Abstract
Oxymatrine (OMT) plays a significant role in chemical agents induced intestinal injury. However, its functional role in L-arginine (Arg) induced acute pancreatitis (AP) following intestinal injury and the corresponding molecular mechanism are unclear to our knowledge. We investigate OMT function in Arg induced AP following intestinal injury in vivo and vitro. OMT (4 mg/ml) decreased Arg (from 100 to 600 µM) induced IEC-6 cells growth in dose-dependent manner and inhibited Arg induced the increase of pAKT, bcl2, and the decrease of Bax. Meanwhile, OMT inhibited Arg (600 µM) induced the increase of pro-inflammatory cytokines TNF-a, IL-6, IL-1β, and NFkBp65 and the decrease of anti-inflammatory cytokine IL-10 expression. Moreover, the change of tight junction proteins claudins 1–4 expression induced by Arg was also reversed by OMT. Consistent with the results in vitro, OMT (50 mg/kg) inhibited Arg (250 mg/100 g) induced AP following intestinal injury in vivo. In detail, OMT resisted Arg induced inflammatory histology of both pancreas and intestine and inhibited Arg induced the change of pAKT, Bax, bcl2, TNF-a, IL-6, IL-1β, IL-10, NFkBp65, claudin 1–4 expression. Moreover, OMT inhibited Arg induced NFkBp65 and ICAM-1 expression in vivo by IHC. Oxymatrine improves Arg-induced acute pancreatitis following intestinal injury via inhibiting AKT/NFkB and claudins signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute pancreatitis (AP) is a potentially serious disease characterized by intrapancreatic activation of multiple proteases and inflammatory reactions in the pancreas and intestine (Chen et al. 2017a, b). The integrity of intestinal barrier is closely related to the degree of AP severity and the origin of systemic inflammation (Chen et al. 2017a, b). However, the pathogenesis of AP with inflammatory mediators (Mayer et al. 2000) and the disfunction of intestinal barrier (Meriläinen et al. 2012) has not been fully elucidated to our knowledge.
L-arginine (Arg) plays a significant role in protein synthesis and cellular functions regulation, including immune response, hormone secretion, and wound healing (Visigalli et al. 2010). The Arg induced AP model in rats has been successfully constructed in previous studies (Chen et al. 2017a, b; Zhang et al. 2012). Meanwhile, our previous first study showed oxymatrine (OMT) reversed Arg induced AP in vivo (Zhang et al. 2012). OMT (Fig. 1), as an alkaloid compound extracted from the root of Sophora flavescens Ait, has exhibited various pharmacological activities, including antiviral (Ma et al. 2013), anti-fibrosis (Liang et al. 2010), anti-inflammation (Wen et al. 2014), immune regulation (Chai et al. 2012), anti-proliferation (Yao and Wang 2014), and antitumor activities (Li et al. 2017). Recently, increasing studies focus on the anti-inflammatory effects of OMT in vitro and vivo (Guzman et al. 2013). For example, OMT is related to the TLR4/MyD88/NF-κB signaling pathway in pancreatic microvascular endothelial cells can alleviate inflammatory responses (Lu et al. 2017). OMT protects against DSS-induced colitis involving PI3K/AKT signaling pathway in BALB/c mice (Chen et al. 2017a, b); OMT improves intestinal epithelial barrier function via NFkB-mediated signaling in CCl4-Induced cirrhotic rats (Wen et al. 2014). However, the definite role of OMT in AP caused disfunction of intestinal barrier has been rarely reported to the best of our knowledge, which is significant to reveal the new treatment for AP.
Oxymatrine (matrine oxide, matrine N-oxide, matrine 1-oxide) is one of many quinolizidine alkaloid compounds extracted from the root of Sophora flavescens, a Chinese herb (Ku Shen in Chinese). Its IUPAC Name: (7aS,13aR,13bR,13cS)dodecahydro-1H,5H,10H-dipyrido[2,1-f:3′,2′,1′-ij][1,6]naphthyridin-10-one 4-oxide
Materials and methods
Animals
Adult Wistar rats weighting from 250 to 300 g were purchased from Beijing Vital River Laboratory Animal Technology (Beijing, China). Animals were maintained according to institutional regulations in facilities approved by the Animal Care Committee of China Medical University in accordance with Chinese government guidelines for animal experiments.
Experimental protocol
As described in our previous study (Zhang et al. 2012), 24 rats were divided into three groups: control group (saline treatment) (n = 8), AP group (Arg treatment) (n = 8), and OMT group (Arg combining OMT treatment) (n = 8). Each group comprised 10 rats. 250 mg/100 g Arg (Sigma, St. Louis, MO, USA) as a 20% solution in 0.15 M physiological saline was gave (i.p) at an interval of 1 h in AP group. The control group received an equal volume of 0.15 M physiological saline at the same time points. Arg induced AP rats received (i.p) 50 mg/kg OMT (Abcam, Cambridge, UK) every 6 h (OMT group). All rats were killed at 48 h, the pancreas and 5 cm of terminal ileums were removed for the late hematoxylin-eosin (HE), immunohistochemistry (IHC), PCR, and western blot (WB) assays.
Cell culture and treatment
The non-transformed rat small intestinal IEC-6 cells (Bena Culture Collection, Beijing, China) were cultured as previously described (Jobin et al. 1997). Cells were plated in 6-well/96-well plates for late PCR, WB, and MTT assays with Arg (600 µM) or OMT (4 mg/ml) treatments as described in result section and previous study (Guzman et al. 2013). Cells were starved for 2 h with serum-free mediua prior to Arg or OMT incubation.
Morphological examination
Ileums specimens were stained with HE and blindly examined under microscopy. As described previously (Howarth et al. 1996), a total score was derived from the sum of 11 histologic criteria, including villus fusion and stunting, surface enterocytes, damage of the brush border, decrease in the number of goblet cells, crypt loss, architectural disruption, injury of crypt cells, crypt abscess formation, infiltration of polymorphonuclear cells and lymphocytes, dilatation of lymphatics and capillaries, and thickening and edema of the submucosal and muscularis external layers. Each histologic variable was evaluated by three professional pathologists and scored from 0 (normal) to 3 (maximal damage) to finally give a maximum possible 33 scores for each intestinal sample.
MTT assays
IEC-6 cells growth under the various doses of Arg was detected by MTT. Briefly, IEC-6 cells were harvested, counted, and then seeded into 96-well plates at the density of 6000 viable cells per well overnight. After starvation (2 h), Arg were added to the mediua with various concentrations and time shown in the “Results Section”. Fifteen microliter of MTT (5 mg/ml in PBS, Sigma) was added and the cells were incubated for 4 h at 37 °C. The mediua was then removed and 100 µl of dimethyl sulphoxide (Sigma) was added to each well for 20 min. Per experiment group at a wavelength of 570 nm in an ELISA 96-well microtiter plate reader (BIORAD680, USA). Experiments were performed in triplicates, and data were presented as the percentage of treated cells compared with control cells.
Real-time PCR
Total RNA was extracted from IEC-6 cells and intestinal tissue samples with or without Arg (600 µM) and OMT (4 mg/ml) treatments using TRIZOL reagent under the manufacturer’s instruction (Takara Bio, Otsu, Japan). For qRT-PCR in vitro, the control group received 0.15 M physiological saline for 4 days; AP group received 600 µM Arg for 4 days; OMT group received 600 µM Arg for 4 days combining with OMT (4 mg/ml) treatment for the last 2 days. For qRT-PCR in vivo, RNA from intestinal tissues was extracted from control, AP and OMT groups, respectively. cDNA was synthesized from total RNA by using the Expand Reverse Transcriptase Kit (Thermo). The expression of target genes was analyzed in a Light Cycler 2.0 kit (Takara). The conditions were as follows: 95 °C for 30 s, 40 cycles of 95 °C for 5 s, and 60 °C for 30 s. The primers were summarized in Table 1. DdH2O was used as a negative control. Quality of the PCR products was monitored by post-PCR melt-curve analysis. The expression of these target genes was quantified using the 2-△Ct (△Ct = △Ctarget gene-△CGAPDH) method. Each experiment was repeated three times.
Western blot
Total protein lysates were prepared from IEC-6 cells and intestinal tissues with or without Arg (600 µM) and OMT (4 mg/ml) treatments. For WB in vitro, the control group received 0.15 M physiological saline for 4 days; AP group received 600 µM Arg for 4 days; OMT group received 600 µM Arg for 4 days combining with OMT (4 mg/ml) treatment for the last 2 days. For WB in vivo, protein from intestinal tissues was extracted from control, AP, and OMT groups, respectively. Samples were loaded onto 10% SDS-polyacrylamide gels, transferred to polyvinylidene difluoride membranes (Millipore Corp, Bedford, MA, USA) and incubated with primary NFkBp65 (Cell Signaling Technology, Beverly, MA, USA), IkBa (Cell Signaling Technology), pAKT (Cell Signaling Technology), AKT(Cell Signaling Technology), Bcl2 (Proteintech, Chicago, IL, USA), Bax (Proteintech), claudins 1–4 (Abcam, Cambridge, UK) and GAPDH (Proteintech) antibodies overnight at 4 °C. Membranes were incubated with horseradish peroxidase-conjugated monoclonal secondary antibody (Santa Cruz) at room temperature for 1.5 h, respectively. Immunoreactive protein bands were visualized with an ECL kit (Millipore, Bedford, MA, USA). Each experiment was repeated three times.
Immunohistochemistry
Immunohistochemistry (IHC) was performed as described previously (Zhang et al). Formalin-fixed, paraffin-embedded 4 μm thick intestinal sections were treated with freshly prepared 3% H2O2 in methanol avoiding light for 30 min at room temperature. Nonspecific antibody binding was then blocked using a specific blocking reagent for 20 min. NFkBp65 and ICAM-1 (intercellular cell adhesion molecule 1, ICAM-1) (Abcam) antibodies were incubated overnight at 4 °C. The corresponding secondary antibodies were incubated at room temperature for 30 min. Reaction products were visualized by incubation with 3,3′-diaminobenzidine and then counterstained with hematoxylin. Staining intensity was scored as 0 (negative), 1 (weak), 2 (medium), and 3 (strong). Extent of staining was scored as 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%), and 4 (76–100%) according to the percentage of the carcinoma involved area that was positively stained. The final staining scores were determined by three professional pathologists.
Statistical analysis
Statistical analysis was performed using SPSS software, version 13.0 (SPSS, Chicago, IL, USA). The differences of MTT assays were compared through paired sample t-test. The differential expression of target proteins in IHC analysis were compared using paired sample non-parametric test. The differences of qRT-PCR and WB analysis were expressed as mean ± SE and compared using Student’s t-test. A value of P < 0.05 indicated statistic significant.
Results and discussion
OMT inhibits Arg-induced cell growth, inflammation and disregulation of tight junction claudins in vitro
MTT showed that Arg enhanced cell growth in does-dependent and time-dependent manner (P < 0.05). As the increasing concentration and the prolonging action time, absorbance (OD) exhibited significantly increase, especially in 600 µM of Arg following 4 days (P < 0.01) (Fig. 2a). However, OMT (4 mg/ml) significantly inhibited Arg induced cell growth in Arg-does dependent manner (P < 0.05) (Fig. 2b). Based on above results, we used the same situation for late PCR and WB assays.
OMT inhibited Arg induced cell growth and inflammation in vitro. a Arg induced cell growth in IEC-6 cells in dose-dependent manner. b OMT inhibited Arg induced cell growth in Arg dose-dependent manner. c The protein levels of NFkBp65, IkBa, pAKT, AKT, Bcl2, Bax and claudin 1-4 in the control, AP and OMT groups in vitro by WB assays. Bars indicate ± S.E. *P < 0.05; **P < 0.01 compared with the control. OMT, oxymatrine; Arg, L-arginine; AP, acute pancreatitis; WB, western blot
Arg (600 µM) significantly increased pAKT, Bcl2 and NFkBp65 but decreased Bax expression. However, OMT reversed Arg induced change of above proteins. It indicated that OMT inhibits Arg-induced cell growth via AKT and Bcl2/Bax pathway (Fig. 2c). Meanwhile, Arg significantly induced claudin 2 but inhibited claudin1, 3, and 4 protein expressions. OMT also reversed Arg induced change of above proteins (Fig. 3c).
qRT-PCR showed that Arg (600 µM) significantly induced pro-inflammatory cytokines TNF-a, IL-6, and IL-1β and inhibited anti-inflammatory IL-10 mRNA expression, respectively. However, OMT inhibited Arg induced the change of TNF-a, IL-6, IL-1β, NFkBp65, and IL-10 expression (Fig. 3).
Histopathology and morphology of the pancreas and the small intestine
We first found that Arg (250 mg/ml) successfully induced varying degrees of AP following the disruption of the intestinal barrier in eight rats.
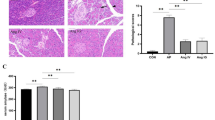
The control group exhibited the normal histological features of the pancreas (a normal architecture filled with acinar cells) (Fig. 4a). AP group revealed a tissue damage characterized by edema, inflammatory cell infiltrates, and acinar cell necrosis (Fig. 4a). However, AP with OMT treatment exhibited a significant amelioration of pancreatic injury (low inflammatory cell infiltrates and relative complete acinus morphology) (Fig. 4a).
The inflammatory histologic changes of pancreas and intestine in control, AP, and OMT groups, respectively. a Pancreatic histopathology in the in control, AP, and OMT groups (×200). b Intestinal histopathology in the intestine in control, AP and OMT groups (×200). c Histopathologic scoring of intestinal injury was plotted in control, AP, and OMT groups. Bars indicate ± S.E.*P < 0.05; **P < 0.01 compared with the control
Histological examination of intestinal tissue from the control group rats revealed normal mucosal architecture with similar histologic scores (Fig. 4b, c). A representative photograph from the AP group rats showed significant histologic injury to the intestinal mucosa with denuded villi, disintegration of the lamina propria, exposed capillaries, and neutrophil and macrophage infiltration (Fig. 4b, c). Histological examination of intestinal tissue from the OMT group rats revealed only capillary congestion and mild epithelial lifting from the lamina propria (Fig. 4b, c).
Taking together, OMT attenuates Arg induced pancreatic and intestinal damage.
OMT inhibits Arg-induced inflammatory and the change of pAKT, bcl2/bax, and claudins expression in vivo
The significant increase of TNF-a, IL-6, and IL-1β and the decrease of IL-10 mRNA levels was shown in intestinal tissues in AP group compared with the control group. However, OMT reversed the change of above inflammatory cytokines induced by Arg (Fig. 5).
Meanwhile, the significantly increase of NFkBp65, pAKT, Bcl2 and the decrease of bax protein levels in intestinal tissues was shown in AP group compared with control group, which was reversed by OMT treatment (Fig. 6).
Claudin family is the key member of the tight junction proteins, which plays a significant role in maintenance of the epithelial barrier function. Consistent with the results in vitro, Arg significantly induced claudin 2 but inhibited claudin 1, 3, and 4 protein expression. However, OMT reversed the change of claudins expression induced by Arg (Fig. 6), which indicated that OMT inhibited Arg-induced AP related intestinal injury involving claudin signaling.
OMT inhibits Arg-induced NFkBp65 and ICAM-1 expression in vivo by IHC
NFkBp65 was localized in both cytoplasm and nuclear in intestinal tissue, while ICAM-1 was mainly localized in the cytoplasm (Fig. 7).
AP group showed significantly increase of NFkBp65 and ICAM-1 expression in intestinal tissues compared with control group (P < 0.01; P < 0.01, respectively). However, OMT partially reversed Arg induced NFkBp65 and ICAM-1 expression (P < 0.05; P < 0.05, respectively) (Fig. 7), which were consistent with the results in vitro.
OMT has been reported to provide a protective effect in agents-induced liver, heart, brain and intestinal injury of various animal models (Wen et al. 2014; Hong-Li et al. 2008; Fan et al. 2008). However, the role of OMT in Arg induced AP following intestinal barrier injury has been rarely reported to our knowledge. In current study, OMT reverses Arg induced AP following intestinal barrier in vitro and vivo.
Accumulating evidence indicates that pro-inflammatory cytokines are released from activated pancreatic macrophages in different AP animal models (Norman 1998). OMT mediated Arg induced AP resulted in a significant amelioration of histologic injury of pancreas in current study. Taking together with our previous study (Zhang et al. 2012), OMT inhibits Arg induce AP via inhibiting pro-inflammatory cytokines release, which was consistent with previous study (Yılmaz et al. 2016).
Disfunction of intestinal bacterial including increased mucosal permeability, reduced bowel motility, and impaired host defenses, have been identified as possible causes for bacterial translocation and the severity of AP (Lu et al. 2017). We first found that OMT inhibited Arg induced cell growth and inflammation in vitro accompanied by the change of pAKT, bcl2, TNF-a, IL-6, IL-1β, NFkBp65, Bax, and IL-10 expressions. Though above results in vitro have not been reported yet to our knowledge, increased levels of TLR4, MyD88, and NF-κB p65 induced by LPS stimulation, are significantly inhibited by OMT pretreatment in MS1 cells (Lu et al. 2017). OMT also inhibits LPS-induced pro-inflammatory (Cxcl2, TNF-a, and IL-6) cytokines expression in IEC-6 cells (Guzman et al. 2013).
Consistent with the results in vitro, OMT inhibited Arg induced AP caused intestinal injury in vivo. OMT also act as a protective role in other agents-induced intestinal injury. OMT improves intestinal barrier function via NFkB-mediated signaling pathway in cirrhosis-associated intestinal mucosal damage (Wen et al. 2014). OMT protects against DSS-induced colitis via inhibiting the PI3K/AKT signaling pathway (Chen et al. 2017a, b). OMT attenuates intestinal ischemia/reperfusion injury in rats via inhibiting TNF-α and phosphorylated p38/MAPK signaling (Zhao et al. 2008). OMT prevents intestinal inflammation through blockade of IL-6 and IL-1b in vivo (Guzman et al. 2013). ICAM-1, as a significant inflammatory mediator in colonic epithelial cells, was activated by Arg. However, OMT reversed Arg induced ICAM-1 overexpression in current study. TNF-a, IL-6, IL-10, and ICAM-1 are closely regulated by the key inflammatory mediator of NFkB (Wang et al. 2011; Santos et al. 2016; Al-Hanbali et al. 2009). Meanwhile, a strong biological-link between NFkB and the AKT pathway in inflammatory modulation is shown in a lot research (Hussain et al. 2012; Han et al. 2017). Thus, we conclude that OMT improves Arg-induced AP related intestinal injury involving AKT/NFkB signaling.
Finally, OMT reversed Arg induced change of claudin 1–4 levels in vitro and vivo. The channel and barrier functions of claudins have been well studied previously (Günzel and Yu 2013; Krug et al. 2014). Intestinal injury caused claudins dysregulation is involved both in expression changes and different distributions (Barmeyer et al. 2015). For example, the increase of claudin 1 and 2 and the decrease of claudin 3 and 4 are shown in ulcerative colitis (Oshima et al. 2008; Poritz et al. 2011; Weber et al. 2008; Prasad et al. 2005; Thuijls et al. 2010). A close interaction between claudins and NFkB-mediated inflammatory response is well investigated. NFkB-dependent decrease in the Sox18-Claudin 5 axis is essentially involved in the disruption of human endothelial cells barrier integrity associated with LPS-mediated acute lung injury (Gross et al. 2017). The increasing claudins expression was dependent on NFkB activation in gastric AGS cancer cells (Chavarría-Velázquez et al. 2018). Meanwhile, claudin 5, 7, and 18 suppress proliferation via inhibiting pAKT in human lung squamous cell carcinoma (Akizuki et al. 2017). Claudin-3 overexpression in promoting the malignant potential of colorectal cancer cells, which is potentially regulated by the EGF-activated ERK1/2 and PI3K-Akt pathways (De Souza et al. 2013). Taking together, OMT might inhibit Arg-induced AP following intestinal injury by AKT/NFkB mediated claudin activity.
Conclusions
We first find that OMT inhibits Arg induced AP and intestinal injury in vitro and vivo via regulating AKT/NFkB and claudins signaling. Various signaling pathways (TGF-β1/Smad3, JNK/MAPK, TLR4 signaling, and COX-2/PGI2) are associated with OMT function in ischemia reperfusion injury. However, the corresponding molecular mechanism is poorly understood and should be investigated in our future study.
Abbreviations
- OMT:
-
Oxymatrine
- Arg:
-
L-arginine
- AP:
-
Acute pancreatitis
- qRT-PCR:
-
Real-time PCR
- WB:
-
Western Blot
- IHC:
-
Immunohistochemistry
- HE:
-
hematoxylin-eosin
References
Al-Hanbali M, Ali D, Bustami M, Abdel-Malek S, Al-Hanbali R, Alhussainy T, Qadan F (2009) Epicatechin suppresses IL-6, IL-8 and enhances IL-10 production with NF-kappaB nuclear translocation in whole blood stimulated system. Neuro Endocrinol Lett 30(1):131–138
Akizuki R, Shimobaba S, Matsunaga T, Endo S, Ikari A (2017) Claudin-5, -7, and -18 suppress proliferation mediated by inhibition of phosphorylation of Akt in human lung squamous cell carcinoma. Biochim Biophys Acta 1864(2):293–302
Barmeyer C, Schulzke JD, Fromm M (2015) Claudin-related intestinal diseases. Semin Cell Dev Biol 42:30–38
Chen X, Zhao HX, Bai C, Zhou XY (2017a) Blockade of high-mobility group box 1 attenuates intestinal mucosal barrier dysfunction in experimental acute pancreatitis. Sci Rep 7(1):6799
Chai NL, Fu Q, Shi H, Cai CH, Wan J, Xu SP, Wu BY (2012) Oxymatrine liposome attenuates hepatic fibrosis via targeting hepatic stellate cells. World J Gastroenterol 18(31):4199–4206
Chen Q, Duan X, Fan H, Xu M, Tang Q, Zhang L, Shou Z, Liu X, Zuo D, Yang J, Deng S, Dong Y, Wu H, Liu Y, Nan Z (2017b) Oxymatrine protects against DSS-induced colitis via inhibiting the PI3K/AKT signaling pathway. Int Immunopharmacol 53:149–157
Chavarría-Velázquez CO, Torres-Martínez AC, Montaño LF, Rendón-Huerta EP (2018) TLR2 activation induced by H. pylori LPS promotes the differential expression of claudin-4, -6, -7 and -9 via either STAT3 and ERK1/2 in AGS cells. Immunobiology 223(1):38–48
De Souza WF, Fortunato-Miranda N, Robbs BK, de Araujo WM, de-Freitas-Junior JC, Bastos LG (2013) Claudin-3 overexpression increases the malignant potential of colorectal cancer cells: roles of ERK1/2 and PI3K-Akt as modulators of EGFR signaling. PLoS One 8(9):e74994
Guzman JR, Koo JS, Goldsmith JR, Mühlbauer M, Narula A, Jobin C (2013) Oxymatrine prevents NF-κB nuclear translocation and ameliorates acute intestinal inflammation. Sci Rep 3:1629
Gross CM, Kellner M, Wang T, Lu Q, Sun X, Zemskov EA, Noonepalle S, Kangath A, Kumar S, Gonzalez-Garay M, Desai AA, Aggarwal S, Gorshkov B, Klinger C, Verin AD, Catravas JD, Jacobson JR, Yuan JX, Rafikov R, Garcia JGN, Black SM (2017) LPS Induced Acute Lung Injury Involves the NF-κB-mediated Downregulation of SOX18. Am J Respir Cell Mol Biol 58(5):614–624
Günzel D, Yu AS (2013) Claudins and the modulation of tight junction permeability. Physiol Rev 93(2):525–569
Jobin C, Haskill S, Mayer L, Panja A, Sartor RB (1997) Evidence for altered regulation of I kappa B alpha degradation in human colonic epithelial cells. J Immunol 158(1):226–234
Fan H, Chen R, Shen L, Lv J, Xiong P, Shou Z, Zhuang X (2008) Oxymatrine improves TNBS-induced colitis in rats by inhibiting the expression of NF-kappaB p65. J Huazhong Univ Sci Technol Med Sci 28(4):415–420
Howarth GS, Francis GL, Cool JC, Xu X, Byard RW, Read LC (1996) Milk growth factors enriched from cheese whey ameliorate intestinal damage by methotrexate when administered orally to rats. J Nutr 126(10):2519–2530
Hong-Li S, Lei L, Lei S, Dan Z, De-Li D, Guo-Fen Q, Yan L, Wen-Feng C, Bao-Feng Y (2008) Cardioprotective effects and underlying mechanisms of oxymatrine against Ischemic myocardial injuries of rats. Phytother Res 22(7):985–989
Hussain AR, Ahmed SO, Ahmed M, Khan OS, Al Abdulmohsen S, Platanias LC, Al-Kuraya KS, Uddin S (2012) Cross-talk between NFkB and the PI3-kinase/AKT pathway can be targeted in primary effusion lymphoma (PEL) cell lines for efficient apoptosis. PLoS One 7(6):e39945.
Han J, Chen D, Liu D, Zhu Y (2017) Modafinil attenuates inflammation via inhibiting Akt/NF-κB pathway in apoE-deficient mouse model of atherosclerosis. Inflammopharmacology 26(2):385–393
Krug SM, Schulzke JD, Fromm M (2014) Tight junction, selective permeability, and related diseases. Semin Cell Dev Biol 36:166–176
Liang JX, Qu XF, Zeng WT, Zhu KL, Zhang H, Wei JJ (2010) Mechanism of oxymatrine in preventing hepatic fibrosis formation in patients with chronic hepatitis B. Nan Fang Yi Ke Da Xue Xue Bao 30(8):1871–1873
Li W, Yu X, Tan S, Liu W, Zhou L, Liu H (2017) Oxymatrine inhibits non-small cell lung cancer via suppression of EGFR signaling pathway. Cancer Med 7(1):208–218
Lu M, Zhang Q, Chen K, Xu W, Xiang X, Xia S (2017) The regulatory effect of oxymatrine on the TLR4/MyD88/NF-κB signaling pathway in lipopolysaccharide-induced MS1 cells. Phytomedicine 36:153–159
Mayer J, Rau B, Gansauge F, Beger HG (2000) Inflammatory mediators in human acute pancreatitis: clinical and pathophysiological implications. Gut 47(4):546–552
Meriläinen S, Mäkelä J, Koivukangas V, Jensen HA, Rimpiläinen E, Yannopoulos F, Mäkelä T, Alestalo K, Vakkala M, Koskenkari J, Ohtonen P, Koskela M, Lehenkari P, Karttunen T, Juvonen T (2012) Intestinal bacterial translocation and tight junction structure in acute porcine pancreatitis. Hepatogastroenterology 59(114):599–606
Ma ZJ, Li Q, Wang JB, Zhao YL, Zhong YW, Bai YF, Wang RL, Li JY, Yang HY, Zeng LN, Pu SB, Liu FF, Xiao DK, Xia XH, Xiao XH (2013) Combining oxymatrine or matrine with lamivudine increased its antireplication effect against the hepatitis B virus in vitro. Evid Based Complement Alternat Med https://doi.org/10.1155/2013/186573
Norman J (1998) The role of cytokines in the pathogenesis of acute pancreatitis. Am J Surg 175(1):76–83
Oshima T, Miwa H, Joh T (2008) Changes in the expression of claudins in active ulcerative colitis. J Gastroenterol Hepatol https://doi.org/10.1111/j.1440-1746
Poritz LS, Harris LR, Kelly AA, Koltun WA (2011) Increase in the tight junction proteinclaudin-1 in intestinal inflammation. Dig Dis Sci 56:2802–2809
Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, Collins JE (2005) Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest 85:1139–1162
Santos AC, Correia CA, de Oliveira DC, Nogueira-Pedro A, Borelli P, Fock RA (2016) Intravenous glutamine administration modulates TNF-α/IL-10 ratio and attenuates NFkB phosphorylation in a protein malnutrition model. Inflammation 39(6):1883–1891
Thuijls G, Derikx JP, de Haan JJ, Grootjans J, de Bruine A, Masclee AA, Heineman E, Buurman WA (2010) Urine-based detection of intestinal tight junction loss. J Clin Gastroenterol 44:e14–e19
Wen JB, Zhu FQ, Chen WG, Jiang LP, Chen J, Hu ZP, Huang YJ, Zhou ZW, Wang GL, Lin H, Zhou SF (2014) Oxymatrine improves intestinal epithelial barrier function involving NF-κB-mediated signaling pathway in CCl4-induced cirrhotic rats. PLoS One 9(8):e106082
Wang W, Deng M, Liu X, Ai W, Tang Q, Hu J (2011) TLR4 activation induces nontolerant inflammatory response in endothelial cells. Inflammation 34(6):509–518
Visigalli R, Barilli A, Parolari A, Sala R, Rotoli BM, Bussolati O, Gazzola GC, Dall’Asta V (2010) Regulation of arginine transport and metabolism by protein kinase Calpha in endothelial cells: stimulation of CAT2 transporters and arginase activity. J Mol Cell Cardiol 49(2):260–267
Weber CR, Nalle SC, Tretiakova M, Rubin DT, Turner JR (2008) Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab Invest 88:1110–1120
Yao N, Wang X (2014) In vitro immunomodulatory activity of oxymatrine on Toll-like receptor 9 signal pathway in chronic hepatitis B. Am J Chin Med 42(6):1399–1410
Yılmaz EE, Bozdağ Z, Ibiloğlu I, Arıkanoğlu Z, Yazgan ÜC, Kaplan I, Gümüş M, Atamanalp SS (2016) Therapeutic effects of ellagic acid on L-arginin induced acute pancreatitis. Acta Cir Bras 31(6):396–401
Zhang Z, Wang Y, Dong M, Cui J, Rong D, Dong Q (2012) Oxymatrine ameliorates L-arginine-induced acute pancreatitis in rats. Inflammation 35(2):605–613
Zhao J, Yu S, Tong L, Zhang F, Jiang X, Pan S, Jiang H, Sun X (2008) Oxymatrine attenuates intestinal ischemia/reperfusion injury in rats. Surg Today 38(10):931–937
Acknowledgements
We thank for the Central Laboratory and General Laboratory of the First Hospital of China Medical University for technical supports. This work was supported by Natural Science Foundation from Liaoning (20170540540) and by the science and technology program from Shenyang (population and health special project, 17-230-9-36).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Zang, H., Zhang, Z., Liu, Q. et al. Oxymatrine improves L-arginine-induced acute pancreatitis related intestinal injury via regulating AKT/NFkB and claudins signaling. Med Chem Res 28, 116–124 (2019). https://doi.org/10.1007/s00044-018-2269-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-018-2269-7