Abstract
Diabetic nephropathy (DN) is a major cause of type 2 diabetes mellitus (T2DM) mortality. Innate immunity has been shown to be closely associated with the occurrence and progression of T2DM-associated complications. In this study, we investigated the expression of Toll-like receptor 4 (TLR4) and CD14+CD16+ monocytes in patients with T2DM and DN patients with uremia and TLR4 response to lipopolysaccharide (LPS), and to further explore the potential effects of inflammatory immune response in T2DM and DN uremia. Thirty DN patients with uremia, 28 T2DM patients, and 20 healthy volunteers were enrolled for the determination of CD14+CD16+ fluorescence intensity and TLR4 expression on monocytes by using peripheral blood flow cytometry. Serum C-reactive protein (CRP) level was determined by using the immunoturbidimetry. Peripheral blood mononuclear cells (PBMCs) were isolated and stimulated with LPS for 24 h. monocytes were collected to detect NF-κB p65 and phosphorylated STAT5(p-STAT5) expressions by using Western blotting. Supernatants were sampled for the determination of interleukin-6 (IL-6) concentration by using ELISA. Compared to normal control, T2DM patients and DN uremic patients had a significantly higher CD14+CD16+ fluorescence intensity, TLR4 expression, serum IL-6 and CRP level, whilst these biomarkers were more upregulated in DN uremic patients than in T2DM patients. Following the exposure to LPS, PBMCs showed a significant upregulation in NF-κB-p65 and p-STAT5 expression and a remarked increase in Supernatants IL-6 level, in a positive correlation with disease severity. Our results suggest that the disturbance in proinflammatory CD14+CD16+ monocytes occurs in T2DM and DN uremic patients. Such immunological dysfunction may be related to the activation of TLR4/NF-κB and STAT5 signaling pathways underlying the immune abnormalities of CD14+CD16+ monocytes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
With an increasing incidence worldwide, type 2 diabetes mellitus (T2DM) and diabetic nephropathy (DN) have become a major medical condition that seriously threatens human life and adversely impacts on quality of life. Therefore, the prevention and treatment of T2DM and DN require further investigation. In clinical practice, DN progresses into uremia inevitably in some patients although their glucose, blood pressure, and serum lipid are well controlled. The pathogenesis of DN is associated with oxidative stress, renin–angiotensin system, and disturbances of glucose and lipid, whereas many other contributing factors may well be also involved.
It has been extensively documented that chronic mild inflammatory response is present and mediated by cytokines in diabetic patients, whilst the activation of non-specific immunity participates in the occurrence and progression of T2DM and T2DM-associated complications. The pathological mechanisms of non-specific immunity consist of genetic mutations, proinflammatory monocytes/macrophages, chronic infection, Toll-like receptor (TLR), and upregulation of macrophage migration inhibitory factor.
IL-6 has been suggested as an important cytokine in the development of T2DM and its complications [1, 2]. Furthermore, NF-κB [4] and STAT5 [28] are the two key signaling pathways closely involved in regulation of inflammatory responses.
CD14+CD16+ monocytes are thought to be a proinflammatory monocyte subpopulation. The expression profiles of CD14+CD16+monocytes have been rarely reported in T2DM and DN complicated with chronic infection. In this study, we investigated the differential expressions of TLR4 and CD14+CD16+ monocytes and regulatory response of monocytes to lipopolysaccharide (LPS) between T2DM patients and DN uremic patients, and investigated the potential mechanisms of inflammatory immune response in the pathogenesis of T2DM and DN uremia.
SUBJECTS AND METHODS
Patients
Healthy volunteers (n = 20) were enrolled between 2009 and 2011, including ten males and ten females. Eligible control subjects had no history of diabetes mellitus, hypertension, coronary heart disease, kidney disease, or other endocrine/metabolic disorders, or a family history of diabetes mellitus, would be excluded. T2DM patients (T2DM group, n = 28) and uremic DN patients not on dialysis (DN group, n = 30) who visited Department of Nephrology and Department of Endocrinology at Chongqing Medical University were concomitantly enrolled between May 2009 and February 2011. the T2DM group consisted of 15 males and 13 females, whilst the uremic DN group included 16 males and 14 females. The diagnosis of T2DM followed WHO Diagnostic Criteria and Classification of Diabetes Mellitus 1999. The eligibility criteria were as follows: serum 25-hydroxy vitamin D (25[OH]VD), 21–29 ng/ml; glutamic acid decarboxylase antibody (GADA) level, below 1.0; fasting plasma glucose, below 7.0 mmol/l; and HbA1c, below 6.5%. The diagnostic criteria for DN complicated with chronic renal failure (uremia) were as follows: serum creatine >707 μmol/l, or endogenous creatine clearance rate <10 ml/min, and/or 24-h urinary albumin excretion >300 mg, and in stable condition (see Table 1). The exclusion criteria included type 1 or secondary diabetes mellitus, acute infections, cardiac or hepatic dysfunction, acute diabetic complications or infections within 3 months prior to the study, being pregnant or lactating, being complicated with other connective tissue diseases or autoimmune diseases, suffering from malignant tumors, cardio-cerebrovascular accidents, or asthma, or taking medications potentially interfering with the study. All subjects were of Chinese Han ethnicity but not biologically related. The study protocol was approved by the Institutional Review Board at the Affiliated Hospital of Chongqing Medical University, and all study subjects volunteered to give informed consents prior to enrollment.
Blood Sample Collection and Human Monocyte Isolation and Culture
Blood samples were drawn from the patients and controls after an 8-h fasting period and collected into heparinized tubes. Serum was obtained and stored at −80°C for biochemical tests. Then, peripheral blood mononuclear cells (PBMCs) were freshly isolated from the heparinized whole blood by Ficoll-Hypaque density gradient. Subsequently, cells were washed with non-serum 1640 Culture Medium (Gibco, USA), and the cells were washed twice, resuspended in RPMI-1640 (GIBCO, USA) containing 10% (v/v) heat-inactivated FCS, 50 mg/ml streptomycin and 50 U/ml penicillin, and subsequently seeded at 1 × 106 per cell in 6-well plate and incubated at 37.0°C in a humidified 5% CO2.
PBMCs were collected from control group (n = 20), T2DM group (n = 28), and uremic DN group (n = 30) and synchronized for 24 h. PBMCs were exposed to LPS for 24 h, at a final concentration of 1 μg/ml, followed by the collection of PBMCs and supernatants. The concentration of LPS exposure was optimized through the preliminary study and referred to previous reports [20, 21].
Flow Cytometric Analysis of CD14+CD16+ Monocyte and TLR4 Expression
Peripheral whole blood was pretreated with EDTA and added into flow cytometry tubes (150 μl per tube), supplemented with CD14-PE, CD16-FITC and TLR4(CD284)-FITC (10 μl per tube; AbD Serotec, Oxford, UK), respectively. A tube was supplemented without fluorescent antibody as blank control. The flow cytometry samples were vortexed well and incubated for 30 min at room temperature and in a lightproof manner. One milliliter erythrocyte lysis buffer was added into and mixed with blood cells for 10 min. Cells were centrifuged in 1 ml PBS plus 1% BSA at 1,600 rev/min for 5 min. The supernatants were discarded, and cells were rinsed for twice prior to the fixation with paraformaldehyde for 10 min. Mean fluorescent intensities (MFIs) of CD14+CD16+ and TLR4 were analyzed by using flow cytometry.
Enzyme-Linked Immunosorbent Assay (ELISA) Detection of Serum 25(OH)D3, IL-6
The serum levels of 25(OH)D3 and IL-6 were measured by ELISA according to the manufacturer’s instructions (PeproTech, USA). Next, the serum levels of GADA was measured by ELISA according to the manufacturer’s instructions (BioMerica Inc.USA).The minimum detectable concentrations were 3.125 pg/ml (25(OH)D3) and 0.7 pg/ml (IL-6), respectively. All samples were measured in duplicate.
Immunoturbidimetry Measurement of Serum High-Sensitivity C-Reactive Protein (hsCRP), Serum Endotoxin Assay and Other Biochemical Measurements
C-reactive protein (CRP) was measured by immunoturbidimetry using latex particle-enhanced immunoturbidimetry (Shanghai Biotechnology and CoxBio China, Shanghai, China). Serum endotoxin level was determined by quantitative colorimetric limulus test. The endotoxin detection reagent kit was purchased from Xiamen Houshiji, Ltd. (China). The measurements were performed according to the manufacturers’ protocols. Serum albumin, triglycerides, blood glucose, creatine kinase MB, troponin, urea and creatinine were detected with an automatic biochemical analyzer (Hitachi 7600; Hitachi, Tokyo, Japan).
Western Blotting of NF-κB p65, IκB, STAT5, p-STAT5
After the cells were treated with LPS from patients and controls for 24 h, ice-cold protein extraction buffer (Nanjing KeyGEN, China) supplemented with 1% protease inhibitor and 1% phosphorylate inhibitor was added for protein extraction. A BCA protein concentration detection kit(Nanjing KeyGEN, China) was used to determine protein concentration. The protein sample was mixed with sample loading buffer and heated to 100°C for 5 min. Proteins were then separated by electrophoresis in 6% or 10% Tris-glycine polyacrylamide gradient gels. Separated proteins were then transferred onto nitrocellulose membrane (Invitrogen), and blocked for 1 h with TBS–Tween containing 5% bovine serum albumin. Next, the membrane was incubated overnight at 4°C with specific primary antibodies for Anti- NF-κB p65, Anti- IκB, Anti-STAT5 and Anti-p-STAT5(1:500 dilution) Ab or β-actin (1:5,000 dilution) (antibodies purchased from Santa Cruz Company). After washing, the membrane was incubated at room temperature for 2 h with horseradish peroxidase (HRP)-labeled secondary goat anti-rabbit antibody (1:3,000 dilution with blocking buffer; Beijing 4Abio.com, China), and was then used for the detection of protein expression using a chemiluminescent staining reagent kit (Shanghai Beyotime Company, China). The average pixel density was analyzed by UN-SCAN-IT gel analysis software (Silk Scientific, Utah, USA).
Statistical Analysis
Statistical analysis was performed using the statistical package SPSS Version 13.0. Results were expressed as mean±stand deviation (SD). Statistical analysis involved Spearman’s correlation between two continuous variables and one-way analysis of variance (ANOVA) followed by Bonferroni multiple comparisons test. P value below 0.05 was considered significant.
RESULTS
Basic Clinical Characteristics of Patients
There were no significant differences in age, sex, BMI, HBA1c, fasting glucose, DBP, triglyceride, total cholesterol, HDL-cholesterol, routine blood test, GADA or medications among the three groups (P > 0.05). However, 24 h UALB, BUN, SCr, serum CRP and Endotoxin (ET) levels were higher in the DN uremia group compared to the T2DM and control groups (serum CRP and ET levels: DN uremic group, 5.4 ± 2.8 and 0.28 ± 0.13; T2DM group 3.3 ± 1.6 and 0.10 ± 0.05; control group, 1.7 ± 0.7 and 0.03 ± 0.01; P < 0.01; see Table 1), while serum 25(OH)VD levels were lower in T2DM and DN uremic groups (DN uremic group, 23.9 ± 2.6; T2DM group, 24.8 ± 2.6; control group 45.1 ± 7.2; P < 0.01; Table 1). Serum CRP levels were in negative correlation with serum 25(OH)VD levels (r = −0.618, P < 0.05).
Analysis of MFIs of CD14+CD16+ and TLR4 Expression in Monocytes Among the Groups
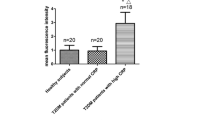
As shown in Fig. 1, MFIs of CD14+CD16+ monocytes were significantly higher in the blood of DN uremic patients (DN uremic patients, 14.4 ± 4.0; T2DM patients, 6.9 ± 2.3; healthy subjects, 4.6 ± 1.5; P < 0.01), and the MFIs of TLR4 in monocytes were higher when compared to T2DM and healthy subjects (DN uremic patients, 2.8 ± 0.9; T2DM patients, 2.1 ± 0.8; healthy subjects, 1.6 ± 0.7; P < 0.05).And both MFIs of CD14+CD16+ monocytes and TLR4 in T2DM were higher when compared to healthy subjects (P < 0.01).
MFIs of CD14+CD16+ and TLR4 expression in monocytes from three groups. The representative photographs of flow cytometry analysis MFIs of CD14+CD16+ and TLR4 expression in monocytes of (a) healthy subjects, (b) type 2 diabetes (T2DM) patients and (c) diabetic nephropathy (DN) uremic patients. a Identification of monocytes displayed in a representative FSC/SSC dot plot. b Green lines represent staining with isotype matched control antibody. c A representative flow cytometry chart of CD16 expression by CD14-positive monocytes. d A representative flow cytometry chart of TLR4 expression by CD14-positive monocytes. (d) A flow cytometry chart of TLR4 and CD16+ expression by CD14-positive monocytes. MFI of CD14+CD16+ = c/b; MFI of TLR4 = d/b; Values are mean ± standard deviation. *P < 0.01 vs. healthy subjects; **P < 0.05 vs. healthy subjects; #P < 0.01 vs. T2DM patients; ##P < 0.05 vs. T2DM patients.
Western Blotting of NF-κB p65, IκB, STAT5 and p-STAT5 Proteins
As TLR4 recruit various protein kinases after binding ligands, it leads to activation of NF-κB p65 and the phosphorylation of STAT5 (p-STAT5). The levels of NF-κB p65 and p-STAT5 were measured by Western blotting using special mAb. As shown in Fig. 2a and b, there was no significant difference in the baseline total STAT5 level between controls (0.889 ± 0.085), T2DM patients (0.944 ± 0.016) and patients with DN uremia (0.968 ± 0.012) (P > 0.05). The changes in NF-κB p65 and p-STAT5 level after LPS stimulation were significantly lower in controls (0.009 ± 0.002 and 0.007 ± 0.004, respectively) than T2DM patients (vs. 0.443 ± 0.100 [P < 0.01] and vs. 0.625 ± 0.153 [P < 0.01], respectively) and in DN uremic patients (vs. 0.515 ± 0.100 [P < 0.01] and vs. 0.981 ± 0.105 [P < 0.01], respectively). The levels of IκB were significantly lower (0.268 ± 0.010 [T2DM patients] and 0.234 ± 0.016 [DN uremic patients], respectively; P < 0.01) compared to the healthy subjects (0.693 ± 0.107).
NF-κB p65, IκB, STAT5 and p-STAT5 proteins expression on LPS-treated monocytes from three groups. a The representative photographs of western blotting of NF-κB p65, IκB, STAT5 and p-STAT5 proteins expression on LPS-treated monocytes from (1) healthy subjects, (2) type 2 diabetes (T2DM) patients and (3) diabetic nephropathy (DN) uremic patients. b Western blotting chart of NF-κB p65, IκB, STAT5 and p-STAT5 proteins expression. Values are mean±standard deviation. *P < 0.01 vs. healthy subjects; #P < 0.05 vs. T2DM patients.
Serum and the Cell Supernatant Effector cytokine IL-6 Levels Among the Groups
As shown in the Fig. 3, Tables 2 and 3, the serum and cell supernatant IL-6 levels on LPS-treated monocytes from the groups were higher in patients with DN uremia (serum levels, 84.8 ± 20.7; cell supernatant levels, 148.9 ± 44.6 pg/ml) and T2DM patients (serum levels, 55.5 ± 17.1; cell supernatant levels, 114.1 ± 16.3 pg/ml) compared to the healthy subjects (serum levels, 11.0 ± 2.0; cell supernatant levels, 66.51 ± 14.95 pg/ml; P < 0.01, respectively). And the IL-6 level higher in patients with DN uremia compared to T2DM patients (P < 0.01).
Serum CRP and IL-6 levels are recognized as the most sensitive indicators of the microinflammatory state that is often considered to be closely associated with T2DM and its complications. For this reason, we investigated the relationship between plasma CRP and IL-6 and circulating CD14+CD16+ monocytes in the T2DM and DN uremic patients. As displayed in Fig. 4, serum levels of CRP were in positive correlation with MFIs of CD14+CD16+ and TLR4 in monocytes (CD14+CD16+ monocytes MFIs, r = 0.553, P < 0.01, Fig. 4a; TLR4 MFIs, r = 0.413, P < 0.01, Fig. 4b). The same pattern was seen between serum IL-6 levels and CD14+CD16+ monocytes MFIs (r = 0.867, P < 0.01, Fig. 4c) and TLR4 MFIs (r = 0.781, P < 0.01, Fig. 4d).
DISCUSSION
It has been shown in experimental studies that monocytes from T2DM patients have a pro-inflammatory profile. Major evidence points towards a link between inflammation and type 2 diabetes, insulin resistance and diabetic complications [1–9]. In this study, we have shown that in diabetic patients, an abnormal monocytes/macrophages behavior exists, with a pro-inflammatory profile. This abnormal profile is mainly true in type 2 diabetic patients. It was suggested that chronic sub-clinical inflammatory and activation of the innate immune system could be linked to type 2 diabetes pathogenesis and also to the development of the common DN [3, 7].
Recent studies have demonstrated that an inflammatory mechanism contributes to the pathogenesis of DN. Monocytes/macrophages are the principal inflammatory cells found in the diabetic kidney, and recent studies have suggested that monocytes/macrophages play an important role in the pathogenesis of glomerulopathy and tubulointerstitial lesions in DN [10–13]. It is consistent with our previous study [14]. It has been reported that vitamin D (VD) and VD receptor (VDR) deficiencies are associated with the occurrence of T2DM and T2DM-associated complications [15–19].
Our research shows that serum ET levels increased in patients with T2DM and DN uremia, and monocyte/macrophages may show a dysfunction in such an ET environment. In our study, uremic DN patients have an increased hsCRP and ET but a significantly lower 25(OH)VD than T2DM patients and control subjects, whilst serum 25(OH)VD is in a negative correlation with hsCRP (r = −0.562, P < 0.05). Such finding is consistent with the report of Devaraj et al. [16]. The MFIs of CD14+CD16+ are (4.8 ± 1.7), (7.8 ± 2.1) and (14.4 ± 3.9) in control subjects, T2DM group, and DN group, respectively, whilst those of TLR4 are (1.6 ± 0.7), (2.1 ± 0.8), and (2.8 ± 0.9), respectively. Furthermore, uremic DN patients have the highest MFIs of CD14+CD16+ and TLR4 (P < 0.01). CD14+CD16+ monocytes increased and TLR4 in monocytes exhibit an upregulated expression and a hyperresponsiveness to LPS in T2DM patients and uremic DN patients. It suggests that aberrant immune activation is present in T2DM patients and uremic DN patients, in agreement with the results of Du et al. [20]. Given the fact that CD14+CD16+ monocytes represent a sensitive marker for inflammation or cellular activation, the role of CD14+CD16+ monocytes in various diseases have received considerable interest in recent years [24–27]. And there is a host of reports [22, 24–27] about increases or decreases in the number of CD14+CD16+ monocytes in various diseases. There was no significant difference in the numbers of CD14+CD16+ monocyte subpopulation frequencies between DM and healthy controls in some reports [23]. But the relationship CD14+CD16+ fluorescence intensity and TLR4 expression on monocytes from T2DM patients and uremic DN patients has been rarely reported.
Our study also shows that monocytes in uremic DN patients and T2DM patients have a significantly higher serum IL-6 levels (84.8 ± 20.7 [DN uremic patients] vs. 55.5 ± 17.1 [T2DM patients] vs. 11.0 ± 2.0 [healthy subjects]; P < 0.01) and IL-6 secretion in response to 24-h LPS stimulation than those in control subjects (148.9 ± 44.6 [DN uremic patients] vs. 114.1 ± 16.3 [T2DM patients] vs. 66.5 ± 15.0 [healthy subjects]; P < 0.01). CD14+CD16+ monocytes exhibit an upregulation and TLR4 expression in association with diabetic severity (positive correlation of CD14+CD16+ and TLR4 MFIs in monocytes with serum creatinine; r = 0.764 and 0.605, respectively; both P < 0.01) and in a positive correlation with microinflammation (serum IL-6 and CRP levels). It is suggested that CD14+CD16+ monocytes dysfunction may also be involved in the inflammatory pathogenesis of T2DM and DN. However, the CD14+CD16+ monocyte functions of T2DM and DN are still to be defined.
Our findings also show that T2DM patients and uremic DN patients exhibit a high-sensitivity response to LPS stimulation, manifesting as, following 24-h LPS exposure, the increased of IL-6 secretion in monocytes and the concurrent activation of intracellular NF-κB-p65 and p-STAT5 signaling pathways. It can be concluded that TLRs, NF-κB and STAT signaling pathways may play various roles in the pathogenesis of diabetes mellitus. CD14+CD16+ monocytes may participate in the occurrence and progression of DN uremia through the mutual interactions of TLRs, NF-κB, and STAT. The systematic characterization of TLRs, NF-κB, and STAT expression and responsiveness variations with inflammatory cytokines in monocytes is helpful for the prevention and treatment of T2DM and DN.
In summary, our study suggests that the disturbance in proinflammatory CD14+CD16+ monocytes occurs in T2DM and DN uremic patients. Such immunological dysfunction may be related to the activation of TLR4/NF-κB and STAT5 signaling pathways underlying the immune abnormalities of CD14+CD16+ monocytes.
References
Navarro-González, J.F., and C. Mora-Fernández. 2008. The role of inflammatory cytokines in diabetic nephropathy. Journal of the American Society of Nephrologists 19(3): 433–442.
Ortiz-Muñoz, G., V. Lopez-Parra, O. Lopez-Franco, et al. 2010. Suppressors of cytokine signaling abrogate diabetic nephropathy. Journal of the American Society of Nephrologists 21: 763–772.
O’Connor, J.C., A. Satpathy, M.E. Hartman, et al. 2005. IL-β mediated innate immunity is amplified in the db/db mouse model of type 2 diabetes. Journal of Immunology 174(8): 4991–4997.
Ko, G.J., Y.S. Kang, S.Y. Han, et al. 2008. Pioglitazone attenuates diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic rats. Nephrology, Dialysis, Transplantation 23(9): 2750–2760.
Mora, C., and J.F. Navarro. 2006. Inflammation and diabetic nephropathy. Current Diabetes Reports 6(6): 463–468.
Navarro, J.F., and C. Mora. 2006. Diabetes, inflammation, proinflammatory cytokines, and diabetic nephropathy. The Scientific World Journal 6: 908–917.
Tuttle, K.R. 2005. Linking metabolism and immunology: diabetic nephropathyis an inflammatory disease. Journal of the American Society of Nephrologists 16(6): 1537–1538.
Kikuchi, Y., T. Imakiire, M. Yamada, et al. 2005. Mizoribine reduces renal injury and macrophage infiltration in non-insulin dependent diabetic rats. Nephrology, Dialysis, Transplantation 20(8): 1573–1581.
Giulietti, A., E. van Etten, L. Overbergh, et al. 2007. Monocytes from type 2 diabetic patients have a pro-inflammatory profile 1,25-dihydroxyvitamin D3 works as anti-inflammatory. Diabetes Research and Clinical Practice 77: 47–57.
Ruster, C., and G. Wolf. 2008. The role of chemokines and chemokine receptors in diabetic nephropathy. Frontiers of Bioscience 13: 944–955.
Chow, F., E. Ozols, D.J. Nikolic-Paterson, et al. 2004. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state, and progressive renal injury. Kidney International 65: 116–128.
Furuta, T., T. Saito, T. Ootaka, et al. 1993. The role of macrophages in diabetic glomerulosclerosis. American Journal of Kidney Diseases 21: 480–485.
Utimura, R., C.K. Fujihara, A.L. Mattar, et al. 2003. Mycophenolate mofetil prevents the development of glomerular injury in experimental diabetes. Kidney International 63: 209–216.
ManLi, L., G. Hua, and Q. Lin. 2009. The expression of TLR4 on peripheral blood monocytes from uremic patients with diabetic nephropathy and its relation with plasma MCP-1 concentration. China Immunology Journal 25(9): 848–850.
Hypponen, E., B.J. Boucher, D.J. Berry, et al. 2008. 25-Hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: a cross sectional study in the 1958 British Birth Cohort. Diabetes 57(2): 298–305.
Devaraj, S., J.-M. Yun, C.R. Duncan-Staley, et al. 2011. Low vitamin D levels correlate with the proinflammatory state in type 1 diabetic subjects with and without microvascular complications. American Society for Clinical Pathology 135: 429–433.
Ozfirat, Z., and T.A. Chowdhury. 2010. Vitamin D deficiency and type 2 diabetes. Postgraduate Medical Journal 86: 18–25.
Kayaniyil, S., R. Vieth, R. Retnakaran, et al. 2010. Association of vitamin D with insulin resistance and β-cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care 33(6): 1379–1381.
Di Cesar, D.J., R. Ploutz-Snyder, R.S. Weinstock, et al. 2006. Vitamin D deficiency is more common in type 2 than in type 1 diabetes. Diabetes Care 29(1): 174.
Du, T., Z.-G. Zhou, S. You, et al. 2009. Modulation of monocyte hyperresponsiveness to TLR ligands by 1,25-dihydroxy-vitamin D3 from LADA and T2DM. Diabetes Research and Clinical Practice 83(2): 208–214.
Sadeghi, K., B. Wessner, U. Laggner, et al. 2006. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. European Journal of Immunology 36: 361–370.
Ziegler-Heitbrock, L. 2007. The CD14+CD16+ blood monocytes: their role in infection and inflammation. Journal of Leukocyte Biology 81: 584–592.
Patino, R., J. Ibarra, A. Rodriguez, et al. 2000. Circulating monocytes in patients with diabetes mellitus, arterial disease, and increased CD14 expression. The American Journal of Cardiology 85: 1288–1291.
Ulrich, C., G.H. Heine, M.K. Gerhart, et al. 2008. Proinflammatory CD14+CD16+ monocytes are associated with subclinical atherosclerosis in renal transplant patients. American Journal of Transplantation 8(1): 103–110.
Barisione, C., S. Garibaldi, G. Ghigliotti, et al. 2010. CD14CD16 monocyte subset levels in heart failure patients. Disease Markers 28(2): 115–124.
Pettersson, A., A. Sabirsh, J. Bristulf, et al. 2005. Pro- and anti-inflammatory substances modulate expression of the leukotriene B4 receptor, BLT1, in human monocytes. Journal of Leukocyte Biology 77: 1018–1025.
Rogacev, K.S., S. Seiler, A.M. Zawada, et al. 2011. CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. European Heart Journal 32(1): 84–92.
Tripathi, P., S. Kurtulus, S. Wojciechowski, et al. 2010. STAT5 is critical to maintain effector CD8+ T cell responses. Journal of Immunology 185: 2116–2124.
Acknowledgements
We thank Professor Qifu Li and Dr Lai Han for their cooperation in patient selection. This study was supported by the outstanding doctoral dissertation fund of Chongqing Medical University and the Key Project of Chongqing Municipal Health Bureau (2010-1-16).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, M., Gan, H., Shen, Q. et al. Proinflammatory CD14+CD16+ Monocytes are Associated with Microinflammation in Patients with Type 2 Diabetes Mellitus and Diabetic Nephropathy Uremia. Inflammation 35, 388–396 (2012). https://doi.org/10.1007/s10753-011-9374-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-011-9374-9