Abstract
Alveolar macrophages (AMs) can initiate lung inflammation by producing pro-inflammatory cytokines and chemokines, but they participate actively in the prevention of inflammation during acute lung injury (ALI). Heme oxygenase-1 (HO-1) is mainly expressed in AMs and has anti-inflammatory properties in ALI, but the anti-inflammatory mechanisms of HO-1 are largely unknown. In this study, AMs were treated with saline, LPS (1 μg/ml), hemin (10 μM), zinc protoporphyrin (ZnPP; 10 μM, 1 h prior to LPS and hemin), SB203580 (10 μM, 1 h prior to LPS and hemin), or their combination up to 24 h. The specific HO-1 inhibitor ZnPP and SB203580 were used to inhibit the effects of HO-1 and the phosphorylated p38 mitogen-activated protein kinase (MAPK), respectively. The protein levels of HO-1 and p38 MAPK were analyzed by western blotting; arginase activity was measured in lysates obtained from cultured cells; nitric oxide production in the extracellular medium of AMs cultured for 24 h was monitored by assessing nitrite levels; the phagocytic ability of macrophage was measured by neutral red uptake. IL-10 of culture supernatants in AMs was determined by enzyme-linked immunosorbent assay. The results indicated that HO-1 induced by hemin increased arginase activity and phagocytic ability and decreased iNOS activity via p38 MAPK pathway in primary rat AMs. These changes and p38 MAPK may be the anti-inflammatory mechanism of HO-1 induced by hemin in primary rat AMs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Alveolar macrophages (AMs) have pro-inflammatory and anti-inflammatory effect during acute lung injury (ALI). In the acute phase of ALI, AMs often release different kinds of pro-inflammatory cytokines, such as IL-1β, IL-8, GM-CSF, and TNF-α, etc. [1]. However, during the later period of ALI, AMs play important roles in preventing inflammation and repairing injury tissue by increasing anti-inflammatory cytokines (such as IL-1ra, IL-10) secretion and phagocytizing neutrophils [2, 3]. The mechanism of anti-inflammation in AMs is largely unknown. If the mechanism of the protective effect of AMs is uncovered, we can manipulate the uncontrolled inflammation during ALI.
Destruction of the alveolar–capillary barrier is a common characteristic for many types of lung injury. Red blood cells may leak into the alveolar spaces and closely contact with AMs. After being retained for certain periods, the red blood cells are destroyed, releasing a large amount of hemin. In previous study, we showed that heme oxygenase-1 (HO-1) exerted a protective effect against lung injury after erythrocyte instillation [4], but the protective effect and mechanisms of HO-1 are yet unknown.
It has been well known that HO-1 protects macrophages against apoptosis, inflammation, and oxidative stress [5, 6]. Many studies indicated that HO-1 attenuated pulmonary inflammation and had beneficial effects in ALI and sepsis [7, 8]. In this research, we tested the hypothesis that hemin could induce expression of HO-1, which in turn could inhibit iNOS and NO production, increasing arginase activity and enhancing phagocytic ability of AMs stimulated with the lipopolysaccharide (LPS). Although several signaling pathways are activated by hemin in AMs, we focused on p38 mitogen-activated protein kinase (MAPK) to determine whether the effect of hemin on LPS-stimulated AMs is mediated through the p38 MAPK signaling pathway. We also studied the possible roles of hemin on IL-10 production.
MATERIALS AND METHODS
Reagents
The following reagents were purchased from the indicated sources: LPS, hemin, zinc protoporphyrin IX (ZnPP), β-NADPH, urea, l-arginine, and α-isonitrosopropiophenone (Sigma-Aldrich, St. Louis, USA); Dulbecco’s minimum essential medium (DMEM) low glucose (Invitrogen, Canada); SB203580 (Promega, American); HO-1 rabbit polyclonal primary antibody (1:1,000 dilution; Millipore, USA); phosphorylated p38 rabbit polyclonal primary antibody (1:750 dilution; Signalway Antibody Co., Ltd., USA); AP-conjugated goat antirabbit or antimouse secondary antibodies (Santa Cruz Biotechnology, CA); and BCIP/NBT Alkaline Phosphatase Color Development Kit (Beyotime Institute of Biotechnology, China).
Alveolar Macrophage Culture
Animal experiments were started after 1 week of acclimation and were performed in accordance with institutional guidelines. AMs were harvested from rats (males, 280~310 g) as previously described [9] and modified. Briefly, animals were euthanized with 120 mg/kg of intraperitoneal pentobarbital. A 14-gauge angiocatheter was inserted into the trachea through a midline neck incision and secured with a 4-0 braided silk suture. A median sternotomy was performed, and the heart–lung block was rapidly excised. Intratracheal lavage of the lungs was performed three times instilling 5-ml aliquots of cold phosphate-buffered saline (PBS). This process yielded a 90% lavage recovery. Collected lavage fluid was centrifuged at 1,000×g for 5 min and the cell pellet was resuspended in low-glucose DMEM containing 10% fetal bovine serum, 25 mM N-(2-hydroxyethyl)piperazine-N-2-ethanesulfonic acid, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells counts and viability were assessed by trypan blue exclusion methods, and DMEM added until a density of 200,000 cells/ml was reached. Then, cells suspension was inoculated to the six-well plates or 24-well plates and 96-well plates according to experiment plan. Cells were cultured at 37°C in a 5% CO2 humidified incubator for 2 h. Nonadherent cells were removed by gentle agitation. After cultured 24 h, AMs were washed with PBS and stimulated with LPS (1 μg/ml), hemin (10 μM), ZnPP (10 μM), and SB203580 (1 μM) or united.
Preparation of Hemin and ZnPP
Both hemin and ZnPP were dissolved in 0.2 N NaOH, adjusted to physiological pH 7.4 with 1 N HCl, aliquoted in dark brown tubes, and frozen at −80°C.
Western Blotting
AMs were lysed in RIPA lysis buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, sodium orthovanadate, sodium fluoride, EDTA, leupeptin, and 1 mM PMSF. Cells were scraped from the plates and sonicated for 30 s. Protein measurements were performed using the BCA protein assay and equal amounts of protein (40 μg) were loaded per lane on 12% SDS-PAGE. Protein was transferred to NC membranes (PALL) and blocked 3 h at room temperature with confining liquid, and then incubated overnight in the appropriate rabbit polyclonal primary antibody against phosphorylated p38 MAPK (1:750 dilution), HO-1 (1:1,000 dilution), or mouse polyclonal primary antibody against actin, respectively. After incubated with primary antibody, the membranes were washed in TBST. Then AP-conjugated goat antirabbit or antimouse secondary antibodies were used at 1:500 dilution. Western blot was developed using BCIP/NBT alkaline phosphatase color development kit, and proteins were visualized.
Nitric Oxide Production
Nitric oxide (NO) production in the extracellular medium of AMs cultured for 24 h was monitored by assessing nitrite levels using NO test kits (Nanjing Jiancheng Bioengineering Institute, China).
Arginase Activity
Arginase activity was measured in lysates obtained from cultured cells as described [10]. At 24 h after stimulation, AMs (about 5 × 105 cells) were lysed with 80 μl Triton X-100 (0.1%). After 30 min, arginase was activated by adding 100 μl Tris–HCl (25 mM) and 35 μl MnCl2 (10 mM), and incubating for 10 min at 56°C. l-Arginine hydrolysis was conducted by incubating cell lysates with 100 μl l-arginine (0.5 M, pH 9.7) at 37°C for 1 h. The reaction was stopped with 800 μl H2SO4 (96%)/H3PO4 (85%)/H2O (1:3:7). The produced urea was quantified at 540 nm after addition of 40 μl α-isonitrosopropiophenone (9%, dissolved in 100% ethanol), followed by heating at 100°C for 20 min. One unit of enzyme is defined as the amount that catalyzes the formation of 1 μmol urea/min.
Phagocytic Assay
The phagocytic ability of macrophage was measured by neutral red uptake which was described by Chen [11]. AMs were cultured with LPS (1 μg/ml), hemin (10 μM), ZnPP (10 μM), and SB203580 (1 μM) or united for 24 h. After extracellular medium were moved, cells were rinsed with PBS. Two hundred microliters neutral red solutions (dissolved in 10 mM PBS with the concentration of 0.09%) was added and incubated for 3 h. The supernatant was discarded, and cells in 96-well plates were washed twice with PBS to remove the neutral red that was not phagocytized by AMs. Then cell lysate (ethanol and 0.01% acetic acid at the ratio of 1:1, 200 μl/well) was added to lyse cells. After AMs were incubated in 4°C overnight, the optical density at 540 nm was measured by a microplate reader. OD values represent phagocytic ability.
IL-10 Assays
The concentration of IL-10 was measured in supernatants obtained from six-well culture plates using a standard sandwich ELISA following the manufacturer’s protocols (Wuhan Boster Biological Technology, China).
Statistical Analysis
Results were expressed as \( \overline \chi \pm {\text{SD}} \) and compared using SPSS 13.0. Data were compared by one-way analysis of variance among groups followed by the Student–Neuman–Keul test for post hoc comparisons. Differences were determined to be statistically significant when P < 0.05 was attained.
RESULTS
HO-1 Protein Levels Induced by Hemin in Rat AMs
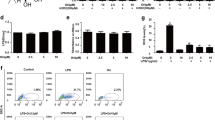
To examine the effect of Hemin on HO-1 in AMs, we determined HO-1 protein levels in rat AMs. As shown in Fig. 1, hemin increased HO-1 protein expression in rat AMs (P < 0.05 versus LPS group), the combination of hemin and LPS further increased HO-1 protein expression levels (P < 0.05 versus LPS group). Next we would test the functional changes of rat AMs with overexpression of HO-1.
Different HO-1 expression levels in AMs. AMs were stimulated for 24 h with saline, LPS (1 μg/ml), hemin (10 μM), or their combination, as indicated. Cell lysates were collected and were electrophoresed, transferred to nitrocellulose membrane, and probed for HO-1 or β-actin (as internal control) expression. a Representative banding from triplicate experiments was offered. b Densitometric analysis for levels of HO-1 protein relative to β-actin are presented as \( \overline \chi \pm {\text{SD}} \). n = 3. *P < 0.05 versus LPS group, #P < 0.05 versus the hemin group.
The Changes of NO Production, Arginase Activity, and Phagocytic Ability in Rat AMs
To study the effect of HO-1 on the functional changes of rat AMs, we determine the changes of NO production, arginase activity, and phagocytic ability in rat AMs. Hemin decreased NO production and increased arginase activity and phagocytosis in LPS-stimulated AMs (P < 0.05, compared with the LPS group, Table 1), While pretreatment with ZnPP, the specific HO-1 inhibitor significantly reversed the effects of hemin on NO production, arginase activity, and phagocytic ability in LPS-stimulated AMs (P < 0.05, compared with the LPS + Hemin group, Table 1).
The Levels of p38 MAPK Expression in AMs
To determine whether the effect of hemin on LPS-stimulated AMs is mediated by a p38 MAPK signaling pathway, we measured the levels of p38 MAPK expression in AMs. AMs were incubated with hemin (10 μM) for 0 min, 15 min, 30 min, 1 h, 3 h, 6 h, 12 h, and 24 h, respectively. The protein levels of phosphorylated p38 MAPK were measured by western blot analysis as described in the “MATERIALS AND METHODS” section. As shown in Fig. 2, hemin increased the phosphorylation of p38 MAPK in a time-dependent manner (Fig. 2) and peaked at 30 min (P < 0.05 versus other groups).
Hemin-induced p38 MAPK phosphorylation in AMs in a time-dependent manner. AMs were incubated with hemin (10 μM) for 0 min, 15 min, 30 min, 1 h, 3 h, 6 h, 12 h, and 24 h. The levels of phosphorylation p38 MAPK were measured by western blot analysis as described in the “MATERIALS AND METHODS” section. a Representative banding from triplicate experiments was offered. b Densitometric analysis for levels of HO-1 protein relative to β-actin are presented as \( \overline \chi \pm {\text{SD}} \). n = 3. *P < 0.05 versus other groups.
P38 MAPK Mediated the Effect of Hemin on Rat AMs
After the determination of p38 MAPK protein level, we employed SB203580 (a specific inhibitor of p-p38 MAPK) to investigate the effect of SB203580 on p38 MARK signal pathway in hemin-activated rat AMs. As shown in Fig. 3, SB203580 abolished the effect of hemin-induced HO-1 expression. In LPS + hemin + SB203580 group, there were higher NO production, lower phagocytic ability, and arginase activity in rat AMs (P < 0.05, versus LPS + hemin group, Table 1). Therefore, we proposed that the effect of hemin on the LPS-stimulated AMs might be mediated by p38 MAPK.
Hemin-induced HO-1 expression via p38 MAPK pathway. AMs were pretreated for 1 h with the p38 MAPK inhibitor SB203580 (1 μM) prior to LPS (1 μg/ml) and hemin (10 μM) exposure for 24 h. Cell lysates were collected and were electrophoresed, transferred to nitrocellulose membrane, and probed for HO-1 or β-actin (as internal control) expression. a Representative banding from triplicate experiments was offered. b Densitometric analysis for levels of HO-1 protein relative to β-actin are presented as \( \overline \chi \pm {\text{SD}} \). n = 3. *P < 0.05 versus LPS group, #P < 0.05 versus LPS group, §P < 0.05 versus LPS + hemin group.
The Concentration of IL-10
Because IL-10 is a major anti-inflammatory cytokine secreted by AMs, we studied whether HO-1 could increase the concentration of IL-10 in rat AMs. LPS induced IL-10 secretion in rat AMs (P < 0.05, versus other groups, Fig. 4). No statistically significant difference was found between other groups. SB 203580 and ZnPP pretreatment had no effect of IL-10 secreting on hemin (P < 0.05, versus LPS group, Fig. 4).
The levels of IL-10 in the extracellular medium of primary rat AMs. AMs were stimulated for 24 h with saline, LPS (1 μg/ml), hemin (10 μM), ZnPP (10 μM, 1 h prior to LPS and hemin), SB203580 (10 μM, 1 h prior to LPS or hemin), or their combination as indicated. Culture supernatants were collected, centrifuged, and assayed for IL-10 expression by ELISA. Data are presented as the \( \overline \chi \pm {\text{SD}} \) of triplicate determination. #P < 0.05 versus other groups.
DISCUSSION
In the present study, we demonstrated that hemin significantly upregulated HO-1 expression in primary rat AMs and the pretreatment with hemin significantly inhibited LPS-induced NO production, developed the arginase activity, and enhanced phagocytose ability in rat AMs. Furthermore, we showed that these effects were markedly reversed by a HO-1 specific inhibitor, ZnPP; we also found that p38 MAPK may be involved in the effect of hemin on rat AMs (Fig. 5).
A schematic representation about the signal mechanism and effect of hemin on primary rat AMs. Hemin induced HO-1 expression via the p38 MAPK pathway, which can be blocked by SB203580 (a specific inhibitors of p-p38 MAPK). Overexpression of HO-1 decreased iNOS activity, increased arginase activity and phagocytic ability, which be confirmed by ZnPP (a HO-1 inhibitor).
HO is the rate-limiting enzyme in heme catabolism, leading to the generation of biliverdin, free iron, and carbon monoxide (CO). Three HO isoforms (i.e., HO-1, HO-2, and HO-3) have been identified. HO-1 (32 kDa) is a stress-inducible protein, and HO-2 (36 kDa) is constitutively synthesized and exists primarily in the brain and testis, whereas HO-3 (33 kDa) is less well characterized [12]. HO-1 and its byproducts have anti-inflammatory, antiapoptotic, and antiproliferative effects [13, 14]. HO-1 is highly expressed in airways induced by oxidative stress, including hyperoxia, hypoxia, endotoxemia, heavy metal exposure, bleomycin, diesel exhaust particles, and allergen exposure [15–18]. HO-1 plays an important role in preventing airway inflammation. In this research, we found that hemin could upregulate HO-1 expression, which was coincided with decreased iNOS activity, increased arginase activity, and phagocytic ability. The action of hemin is HO-1 specific because the effects were prevented by ZnPP. These data suggested that the anti-inflammatory activity of HO-1 is directly related to iNOS activity, arginase activity, and phagocytic ability.
The small amount of NO produced by constitutive NOS, including eNOS and nNOS, is an important regulator of physical homeostasis, whereas the large amount of NO produced by iNOS has been closely correlated with the pathophysiology in a variety of diseases and inflammation. Our results demonstrated the suppressive effects of HO-1 on iNOS activity, consistent with the previous observations [19–22]. Macrophages metabolize arginine to produce either NO through iNOS or ornithine through arginase producing divergent biological effects [23]. These two cross-regulated metabolic processes may reflect opposite functional states of macrophages. Basically, NO had cytotoxic and cytostatic activity while the products of arginase pathway promoted cell proliferation [24]. It has been reported that the anti-inflammatory activity of Echinacea results in inhibition of NO production and increased arginase activity [25]. In this study, we also observed the opposite regulatory effects of HO-1 on NO production and arginase activity.
AMs, predominated by arginase pathway, contribute to scavenge debris, phagocytosing apoptotic cells after inflammatory injury and orchestrating tissue remodeling and repair through the production of extracellular matrix proteins [26]. Dexamethasone increased phagocytic ability of macrophages against the inflammation [27]. Here we found that HO-1 also increased the phagocytic ability of AMs. So it is possible that HO-1 may play an anti-inflammatory role by increasing phagocytic ability of AMs.
The stress-activated kinases of the p38 MAPK family are serine–threonine kinases that are activated by environmental stresses such as heat, UV irradiation, or osmotic stress and various pro-inflammatory and stressful stimuli [28, 29]. P38 MAPK has multiple roles in innate immune responses. Numerous researches indicated that p38 MAPK was involved in pro-inflammatory reaction and would be a potential therapeutic target for the inflammatory disease [30]. But p38 MAPK also had anti-inflammatory reaction in macrophages [31–33]. Several studies revealed that HO-1/CO exhibited anti-inflammatory and antiapoptotic functions partly mediated by the activation of p38 MAPK [34–36]. In this research, we found that the protective role of HO-1 could be p38 MAPK dependent in rat AMs.
IL-10 is an important anti-inflammatory cytokine of AMs. IL-10 induces the expression of HO-1 in murine macrophages and human macrophages [37]. Moreover, HO-1 mediated the anti-inflammatory effect of interleukin-10 in mice [38]. Here we found that HO-1 induction did not increase the content of IL-10 in primary rat AMs, suggesting that HO-1 and its products (biliverdin, free iron, and carbon monoxide) could play anti-inflammatory roles independent of IL-10.
In conclusion, we have demonstrated that in vitro hemin significantly increased HO-1 expression, which in turn enhanced HO-1 expression, decreased NO production, increased the arginase activity, and improved phagocytic ability in LPS-stimulated rat AMs. These effects of hemin on rat AMs may be mediated by HO-1 induction and associated with activation of p38 MAPK. These findings support the concept that HO-1 upregulation in AMs is an anti-inflammatory mechanism.
References
Zhao, M., L.G. Fernandez, A. Doctor, A.K. Sharma, A. Zarbock, and C.G. Tribble. 2006. Alveolar macrophage activation is a key initiation signal for acute lung ischemia-reperfusion injury. American Journal of Physiology. Lung Cellular and Molecular Physiology 291: L1018–L1026.
Matute, B.G., and T.R. Martin. 2003. Science review: apoptosis in acute lung injury. Critical Care 7: 355–358.
Miyake, Y., H. Kaise, K. Isono, H. Koseki, K. Kohno, and M. Tanaka. 2007. Protective role of macrophages in noninflammatory lung injury caused by selective ablation of alveolar epithelial type II cells. Journal of Immunology 178: 5001–5009.
Pang, Q.F., Q.M. Zhou, S. Zeng, L.D. Dou, J. Yong, and Y.M. Zeng. 2008. Protective effect of heme oxygenase-1 on lung injury induced by erythrocyte instillation in rats. Chinese Medical Journal 121: 1688–1692.
Deshane, J., M. Wright, and A. Agarwal. 2005. Heme oxygenase-1 expression in disease states. Acta Biochimica 52: 273–284.
Kirkby, K.A., and C.A. Adin. 2006. Products of heme oxygenase and their potential therapeutic applications. American Journal of Physiology. Renal Physiology 290: F563–F571.
Takaki, S., N. Takeyama, Y. Kajita, T. Yabuki, H. Noguchi, and Y. Miki. 2010. Beneficial effects of the heme oxygenase-1/carbon monoxide system in patients with severe sepsis/septic shock. Intensive Care Medicine 36: 42–48.
Morse, D., L. Lin, A.M. Choi, and S.W. Ryter. 2009. Heme oxygenase-1, a critical arbitrator of cell death pathways in lung injury and disease. Free Radical Biology & Medicine 47: 1–12.
McCourtie, A.S., H.E. Merry, A.S. Farivar, C.H. Goss, and M.S. Mulligan. 2008. Alveolar macrophage secretory products augment the response of rat pulmonary artery endothelial cells to hypoxia and reoxygenation. The Annals of Thoracic Surgery 85: 1056–1060.
Meyer, M., F. Huaux, X. Gavilanes, S. van den Brûle, P. Lebecque, S. Lo Re, and D. Lison. 2009. Azithromycin reduces exaggerated cytokine production by M1 alveolar macrophages in cystic fibrosis. American Journal of Respiratory Cell and Molecular Biology 41: 590–602.
Chen, W., W. Zhang, W. Shen, and K. Wang. 2010. Effects of the acid polysaccharide fraction isolated from a cultivated Cordyceps sinensis on macrophages in vitro. Cellular Immunology 262: 69–74.
Otterbein, L.E., and A.M. Choi. 2000. Heme oxygenase: colors of defense against cellular stress. American Journal of Physiology 270: L1029–L1037.
Otterbein, L.E., M.P. Soares, K. Yamashita, and F.H. Bach. 2003. Heme oxygenase-1: unleashing the protective properties of heme. Trends in Immunology 24: 449–455.
Kim, H.P., S.W. Ryter, and A.M. Choi. 2006. Co as a cellular signaling molecule. Annual Review of Pharmacology and Toxicology 46: 411–449.
Carraway, M.S., A.J. Ghio, J.D. Carter, and C.A. Piantadosi. 2000. Expression of heme oxygenase-1 in the lung in chronic hypoxia. American Journal of Respiratory Cell and Molecular Biology 278: L806–L812.
Carraway, M.S., A.J. Ghio, J.L. Taylor, and C.A. Piantadosi. 1998. Induction of ferritin and heme oxygenase-1 by endotoxin in the lung. American Journal of Lung Cellular and Molecular Physiology 275: L583–L592.
Eyssen-Hernandez, R., A. Ladoux, and C. Frelin. 1996. Differential regulation of cardiac heme oxygenase-1 and vascular endothelial growth factor mRNA expressions by hemin, heavy metals, heat shock and anoxia. FEBS Letters 382: 229–233.
Ferrándiz, M.L., and I. Devesa. 2008. Inducers of heme oxygenase-1. Current Pharmaceutical Design 14: 473–486.
Ashino, T., R. Yamanaka, M. Yamamoto, H. Shimokawa, K. Sekikawa, and Y. Iwakura. 2008. Negative feedback regulation of lipopolysaccharide-induced inducible nitric oxide synthase gene expression by heme oxygenase-1 induction in macrophages. Molecular Immunology 45: 2106–2115.
Lin, H.Y., S.H. Juan, S.C. Shen, F.L. Hsu, and Y.C. Chen. 2003. Inhibition of lipopolysaccharide induced nitric oxide production by flavonoids in RAW264.7 macrophages involves heme oxygenase-1. Biochemical Pharmacology 66: 1821–1832.
Lin, H.Y., S.C. Shen, and Y.C. Chen. 2005. Anti-inflammatory effect of heme oxygenase 1: glycosylation and nitric oxide inhibition in macrophages. Journal of Cellular Physiology 202: 579–590.
Maestrelli, P., C. Páska, M. Saetta, G. Turato, Y. Nowicki, and S. Monti. 2003. Decreased haem oxygenase-1 and increased inducible nitric oxide synthase in the lung of severe COPD patients. European Respiratory Journal 21: 971–976.
Wang, W.W., C.P. Jenkinson, J.M. Griscavage, R.M. Kern, N.S. Arabolos, and R.E. Byrns. 1995. Co-induction of arginase and nitric oxide synthase in murine macrophages activated by lipopolysaccharide. Biochemical and Biophysical Research Communications 210: 1009–1016.
Meurs, H., H. Maarsingh, and J. Zaagsma. 2003. Arginase and asthma: novel insights into nitric oxide homeostasis and airway hyperresponsiveness. Trends in Pharmacological Sciences 24: 450–455.
Zhai, Z., A. Solco, L. Wu, E.S. Wurtele, M.L. Kohut, P.A. Murphy, and J.E. Cunnick. 2009. Echinacea increases arginase activity and has anti-inflammatory properties in RAW 264.7 macrophage cells, indicative of alternative macrophage activation. Journal of Ethnopharmacology 122: 76–85.
Mantovani, A., S. Sozzani, M. Locati, P. Allavena, and A. Sica. 2002. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in Immunology 23: 549–555.
Gratchev, A., J. Kzhyshkowska, J. Utikal, and S. Goerdt. 2005. Interleukin-4 and dexamethasone counterregulate extracellular matrix remodelling and phagocytosis in type-2 macrophages. Scandinavian Journal of Immunology 61: 10–17.
Lee, J.C., J.T. Laydon, and P.C. McDonnell. 1994. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372: 739–746.
Han, J., J.D. Lee, L. Bibbs, and R.J. Ulevitch. 1994. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265: 808–811.
Yong, H.Y., M.S. Koh, and A. Moon. 2009. The p38 MAPK inhibitors for the treatment of inflammatory diseases and cancer. Expert Opinion Investigation 18: 1893–1905.
Chanteux, H., A.C. Guisset, C. Pilette, and Y. Sibille. 2002. LPS induces IL-10 production by human alveolar macrophages via MAPKinases- and Sp1-dependent mechanisms. Respiratory Research 8: 71.
Schwacha, M.G., I.H. Chaudey, and M. Alexander. 2003. Regulation of macrophage IL-10 production postinjury via beta2 integrin signaling and the P38 MAP kinase pathway. Shock 20: 529–535.
Seimon, T.A., Y. Wang, S. Han, and T. Senokuchi. 2009. Macrophage deficiency of p38alpha MAPK promotes apoptosis and plaque necrosis in advanced atherosclerotic lesions in mice. The Journal of Clinical Investigation 119: 886–898.
Li, Y.Z., Y.X. Zhong, X. Fang, and C.C. Bang. 2008. Effect of radix paeoniae rubra on expression of p38 MAPK/iNOS/HO-1 in rats with lipopolysaccharide-induced acute lung injury. Chinese Medical Journal 121: 1688–1692.
Wang, X.M., H.P. Kim, K. Nakahira, S.W. Ryter, and A.M. Choi. 2009. The heme oxygenase-1/carbon monoxide pathway suppresses TLR4 signaling by regulating the interaction of TLR4 with caveolin-1. Journal of Immunology 182: 3809–3818.
Williams, J.A., C.H. Pontzer, and E. Shacter. 2000. Regulation of macrophage interleukin-6 (IL-6) and IL-10 expression by prostaglandin E2: the role of p38 mitogen-activated protein kinase. Journal of Interferon and Cytokine Research 20: 291–298.
Ricchetti, G.A., L.M. Williams, and B.M. Foxwell. 2004. Heme oxygenase 1 expression induced by IL-10 requires STAT-3 and phosphoinositol-3 kinase and is inhibited by lipopolysaccharide. Journal of Leukocyte Biology 76: 719–726.
Lee, T.S., and L.Y. Chau. 2002. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nature Medicine 8: 240–246.
Acknowledgments
This work was supported by the following grant the Priority Academic Program Development of Jiangsu Higher Education Institutions, Peak of the six personnel in Jiangsu Province, and Jiangsu Province Graduate Education Innovation project no. CX09S_035Z and Xuzhou Medical College president’s fund (no. 09KJZ07).
Conflict of Interest
All of the authors declared that there was not any actual or potential conflict of interest in this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hualin, C., Wenli, X., Dapeng, L. et al. The Anti-inflammatory Mechanism of Heme Oxygenase-1 Induced by Hemin in Primary Rat Alveolar Macrophages. Inflammation 35, 1087–1093 (2012). https://doi.org/10.1007/s10753-011-9415-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-011-9415-4