Abstract
Resveratrol has a preventive potential on bleomycin-induced pulmonary fibrosis in prophylactic use; however, it was not studied in the treatment of the fibrosis. This study investigated the role of resveratrol on the treatment of bleomycin-induced pulmonary fibrosis. Intratracheal bleomycin (2.5 mg/kg) was given in fibrosis groups and saline in controls. First dose of resveratrol was given 14 days after bleomycin and continued until sacrifice. On 29th day, fibrosis in lung was estimated by Aschoft’s criteria and hydroxyproline content. Bleomycine increased the fibrosis score (3.70 ± 1.04) and hydroxyproline levels (4.99 ± 0.90 mg/g tissue) as compared to control rats (1.02 ± 0.61 and 1.88 ± 0.59 mg/g), respectively. These were reduced to 3.16 ± 1.58 (P = 0.0001) and 3.08 ± 0.73 (P > 0.05), respectively, by resveratrol. Tissue malondialdehyde levels in the bleomycin-treated rats were higher (0.55 ± 0.22 nmol/mg protein) than that of control rats (0.16 ± 0.07; P = 0.0001) and this was reduced to 0.16 ± 0.06 by resveratrol (P = 0.0001). Tissue total antioxidant capacity is reduced (0.027 ± 0.01) by bleomycine administration when compared control rats (0.055 ± 0.012 mmol Trolox Equiv/mg protein; P = 0.0001) and increased to 0.041 ± 0.008 (P = 0.001) by resveratrol. We concluded that resveratrol has some promising potential on the treatment of bleomycin-induced pulmonary fibrosis in rats. However, different doses of the drug should be further studied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Management of idiopathic pulmonary fibrosis (IPF) is highly debatable and no curative treatment has been developed so far. Despite of many clinical studies on antioxidant and immunosuppressive drugs in the treatment of IPF, none of them cure the disease or change the disease progression. Since it is suggested that unrevealed insults initiating the cycle of chronic inflammation leading fibrosis play major role in the pathogenesis of IPF, making the hypothesis that preventing the inflammatory cascade before parenchymal damage, fibrosis might be reduced and anti-inflammatory therapy seems to be rational. Corticosteroids, immunosuppressive, or cytotoxic agents are first choice drugs in common practice. However, benefit of these treatment options is unclear and has potential serious side effects [1]. The American Thoracic Society and the European Respiratory Society international consensus statement recommends combined therapy (corticosteroid and either azathioprine or cyclophosphamide) for initial therapy of the patients with IPF. However, this statement also suggested that existing therapy for IPF has limited benefits and it is necessary to develop novel therapies such as the diverse immunosuppressive or antifibrotic agents [2].
Since oxygen radicals leading epithelial injury is one of the possible mechanisms in the pathogenesis of IPF, antioxidant strategies seem to be reasonable. Possible strategies might include delivery of antioxidant enzymes and an antioxidant substance which scavenges free oxygen radical to the lung or body [3].
Bleomycin-induced lung fibrosis is widely used as an experimental model of human IPF. Intratracheal bleomycin application in rats immediately leads the release of proinflammatory cytokines such as interleukin-1, tumor necrosis factor-α, interleukin-6, interferon-γ, following by increases of profibrotic markers such as transforming growth factor-β1, fibronectin, and procollagen-1, and reached peak levels in14 days [4]. Previous studies showed that fibrotic changes occur in approximately 9 days after application of BLM. Therefore, studies related to bleomycin-induced lung fibrosis are classified as preventive when the tested substance given before bleomycin and as therapeutic when they given 7 days after bleomycin [5]. There are also some studies which tested several antioxidant substances such as N-acetylcysteine [6], erdosteine [7], aminoguanidine [8], caffeic acid phenetyl ester [9], melatonin [10], ginkgo biloba [11], and resveratrol [12] in bleomycin-induced lung fibrosis in rats as a prophylactic setting, and found these substances usually reduced or prevented lung fibrosis according to Aschoft’s criteria and lung hydroxyproline content. However, in clinical practice, majority of patients with IPF were diagnosed at delayed stage of the disease. Thus, it is clear that new studies are needed to investigate the effect of the drugs in bleomycin-induced lung fibrosis model simulating human IPF at the late stage as a therapeutic model.
Resveratrol, trans-3,5,4′-trihydroxy stilbene, has potent antioxidative and anti-inflammatory properties [13]. Several vegetal sources particularly grape skin, cranberry, mulberry, lingberry, bilberry, jackfruit, peanut, and the butterfly orchid tree contain resveratrol [14]. Antioxidant mechanism of resveratrol can be explained with (1) competition to coenzyme Q, and reactive oxygen species complex chain of processes in place to reduce oxidative stresses, (2) capturing the superoxide radicals generated in mitochondria, and (3) inhibition of lipid peroxidation induced by fenton reaction products. Anti-inflammatory effects is associated with inhibition of inflammatory mediators, oxidation and leukocyte activation [15]. It has been shown that resveratrol has preventive and curative effects on cardiovascular disease, protection of vascular endothelium, regulation of lipid metabolism, increase in intracellular nitric oxide levels, and inhibiting effect of platelet aggregation [16–18].
As mentioned, Sener et al. [12] previously found that the resveratrol has effective in the prevention of bleomycin-induced pulmonary fibrosis; however, it was not studied in the treatment of the fibrosis. Here, we report the first investigation of resveratrol’s effect on the treatment of bleomycin-induced pulmonary fibrosis in rats.
METHOD
Animals
Twenty-eight adult male Wistar albino rats, weighing approximately 200–250 g were obtained from Fatih University, Faculty of Medicine, Experimental Research Center. Rats were kept at optimal conditions (room temperature between 22 and 25 °C and humidity was kept between 65 and 70 %) for 28 days in standard rat cages and free access standard pellet feed and water ad libitum. A 12-h light/dark cycle was maintained. All procedures involving animals were approved by the institutional committee for animal care.
Animal Model of Bleomycin-Induced Pulmonary Fibrosis
Male Wistar albino rats were intratracheally injected with bleomycine hydrochloride (2.5 mg/kg body weight in 0.25 ml phosphate buffered saline; Nippon Kayaku Co., Tokyo, Japan) as described previously [19]. The rats were sacrificed 29 days after bleomycin injection. Control animals received the same volume of intratracheal saline instead of bleomycin. Pulmonary fibrosis was assessed by lung hydroxyproline content as well as lung histology. Lung histology was performed as described in the following section [20].
Experimental Groups
The animals were randomly divided into four groups (group A, vehicle + bleomycin; group B, bleomycin + resveratrol; C, vehicle + vehicle; D, resveratrol); treatments (vehicle and resveratrol) were administered orally by gavage on a daily basis. Oral dose of resveratrol given as a single dose (10 mg/kg, purchased from terraternal pharmaceutical, London, England) was selected since the dose of the drug significantly prevented lung fibrosis in the previous study [12]. Control groups received vehicle + vehicle, and vehicle + bleomycin. First doses of resveratrol and vehicle were given 14 days after the bleomycin injection and continued until sacrifice.
Bronchoalveolar Lavage
After anesthesia, the lungs were prepared for lavage by cannulating the trachea with a blunt needle attached by a syringe. The lung lavage was obtained by washing the lung four times with 4 ml aliquots of saline through a tracheal cannula. Cell suspensions were concentrated by low speed centrifugation, and the cell pellet that resuspended total cell count were made in a haemocytometer. Differential cell counts were estimated from cytospine preparations by counting 300 cells stained with May–Grünwald–Giemsa.
Lung Histology
After death, lung tissues were fixed by inflation with a buffered 10 % formalin solution for 24 h and embedded in paraffin. Tissues were then sectioned, stained with hematoxylin and eosin, and examined for pulmonary fibrosis. Each successive field was individually assessed for severity of interstitial fibrosis using the semiquantitative grading system described by Ashcroft et al. [20]. The entire lung section was reviewed at a magnification of ×100 for each of the 30–35 microscopic fields needed to review the section. Criteria for grading pulmonary fibrosis were as follows. Grade = 0 normal lung, grade 1 = minimal fibrous thickening of alveolar or bronchial walls, grade 2–3 = moderate thickening of walls without obvious damage to lung architecture, grade 4–5 = increased fibrosis with definite damage to lung architecture and formation of fibrous bands or small fibrous mass, grade 6–7 = severe distortion of structure and large fibrous areas, “honeycomb lung” was placed in this category, grade 8 = total fibrous obliteration of the field. The mean score of all fields was taken as the fibrosis score of that lung section.
Lung Tissue Malondialdehyde Analysis
Lung tissue and serum malondialdehyde (MDA) levels were determined based on the reaction of MDA with thiobarbituric acid (TBA) at 95–100 °C. This is a modified version of the Draper and Hadley method which based on double-boiling method [21]. In the first heat, MDA released from proteins and the total MDA reacts with TBA in the second heat. The color absorbance of TBA-MDA complex is read at 532 nm, MDA concentrations is calculated with molar absorption coefficient. Results were expressed as nanomole per gram wet tissue protein of lung.
Hydroxyproline Determination in Lung Tissue
Hydroxyproline levels in lung tissues were determined spectrophotometrically with Woessner’s method [22].
Total Antioxidant Capacity Measurement in Lung Tissue
Plasma total antioxidant capacity (TAOC) was determined using a novel automated measurement method, developed by Erel [23]. In this method, oxidants present in the sample oxidize the ferrous ion-o-dianisidine complex to ferric ion. The oxidation reaction is enhanced by glycerol molecules, which are abundantly present in the reaction medium. The ferric ion makes a colored complex with xylenol orange in an acidic medium. The color intensity, which can be measured spectrophotometrically, is related to the total amount of oxidant molecules present in the sample. The assay is calibrated with hydrogen peroxide and the results are expressed in terms of micromolar hydrogen peroxide equivalent per liter.
Statistical Analysis
Data were expressed as means ± SEM. Statistical analysis were carried out by analysis of variance followed by appropriate post hoc tests including multiple comparison tests. All analyses were made using the SPSS statistical software package and probability value of less than 0.05 were considered statistically significant.
RESULTS
Effect of Resveratrol on Lung Fibrosis
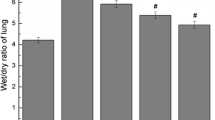
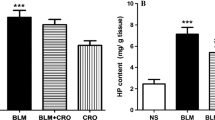
Intratracheal bleomycine administration resulted in an increase of the fibrosis score (3.70 ± 1.04) as determined by Ashcroft’s criteria when compared to control group (1.02 ± 0.61; P < 0.001) as shown in Fig. 1. Administration of resveratrol alone did not generate any changes in lung morphology. However when resveratrol was administered in bleomycin-treated rat slight reduction in this increment of fibrosis score was observed, although it was not reached at statistically significant level (P > 0.05). Similarly, bleomycin increased hydroxyproline levels (4.99 ± 0.90 mg/g tissue) as compared to saline treated rats (1.88 ± 0.59 mg/g; Fig. 1). These increments in fibrosis score and hydroxyproline level were reduced to 3.16 ± 1.58 (P = 0.0001) and 3.08 ± 0.73 (P > 0.05), respectively, by resveratrol treatment (Fig. 2).
Effect of resveratrol on bleomycin-induced increases in fibrosis score and histopathological changes in experimental groups. These figures are representatives of the lungs from animals in each treatment group. Lung tissues were obtained 29 days after instillation of bleomycin and physiologic saline and stained with H&E, viewed at a magnification of ×100. A bar graphic show that the effect of resveratrol on the bleomycin-induced increases of fibrosis scores in the groups (a). Normal appearance of lung histology in control groups treated saline and resveratrol (b, n = 7) and c (n = 7). Extensive inflammation and fibrosis showing in bleomycin group (d, n = 7). An improvement of the bleomycin-induced histological changes was seen in the rat lungs treated with resveratrol (e, n = 7). *Significantly higher (p < 0.001) when compared the all other groups except for bleomycin and resveratrol treated group.
Effect of resveratrol on bleomycin-induced increases in the lung tissue hydroxyproline content in the experimental groups. Bleomycin administration significantly increased the hydroxyproline level in the lung tissue, which was decreased by the treatment with resveratrol. Data are mean ± SD. *Significantly higher (p < 0.001) when compared the all other groups. **Significantly lower than bleomycin group.
Changes in Oxidative Stress and Total Antioxidant Capacity
As depicted in Fig. 3, lung tissue and serum MDA levels (0.55 ± 0.22 nmol/mg protein and 2.58 ± 0.61 μmol/L, respectively) in the rat treated by bleomycin were increased when compared to control rats (0.16 ± 0.07 nmol/mg protein and 0.77 ± 0.24 μmol/L, respectively) and these increases in MDA levels were reduced to 0.16 ± 0.06 nmol/mg protein and 1.59 ± 0.30 μmol/L by resveratrol treatment. As shown in Fig. 4, bleomycin administration leads a decrease in TAOC in lung tissue (0.027 ± 0.010 mmol Trolox Equiv/mg protein) and serum (1.98 ± 0.73 mmol Trolox Equiv/L) in rats, and these reductions were reversed by the treatment of resveratrol (0.041 ± 0.008 mmol Trolox Equiv/mg protein and 2.47 ± 0.48 mmol Trolox Equiv/L, respectively).
Effect resveratrol on bleomycin-induced increases in the tissue (a) and serum (b) malondialdehyde (MDA) levels in the experimental groups. Bleomycin administration significantly increased the MDA levels in the lung tissue, which were significantly decreased by the treatment with resveratrol. Data are mean ± SD. *Significantly higher (p < 0.001) when compared the all other groups.
The effect of resveratro on bleomycin-induced increase in lung tissue (a) and serum (b) total antioxidant capacity (TAOC) in experimental groups. Bleomycin significantly decreased the TAOC in the lung tissue and serum, which were reversed by treatment with resveratrol. Data are mean ± SD. *Significantly lower (p < 0.001) than all other groups.
Total and Differential Cell Count in BAL Fluid
The cells in bronchoalveolar lavage fluid had been analyzed to evaluate the effect of resveratrol on the accumulation of inflammatory cells to the lung induced by bleomycin. Typically, total fluid recovery exceeded 80 % and the percentages of fluid recovered were not significantly different among the groups. The effect of resveratrol on BAL differential and total cell count was shown in Table 1. Bleomycin treatment caused a significant increase in the total cell count in the BAL fluid as compared to control rats and this was significantly reduced by the treatment of resveratrol. The differential cell count showed that neutrophils were markedly augmented in lungs of rats which were exposed to bleomycin and this augmentation was significantly reduced by the treatment of resveratrol.
DISCUSSION
This study primarily demonstrated that bleomycin-induced lung fibrosis which was assessed by an increase in lung tissue hydroxyproline content and semiquantitative fibrosis score was partially reversed by oral resveratrol at a dose of 10 mg/kg. Furthermore, resveratrol was observed to reduce the increase of MDA levels in lung tissue and serum and elevate the decreases of lung tissue TAOC in rats due to bleomycin exposure. Also resveratrol treatment reversed an increase in the total cell count and number of neutrophils in BAL fluids.
Reversal effect of resveratrol on the depletion of lung tissue and serum TAOC and the increments in lung tissue MDA level may possibly result from its antioxidant activity (17). In the present study, the inhibitory effects of resveratrol on the accumulation of leukocytes into the lung manifested by reducing the neutrophils number in BAL fluids may contribute to a further protection of lung from free radical damage produced by leukocytes.
This study used a bleomycin-induced model, which has been extensively applied to analyze the mechanism of pulmonary fibrosis. Although there is no completely satisfactory animal model for human IPF, the bleomycin-induced murine model is relatively well characterized and exhibits certain features of IPF found in the human disease.
Sener et al. [12] found that resveratrol given day 0 after bleomycin significantly prevented experimental lung fibrosis in rat, and they proposed that the one of the probable mechanism of the observed effects of resveratrol may due to its antioxidant and anti-inflammatory properties which was verified an inhibitory effect of resveratrol on the bleomycin-induced increases in the pro-inflammatory cytokines such as TNF alpha, IL-1b, IL-6, and TGF-b in BALF and reduction of lung tissue MDA levels in rats. Similar to this study many previous studies revealed that much more antioxidant substances given early phase have preventive effect on bleomycin-induced lung fibrosis at different degrees in animal models [5]. However, limited studies investigated the role of tested substance in the late phase of lung fibrosis rats which is accepted as an animal model of human IPF. In one of these studies performed by Aoki et al. [24], the effect of follistatin was investigated which is an inhibitor of TGF beta, given early and late phase on histologic changes and hydroxyproline content of the tissue to estimate collagen deposit in the lung. They found that follistatin significantly reduced the hydroxyproline content and no significant difference was observed in the reduction of hydroxyproline content between the early-phase and the late-phase treatment groups. Also, lung fibrosis in rats exposed to bleomycin was markedly improved in follistatin-treated groups.
Kakugawa et al. [25] observed a significant improvement in lung fibrosis score and hydroxyproline content induced by intratracheal bleomycin administration into lung rat by the treatment of pirfenidone, an antifibrotic agent, given 14 days after bleomycin. Our study found that resveratrol significantly reduced oxidative stress and total leukocyte and neutrophils recruitment in lung. However, its effect on lung fibrosis parameter remains limited suggesting that interruption of oxidative stress alone is not a sufficient therapeutic option sole a mechanism in lung fibrosis.
Chaudhary et al. [4] described the time course of inflammation and fibrosis in the bleomycin-induced lung fibrosis model and studied the antiinflammatory and antifibrotic effect of prednisolone and imatinib. Also they stated that the adapted model in their study resembles more closely the clinical setting physicians are faced with, in which patients with fibrosis present long after the resolution of inflammation. They showed that imatinib mesylate given day 9 after bleomycine resulted in significant attenuation of fibrosis, independent on the treatment regimen, whereas prednisolone was only active when treatment occurred during the inflammatory phase of the model. Clearly, only imatinib mesylate is able to interfere with the fibrotic (late) phase. They concluded that the response of the bleomycin model to antifibrotic or antiinflammatory interventions was critically dependent on timing after the initial injury. Piguet et al. reported that TNF alpha receptor antagonist [26], IL-1 receptor antagonist [27], and CD-11a monoclonal antibody [28] as therapeutic use resulted in a significant reduction in collagen content and hydroxyproline levels in lung tissue exposed bleomycin. One major issue is the fact that most agents were administered to the rats in a preventive regimen, before or synchronized with bleomycin. Effectiveness in this setting may reflect more anti-inflammatory action by blocking the early response [12] without influencing the subsequent events causing progressive fibrosis as occurred in the presented study. Therefore, compounds which are successfully administered as “therapeutic treatments” in animal models should be much more promising candidates for clinical use.
Previous studies evaluated antfibrotic effect of resveratrol in cardiac fibrosis model in animals. Olson et al. [29] studied whether resveratrol directly influences the growth and proliferation of cardiac fibroblast (CF) obtained from rat heart and differentiation to the hypersecretory myofibroblast phenotype. They found that resveratrol directly inhibits two critical stages of CF activation, proliferation, and differentiation to myofibroblasts, suggesting that it has antifibrotic properties in the myocardium by limiting CF proliferation and myofibroblast differentiation. Other study by Wang et al. [30] investigated the effectiveness of resveratrol at three different doses (10, 100, and 1,000 mg/kg) on myocardial fibrosis developed following chronic Coxsackie virus-B3 induced myocarditis in mice. This study found that resveratrol alleviated myocardial fibrosis in dose dependent manner. Although an earlier study [12] revealed that resveratrol has a significant preventive effect on the bleomycin-induced lung fibrosis, in our study we observed limited influence of resveratrol as a therapeutic use on lung fibrosis in late phase. This result suggests that resveratrol has power of antioxidant and anti inflammatory property in early or inflammatory phase but its antifibrotic influence has weak. Taken together with presented and Wangs’ results, further studies are needed to investigate whether resveratrol has antifibrotic potential at higher doses than we used, or by intravenous usage.
The limitations of the study are that different doses of resveratrol and its effect on molecular substance such as TGF-beta is not investigated, and lack of comparison among routinely used anti-inflammatory therapy for IPF such as corticosteroid and azathioprine and cyclophosphamide and resveratrol.
Resveratrol (trans-3, 4′, 5-trihydroxystilbene) is a plant-derived polyphenolic phytoalexin produced by the enzyme stilbene synthase in response to environmental stress which is primarily found in the skin of grapes as well as in other fruits and plants, such as raspberries, blueberries, and mulberries. Resveratrol has been shown to exhibit a wide range of health-promoting benefits for the coronary, neurological, hepatic, and cardiovascular systems. It has been shown to inhibit inflammation, viral infection, oxidative stress, and platelet aggregation and the growth of a variety of cancer cells. On the basis of numerous in vitro and in vivo studies [31] which confirmed its ability to modulate various targets and signaling pathways, it is a promising therapy that should be studied in clinical pulmonary fibrosis as well because we do not have yet a better therapy for IPF [32]. We did not investigate the toxic effect of resveratrol. However, previous studies were investigated safety profile of resveratrol and provided evidence that resveratrol is well tolerated and nontoxic in experimental animal at doses of up to 2 g. Human clinical studies have also been performed up to single doses of 5 g without adverse effect. Also doses up to 900 mg/day trans-resveratrol given (as split daily doses) for 2 days were well tolerated with only mild adverse events being reported [33, 34].
In conclusion, our preclinical study demonstrated that the treatment of resveratrol at a dose of 10 mg/kg when administered in the late post bleomycin period (14 day after bleomycin) suppress, at least in part, the lung fibrosis induced by bleomycin exposure in rat. The observed antioxidant influence of resveratrol in this experiment seems to be prominent than antifibrotic potency at this dose. Our results mutually with the recent studies provide in vivo evidence that resvertrol may have therapeutic potential for the treatment of patients with IPF Therefore, further studies are needed to investigate the effect of different doses of resveratrol and the effect of systemic administration. On the other hand, taken together the safety profile of the resveratrol in human studies [34], we plan to try in patient with IPF as a supplementary therapy in our future study. When this data supported with further studies, resveratrol may possibly provide a new and promising therapy for pulmonary fibrosis and other fibrotic diseases in the future.
References
Noth, I., and F.J. Martinez. 2007. Recent advances in idiopathic pulmonary fibrosis. Chest 132: 637–650.
Idiopathic Pulmonary Fibrosis. 2000. Diagnosis and treatment (International Consensus Statement). American Journal of Respiratory and Critical Care Medicine 161: 646–664.
Walters, D.M., H.Y. Cho, and S.R. Kleeberger. 2008. Oxidative stress and antioxidants in the pathogenesis of pulmonary fibrosis: a potential role for Nrf2. Antioxidants & Redox Signaling 10: 321–332.
Chaudhary, N.I., A. Schnapp, and J.E. Park. 2006. Pharmacologic differentiation of inflammation and fibrosis in the rat bleomycin model. American Journal of Respiratory and Critical Care Medicine 173: 769–776.
Moeller, A., K. Ask, D. Warburton, J. Gauldie, and M. Kolb. 2008. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? The International Journal of Biochemistry & Cell Biology 40: 362–382.
Yildirim, Z., M. Kotuk, M. Iraz, I. Kuku, R. Ulu, F. Armutcu, and S. Ozen. 2005. Attenuation of bleomycin-induced lung fibrosis by oral sulfhydryl containing antioxidants in rats: Erdosteine and N-acetylcysteine. Pulmonary Pharmacology and Therapeutic 18: 367–373.
Sogut, S., H. Ozyurt, F. Armutcu, L. Kart, M. Iraz, O. Akyol, S. Ozen, S. Kaplan, I. Temel, and Z. Yildirim. 2004. Erdosteine prevents bleomycin-induced pulmonary fibrosis in rats. European Journal of Pharmacology 494: 213–220.
Yildirim, Z., Y. Turkoz, M. Kotuk, F. Armutcu, A. Gurel, M. Iraz, S. Ozen, I. Aydogdu, and O. Akyol. 2004. Effects of aminoguanidine and antioxidant erdosteine on bleomycin-induced lung fibrosis in rats. Nitric Oxide 11: 156–165.
Ozyurt, H., S. Sogut, Z. Yildirim, L. Kart, M. Iraz, F. Armutçu, I. Temel, S. Ozen, A. Uzun, and O. Akyol. 2004. Inhibitory effect of caffeic acid phenethyl ester (cape) on bleomycine-induced lung fıbrosis in rats. Clinica Chimica Acta 339: 65–75.
Yildirim, Z., M. Kotuk, H. Erdogan, M. Iraz, M. Yagmurca, I. Kuku, and E. Fadillioglu. 2006. Preventive effect of melatonin on bleomycin-induced lung fibrosis in rats. Journal of Pineal Research 40: 27–33.
Iraz, M., H. Erdogan, M. Kotuk, M. Yagmurca, T. Kilic, H. Ermis, E. Fadillioglu, and Z. Yildirim. 2006. Ginkgo biloba inhibits bleomycin-induced lung fibrosis in rats. Pharmacological Research 53: 310–316.
Sener, G., N. Topaloğlu, A.O. Sehirli, F. Ercan, and N. Gedik. 2007. Resveratrol alleviates bleomycin-induced lung injury in rats. Pulmonary Pharmacology & Therapeutics 20: 642–649.
Signorelli, P., and R. Ghidoni. 2005. Resveratrol as an anticancer nutrient: molecular basis, open questions and promises. The Journal of Nutritional Biochemistry 16: 449–466.
Li, Y., Z. Cao, and H. Zhu. 2006. Upregulation of endogenous antioxidants and phase 2 enzymes by the red wine polyphenol, resveratrol in cultured aortic smooth muscle cells leads to cytoprotection against oxidative and electrophilic stres. Pharmacological Research 53: 6–15.
Wenzel, E., and S.V. Germany. 2005. Metabolism and bioavailability of trans-resveratrol. Molecular Nutrition & Food Research 49: 472–481.
De La Lastra, C.A., and I. Villegas. 2007. Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and clinical implications. Biochemical Society Transactions 35: 1156–1160.
Wood, L.G., P.A. Wark, and M.L. Garg. 2010. Antioxidant and antiinflammatory effects of resveratrol in airway disease. Antioxidants & Redox Signaling 13: 1535–1548.
Bradamante, S., L. Barenghi, and A. Villa. 2004. Cardiovascular protective effects of resveratrol. Cardiovascular Drug Reviews 22: 169–188.
Serrano-Mollar, A., D. Closa, N. Prats, S. Blesa, M. Martinez-Losa, J. Cortijo, J.M. Estrela, E.J. Morcillo, and O. Bulbena. 2003. In vivo antioxidant treatment protects against bleomycin-induced lung damage in rats. British Journal of Pharmacology 138: 1037–1048.
Ashcroft, T., J.M. Simpson, and V. Timbrell. 1988. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. Journal of Clinical Pathology 41: 467–470.
Draper, H.H., and M. Hadley. 1990. A review of recent studies on the metabolism of exogenous and endogenous malondialdehyde. Xenobiotica 20: 901–907.
Woessner, J.B. 1961. The determination of hidroxyproline in tissue and protein samples containing small proportions of this amino acid. Arch Biochem Biophysics 93: 440–447.
Erel, O. 2004. A novel automated method to measure total antioxidant response against potent free radical reactions. Clinical Biochemistry 37: 112–119.
Aoki, F., M. Kurabayashi, Y. Hasegawa, and I. Kojima. 2005. Attenuation of bleomycin-induced pulmonary fibrosis by follistatin. American Journal of Respiratory and Critical Care Medicine 172: 713–720.
Kakugawa, T., H. Mukae, T. Hayashi, H. Ishii, K. Abe, T. Fujii, et al. 2004. Pirfenidone attenuates expression of HSP47 in murine bleomycin-induced pulmonary fibrosis. European Respiratory Journal 24: 57–65.
Piguet, P.F., and C. Vesin. 1994. Treatment by human recombinant soluble TNF receptor of pulmonary fibrosis induced by bleomycin or silica in mice. European Respiratory Journal 7: 515–518.
Piguet, P.F., C. Vesin, G.E. Grau, and R.C. Thompson. 1993. Interleukin 1 receptor antagonist (IL-1ra) prevents or cures pulmonary fibrosis elicited in mice by bleomycin or silica. Cytokine 5: 57–61.
Piguet, P.F., H. Rosen, C. Vesin, and G.E. Grau. 1993. Effective treatment of the pulmonary fibrosis elicited in mice by bleomycin or silica with anti-CD-11 antibodies. American Review of Respiratory Disease 147: 435–441.
Olson, E.R., J.E. Naugle, X. Zhang, J.A. Bomser, and J.G. Meszaros. 2005. Inhibition of cardiac fibroblast proliferation and myofibroblast differentiation by resveratrol. American Journal of Physiology-Heart and Circulatory Physiology 288: 1131–1138.
Wang, Z.P., Y.M. Hua, X. Zhang, Y.B. Wang, X.Q. Shi, and M.Y. Li. 2009. Effect of resveratrol on myocardial fibrosis in mice with chronic viral myocarditis. Zhongguo Dang Dai Er Ke Za Zhi 11: 291–295.
Fagone, E., E. Conte, E. Gili, M. Fruciano, M.P. Pistorio, D. Lo Furno, R. Giuffrida, N. Crimi, and C. Vancheri. 2011. Resveratrol inhibits transforming growth factor-β-induced proliferation and differentiation of ex vivo human lung fibroblasts into myofibroblasts through ERK/Akt inhibition and PTEN restoration. Experimental Lung Research 37: 162–174.
Athar, M., J.H. Back, L. Kopelovich, D.R. Bickers, and A.L. Kim. 2009. Multiple molecular targets of resveratrol: anti-carcinogenic mechanisms. Archives of Biochemistry and Biophysics 15(486): 95–102.
Edwards, J.A., M. Beck, C. Riegger, and J. Bausch. 2011. Safety of resveratrol with examples for high purity, trans-resveratrol, resVida(®). Annals of the New York Academy of Sciences 1215: 131–137.
Williams, L.D., G.A. Burdock, J.A. Edwards, M. Beck, and J. Bausch. 2009. Safety studies conducted on high-purity trans-resveratrol in experimental animals. Food and Chemical Toxicology 47: 2170–2182.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akgedik, R., Akgedik, Ş., Karamanlı, H. et al. Effect of Resveratrol on Treatment of Bleomycin-Induced Pulmonary Fibrosis in Rats. Inflammation 35, 1732–1741 (2012). https://doi.org/10.1007/s10753-012-9491-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-012-9491-0