, Y. Ueno, T. Shimosegawa, and K. Sugamura. 2010. Exosome secretion of dendritic cells is regulated by Hrs, an ESCRT-0 protein. Biochemical and Biophysical Research Communications 399: 384–390.
Buschow, S.I., E.N. Nolte-'t Hoen, G. van Niel, M.S. Pols, T. ten Broeke, M. Lauwen, F. Ossendorp, C.J. Melief, G. Raposo, R. Wubbolts, M.H. Wauben, and W. Stoorvogel. 2009. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic 10: 1528–1542.
Johansson, S.M., C. Admyre, A. Scheynius, and S. Gabrielsson. 2008. Different types of in vitro generated human monocyte-derived dendritic cells release exosomes with distinct phenotypes. Immunology 123: 491–499.
Zitvogel, L., A. Regnault, A. Lozier, J. Wolfers, C. Flament, D. Tenza, P. Ricciardi-Castagnoli, G. Raposo, and S. Amigorena. 1998. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell derived exosomes. Nature Medicine 4: 594–600.
Aline, F., D. Bout, S. Amigorena, P. Roingeard, and I. Dimier-Poisson. 2004. Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against T. gondii infection. Infection and Immunity 72: 4127–4137.
Del Cacho, E., M. Gallego, S.H. Lee, H.S. Lillehoj, J. Quilez, E.P. Lillehoj, and C. Sánchez-Acedo. 2012. Induction of protective immunity against Eimeria tenella, Eimeria maxima, and Eimeria acervulina infections using dendritic cell-derived exosomes. Infection and Immunity 80: 1909–1916.
Luketic, L., J. Delanghe, P.T. Sobol, P. Yang, E. Frotten, K.L. Mossman, J. Gauldie, J. Bramson, and Y. Wan. 2007. Antigen presentation by exosomes released from peptide-pulsed dendritic cells is not suppressed by the presence of active CTL. Journal of Immunology 179: 5024–5032.
Escudier, B., T. Dorval, N. Chaput, F. André, M.P. Caby, S. Novault, C. Flament, C. Leboulaire, C. Borg, S. Amigorena, C. Boccaccio, C. Bonnerot, O. Dhellin, M. Movassagh, S. Piperno, C. Robert, V. Serra, N. Valente, J.B. Le Pecq, A. Spatz, O. Lantz, T. Tursz, E. Angevin, and L. Zitvogel. 2005. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. Journal of Translational Medicine 3: 10.
Morse, M.A., J. Garst, T. Osada, S. Khan, A. Hobeika, T.M. Clay, N. Valente, R. Shreeniwas, M.A. Sutton, A. Delcayre, D.H. Hsu, J.B. Le Pecq, and H.K. Lyerly. 2005. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. Journal of Translational Medicine 3: 9.
Pêche, H., M. Heslan, C. Usal, S. Amigorena, and M.C. Cuturi. 2003. Presentation of donor major histocompatibility complex antigens by bone marrow dendritic cell-derived exosomes modulates allograft rejection. Transplantation 76: 1503–1510.
Bianco, N.R., S.H. Kim, M.A. Ruffner, and P.D. Robbins. 2009. Therapeutic effect of exosomes from indoleamine 2,3-dioxygenase-positive dendritic cells in collagen-induced arthritis and delayed-type hypersensitivity disease models. Arthritis & Rheumatism 60: 380–389.
Kim, S.H., N.R. Bianco, W.J. Shufesky, A.E. Morelli, and P.D. Robbins. 2007. Effective treatment of inflammatory disease models with exosomes derived from dendritic cells genetically modified to express IL-4. Journal of Immunology 179: 2242–2249.
Pêche, H., K. Renaudin, G. Beriou, E. Merieau, S. Amigorena, and M.C. Cuturi. 2006. Induction of tolerance by exosomes and short-term immunosuppression in a fully MHC-mismatched rat cardiac allograft model. American Journal of Transplantation 6: 1541–1550.
Kim, S.H., N. Bianco, R. Menon, E.R. Lechman, W.J. Shufesky, A.E. Morelli, and P.D. Robbins. 2006. Exosomes derived from genetically modified DC expressing FasL are anti-inflammatory and immunosuppressive. Molecular Therapy 13: 289–300.
Kim, S.H., E.R. Lechman, N. Bianco, R. Menon, A. Keravala, J. Nash, Z. Mi, S.C. Watkins, A. Gambotto, and P.D. Robbins. 2005. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. Journal of Immunology 174: 6440–6448.
Yang, X., S. Meng, H. Jiang, C. Zhu, and W. Wu. 2011. Exosomes derived from immature bone marrow dendritic cells induce tolerogenicity of intestinal transplantation in rats. Journal of Surgical Research 171: 826–832.
Cai, Z., W. Zhang, F. Yang, L. Yu, Z. Yu, J. Pan, L. Wang, X. Cao, and J. Wang. 2012. Immunosuppressive exosomes from TGF-β1 gene-modified dendritic cells attenuate Th17-mediated inflammatory autoimmune disease by inducing regulatory T cells. Cell Research 22: 607–610.
Simpson, R.J., S.S. Jensen, and J.W. Lim. 2008. Proteomic profiling of exosomes: current perspectives. Proteomics 8: 4083–4099.
Simpson, R.J., J.W. Lim, R.L. Moritz, and S. Mathivanan. 2009. Exosomes: proteomic insights and diagnostic potential. Expert Review of Proteomics 6: 267–283.
Poliakov, A., M. Spilman, T. Dokland, C.L. Amling, and J.A. Mobley. 2009. Structural heterogeneity and protein composition of exosome-like vesicles (prostasomes) in human semen. The Prostate 69: 159–167.
Katzmann, D.J., G. Odorizzi, and S.D. Emr. 2002. Receptor downregulation and multivesicular-body sorting. Nature Reviews Molecular Cell Biology 3: 893–905.
Clayton, A., C.L. Harris, J. Court, M.D. Mason, and B.P. Morgan. 2003. Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59. European Journal of Immunology 33: 522–531.
Lamparski, H.G., A. Metha-Damani, J.Y. Yao, S. Patel, D.H. Hsu, C. Ruegg, and J.B. Le Pecq. 2002. Production and characterization of clinical grade exosomes derived from dendritic cells. Journal of Immunological Methods 270: 211–226.
Hammond, C., L.K. Denzin, M. Pan, J.M. Griffith, H.J. Geuze, and P. Cresswell. 1998. The tetraspan protein CD82 is a resident of MHC class II compartments where it associates with HLA-DR, -DM, and -DO molecules. Journal of Immunology 161: 3282–3291.
Théry, C., A. Regnault, J. Garin, J. Wolfers, L. Zitvogel, P. Ricciardi-Castagnoli, G. Raposo, and S. Amigorena. 1999. Molecular characterization of dendritic cell-derived exosomes: selective accumulation of the heat shock protein hsc73. The Journal of Cell Biology 147: 599–610.
Théry, C., M. Boussac, P. Véron, P. Ricciardi-Castagnoli, G. Raposo, J. Garin, and S. Amigorena. 2001. Proteomic analysis of dendritic cell-derived exosome: a secreted subcellular compartment distinct form apoptotic vesicles. Journal of Immunology 166: 7309–7318.
Théry, C., L. Duban, E. Segura, P. Véron, O. Lantz, and S. Amigorena. 2002. Indirect activation of naïve CD4+ T cells by dendritic cell-derived exosomes. Nature Immunology 3: 1156–1162.
Clayton, A., J. Court, H. Navabi, M. Adams, M.D. Mason, J.A. Hobot, G.R. Newman, and B. Jasani. 2001. Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. Journal of Immunological Methods 247: 163–174.
Hoen, E.N., E.J. Van der Vlist, M. Aalberts, H.C. Mertens, B.J. Bosch, W. Bartelink, E. Mastrobattista, E.V. van Gaal, W. Stoorvogel, G.J. Arkesteijn, and M.H. Wauben. 2012. Quantitative and qualitative flow cytometric analysis of nano-sized cell-derived membrane vesicles. Nanomedicine: Nanotechnology, Biology and Medicine 8: 712–720.
Segura, E., S. Amigorena, and C. Théry. 2005. Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses. Blood Cells, Molecules and Diseases 35: 89–93.
Subra, C., K. Laulagnier, B. Perret, and M. Record. 2007. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie 89: 205–212.
Laulagnier, K., C. Motta, S. Hamdi, S. Roy, F. Fauvelle, J.F. Pageaux, T. Kobayashi, J.P. Salles, B. Perret, C. Bonnerot, and M. Record. 2004. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem Journal 380: 161–171.
Bartel, D.P. 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297.
Turner, M.L., F.M. Schnorfeil, and T. Brocker. 2011. MicroRNAs regulate dendritic cell differentiation and function. Journal of Immunology 187: 3911–3917.
Kosaka, N., H. Iguchi, Y. Yoshioka, F. Takeshita, Y. Matsuki, and T. Ochiya. 2010. Secretory mechanisms and intercellular transfer of microRNAs in living cells. The Journal of Biology Chemistry 285: 17442–17452.
Vickers, K.C., and A.T. Remaley. 2012. Lipid-based carriers of microRNAs and intercellular communication. Current Opinion in Lipidology 23: 91–97.
Montecalvo, A., A.T. Larregina, W.J. Shufesky, D.B. Stolz, M.L. Sullivan, J.M. Karlsson, C.J. Baty, G.A. Gibson, G. Erdos, Z. Wang, J. Milosevic, O.A. Tkacheva, S.J. Divito, R. Jordan, J. Lyons-Weiler, S.C. Watkins, and A.E. Morelli. 2012. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood 119: 756–766.
Mittelbrunn, M., C. Gutiérrez-Vázquez, C. Villarroya-Beltri, S. González, F. Sánchez-Cabo, M.Á. González, A. Bernad, and F. Sánchez-Madrid. 2011. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nature Communications 2: 282.
Stoorvogel, W. 2012. Functional transfer of microRNA by exosomes. Blood 119: 646–648.
Ramachandran, S., and V. Palanisamy. 2012. Horizontal transfer of RNAs: exosomes as mediators of intercellular communication. Wiley Interdisciplinary Reviews: RNA 3: 286–293.
Chen, X., H. Liang, J. Zhang, K. Zen, and C.Y. Zhang. 2012. Horizontal transfer of microRNAs: molecular mechanisms and clinical applications. Protein & Cell 3: 28–37.
Szántó, S., T. Koreny, K. Mikecz, T.T. Glant, Z. Szekanecz, and J. Varga. 2007. Inhibition of indoleamine 2,3-dioxygenase-mediated tryptophan catabolism accelerates collagen-induced arthritis in mice. Arthritis Research & Therapy 9: R50.
Bastos-Amador, P., B. Pérez-Cabezas, N. Izquierdo-Useros, M.C. Puertas, J. Martinez-Picado, R. Pujol-Borrell, M. Naranjo-Gómez, and F.E. Borràs. 2012. Capture of cell-derived microvesicles (exosomes and apoptotic bodies) by human plasmacytoid dendritic cells. Journal of Leukocyte Biology 91: 751–758.
Wolfers, J., A. Lozier, G. Raposo, A. Regnault, C. Théry, C. Masurier, C. Flament, S. Pouzieux, F. Faure, T. Tursz, E. Angevin, S. Amigorena, and L. Zitvogel. 2001. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nature Medicine 7: 297–303.
Fleissner, F., Y. Goerzig, A. Haverich, and T. Thum. 2012. Microvesicles as novel biomarkers and therapeutic targets in transplantation medicine. American Journal of Transplantation 12: 289–297.
Qazi, K.R., U. Gehrmann, E. Domange Jordö, M.C. Karlsson, and S. Gabrielsson. 2009. Antigen-loaded exosomes alone induce Th1-type memory through a B cell-dependent mechanism. Blood 113: 2673–2683.
Delcayre, A., A. Estelles, J. Sperinde, T. Roulon, P. Paz, B. Aguilar, J. Villanueva, S. Khine, and J.B. Le Pecq. 2005. Exosome display technology: applications to the development of new diagnostics and therapeutics. Blood Cells, Molecules, and Diseases 35: 158–168.
Viaud, S., M. Terme, C. Flament, J. Taieb, F. André, S. Novault, B. Escudier, C. Robert, S. Caillat-Zucman, T. Tursz, L. Zitvogel, and N. Chaput. 2009. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha. PLoS One 4: e4942.
Taieb, J., N. Chaput, N. Schartz, S. Roux, S. Novault, C. Ménard, F. Ghiringhelli, M. Terme, A.F. Carpentier, G. Darrasse-Jèze, F. Lemonnier, and L. Zitvogel. 2006. Chemoimmunotherapy of tumors: cyclophosphamide synergizes with exosome based vaccines. Journal of Immunology 176: 2722–2729.
Declaration of Interest
The authors declare that there are no financial, consulting, or personal relationships with other people or organizations that could influence the authors’ work.
Author information
Authors and Affiliations
Corresponding author
Additional information
The content has not been published or submitted for publication elsewhere.
Rights and permissions
About this article
Cite this article
Yin, W., Ouyang, S., Li, Y. et al. Immature Dendritic Cell-Derived Exosomes: a Promise Subcellular Vaccine for Autoimmunity. Inflammation 36, 232–240 (2013). https://doi.org/10.1007/s10753-012-9539-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-012-9539-1