Abstract
Oligodeoxynucleotides (ODN) with CpG motifs (CpG ODN) induce T helper (Th)1-type reaction. We aimed to evaluate the therapeutic effect of CpG ODN in the development of late allergic rhinitis induced by ovalbumin (OVA), which is one of Th2 diseaes, in BALB/c mice. Effects of a single dose of synthetic CpG-ODN (50 μg) intraperitoneally (i.p.) at the priming phase (on day 0) by OVA on the development of late eosinophilic rhinitis at respiratory areas were compared to the control mice treated with its vehicle (ODN without CpG motifs; 50 μg). Animals were again sensitized by OVA (on day 10) i.p., and 4 days after second sensitization animals were challenged by OVA intranasally (on day 14). Four days after challenge, eosinophilic reactions, nasal lesions and local cytokine values were examined. Compared to the control group, the CpG ODN-administration increased production of OVA-specific Th1 cytokine (interferon-γ) and decreased productions of ovalubmin-specific Th2 cytokines [interleukin (IL)-5 and IL-13] in nasal cavity fluids, supernatants of splenocytes and/or sera. Also, eosinophilia and increased total IgE values were decreased in mice treated with the CpG ODN compared to the control group. Moreover, nasal lesions with infiltration of eosinophils were prominently reduced by the CpG ODN-treatment compared to the control mice. The present study suggests that the systemic administration of CpG ODN at the priming phase may reduce local OVA-specific Th2 responses, resulting in decreased nasal pathology in the late allergic eosinophilic rhinitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Allergic airway diseases are prevailing in the word’s population and allergic rhinitis is one of the most common in allergic airway inflammation. According to hygiene hypothesis, declining exposure to infectious agents may increase the risks of suffering from allergic diseases [1, 2]. Allergic airway diseases may consist of an immediate phase, mediated mainly by IgE and mast cells [3], and a late phase, mediated mainly by CD4+ T-helper (Th2) cells and eosinophils in humans with airway allergy and animal models [4–10].
Late allergic rhinitis is a chronic disease associated with nasal inflammation, and edema of the submucosa in nasal and sinus passages, and those changes lead to nasal congestion, rhinorrhea, nasal blockage and reduced olfactory function in humans [11–15]. In addition, histologically, chronic allergic rhinitis is characterized by eosinophil and lymphocyte recruitment into nasal mucosae [16]. There are general agreements that Th2 cytokines, such as interleukin (IL)-4 [17], IL-5 [18] and IL-13 [19] and granulocyte-macrophage colony-stimulating factor [15], correlate with eosinophil infiltration and disease severity. Multiple lines of evidences suggest that allergen-specific CD4+ Th2 cells play an essential role in initiating and generating in immediate and late allergic rhinitis in human.
Bacterial DNA and certain oligodeoxynucleotides containing CpG motifs (CpG ODN), a 6-base DNA motif consisting of an unmethylated CpG dinucleotide flanked by two 5′ purines and two 3′ pyrimidines, have stimulatory effects on murine and human lymphocytes in vitro and murine lymphocytes in vivo [20–25]. For example, lymphocytes stimulated with CpG-ODN secrete interferon (IFN)-γ, IL-6, IL-10, and IL-12 and IL-18, which regulate IFN-γ production, are induced through activation of antigen-presenting cells including dendritic cells, macrophages and NK cells via Toll-like receptor [21, 24]. Thus, it appears that CpG ODN skew immune responses toward the Th1-type, which in turn may suppress allergic airway inflammation by mutual Th1/Th2 inhibitory effects [26]. Although, there are a lot of reports that CpG ODN inhibit the development of asthmatic response in mouse models [27–33], there are a few reports that administration of CpG ODN at the priming [34] and immunizing [35] phases suppress the development of allergic rhinosinusitis in a mouse model of allergic rhinitis. However, effects of CpG ODN on nasal lesions with local Th2 responses in a mouse model of the late allergic rhinitis remain largely unclear. Thus, the present study examined the effect of CpG ODN on the development of the late eosinophilic rhinitis induced by ovalbumin (OVA) in BALB/c mice.
MATERIALS AND METHODS
Animals
Specific pathogen-free, 8-week-old female BALB/c mice (total number of mice; n = 24, Japan Charles River Co., Yokohama, Japan) were used. They were kept at 25 ± 2°C room temperature, 55 ± 10% humidity, and a 12-h light/dark cycle (lighting time 08:00–20:00) and given autoclaved pellets (CE-2, Clea, Tokyo, Japan) and water at libitum. The number of mice used in each set of experiments is given in parenthesis. The animal experiments were approved by Research Ethics Board of the Faculty of Agriculture, Yamaguchi University.
CpG-ODN
The synthetic CpG ODN with two CpG motifs (5′-TCCATGACGTTCCTGACGTT-3′) and control ODN (5′-TCCAGGACTTTCCTCAGGTT-3′) without CpG motifs consisting of 20 bases respectively (endotoxin free and more than 99% purity; Takara Biotechnology, Shiga, Japan) were used as reported previously [36].
Experimental Protocols
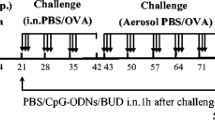
The mice were divided into following two groups. The sensitization and challenge procedures (Fig. 1) were done by the methods described previously [9, 37]. In brief, the animals were sensitized intraperitoneally (i.p.) by the injection of 10 μg of OVA (Grade V: Sigma Chem. Co., MO, USA) in 1.2 mg aluminium hydroxide (Alum.; SERVA, Heidelberg, Germany) adjuvant (OVA/Alum), which was suspended in 0.1 ml phosphate-buffered saline (PBS; pH 7.4), on day 0 and boosted i.p. with 10 μg OVA/Alum on day 10. Also, on day 0, either CpG-ODN (50 μg, n = 14) or control ODN (50 μg, n = 10) were administrated i.p. The injected dose of CpG ODN was determined basically by body weight according to our previous experiments [9, 37]. Four days after the second sensitization (on day 14), the mice were challenged intranasally (both right and left nasal cavities by pipets alternately) with 200 μg of OVA in 50 μl of PBS under anesthesia with ketamine (45 mg/kg BW; Sankyo Co., Tokyo, Japan) and xylazine (8 mg/kg body weight; Bayer Co., Tokyo, Japan) i.p. Four days after the challenge (on day 18), the mice were anesthetized i.p. with over doses of chroroform, and a whole blood was obtained by heart puncture using heparinized syringe. All assays were done on day 18, unless otherwise stated.
Experimental protocol. Animals were sensitized i.p. with 10 μg of OVA mixed with aluminium hydroxide on day 0 and on day 10. On day 0, CpG-ODN (50 μg) and control ODN (50 μg) were administrated i.p. 14 days after the first sensitization, mice were challenged with 200 μg of OVA intranasally. Four days after the challenge (on day 18) all assays were done, unless otherwise stated.
Total Number of Eosinophils, Lymphocytes, Monocytes and Neutrophils in Blood
The absolute number of each leucocyte/microliter per mouse (n = 8; control, n = 14; CpG ODN) was counted, and then the percentage was calculated after blood smear stained with Giemsa solution. Then, the total number of each leucocyte was determined.
Histologic Evaluation of Nasal Lesions
Heads (n = 6; control, n = 10; CpG ODN) were fixed in 10% neutral buffered formalin (pH 7.0) for 2 days, and those were decalcificated in 1 mM Tris–HCl (pH 7.4) with 20% EDTA for 5 days. Nasal region was sliced at the anterior margin of orbit transversely. Figure 2a shows the right side of transverse section. Areas of rectangles (S; lower portion of the nasal septum, F; the floor, L; the lower lateral walls) of respiratory epithelium were examined, since it has been reported that allergic inflammation was mainly localized in respiratory epithelium of these parts in a murine allergic rhinitis model [38, 39]. Paraffin sections (4 μm thickness) were stained with hematoxylin and eosin, alcian blue (AB) and phosphotungstic acid hematoxylin.
Schematic figure of transverse section of nasal region at anterior margin of orbit (right half side; a). Rectangle shows lower portion of the nasal septum (S) and arrowed areas indicate distribution of respiratory epithelium (F; the floor and L; the lower lateral walls), which are the main target cells in this model. (b) The area, which is examined in Fig. 6.
For semiquantitative analysis, the severity and the range of pathologic changes (score index; degree of infiltration of inflammatory cell) per mouse in septal respiratory areas (Fig. 2b; rectangle area of S in Fig. 2a) were scored on 0–4 scale as follows; none (0), weak and partial (1), weak and diffuse (2), moderate and partial (3) and moderate and diffuse (4). Also in the part of Fig. 2b, the thickness of turbinate nasal mucosa (from the top of cilia to the near edge of septal turbinate bone) or epithelium (from the top of cilia to basement membrane) respectively (5 different parts/mouse), and the total number of eosinophil stained with May–Grünwald’s–Giemsa were counted by an eyepiece with a grid, and the number of goblet cells or non-goblet epithelium including ciliated and basal cells were calculated in sections stained with AB. The average of each cell type was calculated by two observers blindly.
Collection of Nasal Cavity Fluids
Nasal cavity fluids (NF) (n = 4 in each group) were collected by the method described previously [40]. After euthanasia, the mouse trachea at the upper level was ligated and then a catheter was guided into the nasopharynx. Then, the nasal passages were gently perfused with 1 ml of cold PBS and collected in a petori dish. NF were centrifuged at 800×g for 10 min at 4°C to separate cells and supernatants.
Isolation of Cervical Lymph Node Cells and Splenic Cells
Splenic and cervical lymph node (LN) (both right and left sides) cells, which respond to nasal inflammation [40], were isolated by the methods described previously [41]. In brief, spleen and lymphnode were aseptically removed and placed in phosphate buffered-balanced salt solution (PBBS; pH 7.4). Single-cell suspension was made by teasing spleens apart with scissors and filtering through a 250-μm disposable syringe (Terumo Co., Tokyo, Japan). Cell suspensions were collected in sterile conical tubes (Assist Trading Co., Tokyo, Japan) after red blood cells were hemolysed by Tris-buffered NH4Cl solution, and washed in PBBS containing 0.1% heat-inactivated fetal calf serum (Gibco, Grand Island, NY, USA) followed by centrifugation at 800×g for 10 min at 4°C. Cells were counted using a hemocytometer and diluted in Dulbecco’s modified Eagles’s medium (DMEM; KC biological, Lenexa, Kans, USA) to a density of 1 × 106 cells/ml. Viability of cells was more than 95% by trypan blue dye exclusion test. LN cells or splenocytes (2 × 105 cells in 200 μl DMEM with FCS, 100 U/ml penicillin and 100 μg/ml streptomycin) mixed with or without OVA (20 μg) were cultured for 48 h at 37°C in a 5% atmosphere and supernatants were obtained.
Measurement of IgE, IL-5, IL-13 and IFN-γ in NF, Cultured LN Cells and Splenic Cells, and/or Blood by ELISA
IL-5 (R & D systems, MN, USA), IL-13 (R & D systems, MN, USA) and IFN-γ (Techre Co., MN, USA) (n = 5 in each cytokine per each group) and IgE (Morinaga Co., Kanagawa, Japan) (n = 10; control, n = 14; CpG ODN) mouse ELISA kits were purchased. The minimal detectable concentration was 2 pg/ml for IL-5, 1.5 pg/ml for IL-13 and 0.7 pg/ml for IFN-γ, and 500 pg/ml for IgE. In addition, total serum IgE values before starting the experiments (day 0) also examined (n = 10; control, n = 14; CpG ODN). In case of cytokines could not be detected, their concentration was estimated as 0.
Statistical Analysis
The data are expressed as the mean of samples examined ±SEM. Unpaired Student’s t-test was used to evaluate the significance of differences; a P-value less than 0.05 was considered significant.
RESULTS
Effects of CpG ODN on the Number of Blood Leucocytes on Day 18
Compared to the control group, the number of blood eosinophils (Fig. 3a) including lymphocytes (Fig. 3b), monocytes (Fig. 3c) and neutrophils (Fig. 3d) was decreased by the administration of CpG ODN (P < 0.05).
Effects of CpG ODN on IgE and IL-13 Values in Sera on Days 0 and 18
IgE (Fig. 4a) and IL-13 (Fig. 4b) values decreased in the mice administrated with the CpG ODN compared to those in the control mice (P < 0.05). In addition, both groups increased IgE values on day 18 compared to those on day 0. IL-5 and IFN-γ in the blood were not detected in both groups (data not shown).
Reduction in serum IgE (a) and IL-13 (b) values by CpG ODN-treatment. Serum IgE values increased by the induction of rhinitis (on day 18) compared to the first priming time (on day 0) in both groups. Alao, serum IgE and IL-13 concentrations in CpG ODN-treated group were reduced compared to those in control group on day 18. Data shown are mean ± SEM. Asterisk P < 0.05 between two groups.
Effects of CpG ODN on IL-13, IL-5 and IFN-γ Values in NF, and Cervical LN Cells and Splenic Cells on Day 18
In the CpG ODN-adminstrated mice compared to the control group, productions of IL-13 (Fig. 5a; P < 0.05) and IL-5 (Fig. 5b; P < 0.05) were decreased, and IFN-γ (Fig. 5c) was slightly increased in NF. In the mice treated with the CpG ODN there was no production of OVA-specific and -nonspecific IL-13 (Fig. 5d) and IL-5 (Fig. 5e), whereas OVA-specific and -nonspecific IL-13 productions and also OVA-specific, but not -nonspecific, IL-5 production were observed in the control group from LN cells. On the other hand, there was no production of IFN-γ (Fig. 5f) in the control mice, whereas there was a low value of OVA-specific and -nonspecific IFN-γ production in the CpG ODN-treated mice (Fig. 5f) from LN cells. Production of OVA-specific IL-13 in the CpG ODN-treated mice was reduced compared to the control mice (Fig. 5g; P < 0.05), and OVA-nonspecific IL-13 production was not found in the two groups (Fig. 5g) in the supernatants of splenic cells. Also OVA-specific IL-5 production was reduced in the CpG ODN-treated mice compared to the control group (Fig. 5h; P < 0.05) and although a small amount of OVA-nonspecific IL-5 (Fig. 5h) production was detected in the control group, there was no non-specific IL-5 production in the CpG ODN-treated group from splenic cells (Fig. 5h). Both of OVA-specific and OVA-nonspecific IFN-γ (Fig. 5i; P < 0.05) productions from splenic cells were increased in the CpG ODN-administrated group compared to the control group.
OVA-specific and -nonspecific cytokine productions in NF, LN cells and splenic cells by CpG ODN-treatment on day 18. Productions of IL-13 (a, d and g), IL-5 (b, e and h) and IFN-γ (c, f and i) in NF (a, b and c), LN (d, e and f) and splenic (g, h and i) cells. OVA−; cultured cells without ovalubumin, OVA+; cultured cells with ovalubumin. In case of cytokines could not be detected, their concentration was estimated as 0. Each value represents the mean ± SEM. Asterisk P < 0.05.
Effects of CpG ODN on the Development of Eosinophilic Rhinitis on Day 18
Compared to the control group, the CpG ODN-administration resulted in decrease in score index (severity of nasal lesion) (Fig. 6a), thickness of nasal mucosa (Fig. 6b) and epithelial layer (Fig. 6c), number of eosinophil infiltrated (Fig. 6d), and number of goblet cells (Fig. 6e) and non-goblet cells (Fig. 6f) (P < 0.05). As shown in Fig. 7, inflammatory lesions were located mainly in mucosa of the areas of respiratory epithelium, but not of olfactory epithelium, at floor near by nasal-associated lymphoid tissue, at septum areas and lateral sides of turbinate. Inflammatory cells reached to the edge of olfactory epithelium contiguous to the respiratory region in the control group. In the control group, epithelial cells in inflamed areas showed hyperplasia, hypertrophy, vacuolar degeneration and widening of epithelial space. Some eosinophils infiltrated into the epithelial layer, leading to the destruction of epithelium (Fig. 7a). Hyperplasia of non-goblet epithelium was often associated with infiltration of eonsinophils and lymphocytes in lamina propria. In some cases, exudations of mucus and cell debris into nasal cavity, and hemorrhage and/or slight fibrosis in lamina propria were found. Congestion and edema were prominent in lamina propria. There were no thickness of basement membrane and hyperplasia of nasal glands. Adhesion of inflammatory cells on the endothelial cells of dilated sinus venous was often found in the area of respiratory epithelium. These lesions were less severe in the CpG ODN-treated group (Fig. 7c), and no lesions in olfactorium epthelium were seen in both groups (Fig. 7b,d).
CpG-ODN-treatment reduces the development of rhinitis. Score index (degree of inflammatory cell infiltration) of lesions (a), thickness of nasal mucosa (b), thickness of epithelium (c), and numbers of eosinophils (d), mucus cells (e) and non-mucus (ciliated and basal) cells in nasal mucosa in septum areas. Each value represents the mean ± SEM. Asterisk P < 0.05.
Representative histologic changes on day 18 in septal respiratory areas of nasal mucosa in a control (a; a long arrow indicates infiltration of eosinophil into epithelial layer) and a CpG ODN-administrated (c) mice. There is no change in olfactorium epithelium (insert; b and d) in both groups. Hematoxylin and eosin. Bar = 50 μm.
As shown in Fig. 8, in the control group hyperplastic and hypertrophic goblet cells having much mucus were recognized (Fig. 8a). Among hyperplastic goblet cells, some had cilia (Fig. 8b,c). Those changes were less prominent and cytoplasmic mucus substance was small in the CpG ODN-administrated group (Fig. 8d). Some cases in both groups had hyaline droplets in goblet and/or ciliated cells. In addition, there was no increase in mast cell infiltration in both groups.
In the control group, increased goblet cells (a; arrows), and goblet cells without cilia (b; arrows) and goblet cells with cilia [c; arrows and a long arrow indicates basal bodies of cillia stained with phosphotungstic acid hematoxylin (PTAH)] are visible. In a CpG ODN-administrated mouse shows decreased number of goblet cells (d; arrows) and size of mucus is small compared to the control group. a; Hematoxylin and eosin, b, c and d; PTAH. a, c and d; the floor of respiratory area near by lymphoid follicles. b; the floor of respiratory area. Bar = 50 μm.
DISCUSSION
The present study clearly demonstrated that systemic administration of CpG ODN at the time of priming reduced the development of late eosinophilic rhinitis associated with reduced OVA-specific Th2 (e.g., IL-5 and IL-13) responses and increased OVA-specific IFN-γ (representative Th1 cytokine) productions systemically and/or locally. Reduced serum IgE values by the treatment of CpG ODN may not play a role in decreased inflammatory reaction of nasal areas, since there was no increased infiltration of mast cells in mice with or without the CpG ODN-treatment. Collectively, the treatment of CpG ODN at the priming phase may sift the imbalance toward the naive Th0 to Th1 type immunity, resulting in the suppression of the development of Th2 immunity by mutual Th1/Th2 inhibitory effects [26]. On the other hand, although there were no detectable amounts of IL-5 and IFN-γ in the blood in both groups, it may be due to reflection of their short half-life. For example, a half-life in the circulation of IFN-γ in mice was 24.37 min [42], and that of IL-5 mRNA-expression was 2.6 h in human T cell line [43].
Among Th2 cytokines, it has been suggested that IL-5 is the principal cytokine for eosinophil maturation, differentiation and survival [15]. Also, IL-13 [19] may contribute infiltration of lymphocytes and eosinophils, goblet cell hypertrophy with mucus hepersecretion and subepithelial basement membrane thickening, which contains types I, III, and V collagen [11], in lower airway allergic inflammation. If that is the case, IL-5 and IL-13 may also operate to develop rhinitis in upper airway. It seems likely, since decreased productions of OVA-specific IL-5 and IL-13 were found in nasal fluids and/or supernatants of cervical LN cells in the CpG ODN-administrated mice, this may be responsible for the decreased infiltration of eosinophils into nasal areas. Also, the present study demonstrated destruction of respiratory epithelium contacted with eosinophils, which may be due to released cytotoxic substances from activated eosinophils (e.g., eosinophil peroxidase etc.) [44, 45]. Thus, decreased eosinophil infiltration may cause less severe epithelial cell damage in the CpG ODN-administrated group. On the other hand, thickening of basement membrane, hyperplasia of nasal glands in inflamed nasal areas and inflammation in the areas of olfactory epithelium in human patients with allergic rhinitis [46] were not found in the present mouse model. Although, we have no explanation for the discrepancy of these histologic changes between human and animal, it must be a property of the species, including anatomical and physiological differences [38].
The present study demonstrated that intensity of inflammation and increased goblet cell hyperplasia in nasal areas in the control mice were decreased by the treatment of CpG ODN. Hyperplasia of ciliated epithelium and goblet cells are common consequences of respiratory inflammation and considered to be protective reaction against inhalted antigens, and perennial allergic rhinitis in human is clinically associated with a significant increase in nasal mucus secreted by submucosal glands and epithelial cells [47]. Also, it has been reported that mucus discharge from the inferior turbinate goblet cells of patients with perennial allergic rhinitis was enhanced by nonhyperplastic increase of nasal goblet cell with an increased functional activity [48] and that goblet cell hyperplasia was seen in human patients with perennial allergic rhinitis [49]. In the present study, hyperplastic goblet cells of the control mice had much mucus in their cytoplasm compared to the CpG ODN-administrated mice, suggesting that both hyperfunction and hyperplasia of goblet cells may occur as the result of the development of rhinitis. In addition, some ciliated cells had mucus droplets, suggesting that they may, at least in part, be derived from ciliated epithelium. Decreased local production of IL-13 by the treatment of CpG ODN may be associated with decreased goblet cell hyperplasia compared to the control group. It seems likely, since it has been reported that IL-13 promotes transdifferentiation from ciliated to goblet cells in lower airway (lung) inflammation in human patients with asthma and animal models with respiratory virus infection [50]. Also, intranasal instillation of OVA-induced metaplastic changes of goblet cells of nasal epithelium of OVA-sensitized rat has been reported [51]. However, it has been suggested that there was no difference in the number of goblet cell between IL-13-deficient-mice and wild type mice, and that goblet cell metaplasia in airway may be induced by different mechanisms, depending on the stimuli [52]. Thus, further study is needed to clarify the mechanisms of decreased goblet cell metaplasia and hyperplasia in rhinitis by the treatment of CpG ODN.
Treatments for allergic airway diseases are mainly focused on the suppression of symptoms. Antihistamine leads to temporary relief [53], and corticosteroids lead to short-term reduction in nasal inflammation [32]. On the other hand, it has been suggested that a treatment of CpG ODN inhibits allergic lung inflammation in presensitized animals for a prolonged period [27]. Also, it has been suggested that CpG ODN may have potential for treatment of an on going allergic asthma by suppressing Th2 responses during IgE-dependent allergic airway reaction and that CpG ODN can reverse Th2-associated allergic lower airway responses [28]. Moreover, it has been reported that immediate and late allergic rhinitis symptoms in previously OVA-sensitized mice were protected by CpG ODN, suggesting availability of CpG ODN on ongoing allergic rhinitis [34] and that CpG ODN conjugated to antigen effectively prevented the development of antigen specific lower airway inflammation for long times [29]. However, these conjugated compounds may allow the antigen to act as a hapten and lead to anti-DNA antibody formation [30]. Thus, alteration from Th2 dominance to Th1 dominance in allergic rhinitis by administration of CpG ODN rather than CpG ODN conjugated antigens may be applicable for one of theraphy in allergic airway inflammation including the late allergic rhinitis, although antigen-specific allergen immunotherapy against grass-pollen was shown to be very effective and has longer lasting effects in human [53, 54].
In conclusion, the present study suggested that reduced Th2 cytokine production may be responsible for the reduced late allergic rhinitis by the systemic treatment of CpG ODN. The alteration of Th2/Th1 balance will be most rewarding when treating allergic rhinitis. The administration of the CpG ODN suggests that this may have a potential to reduce Th2 response in several types of allergens in human, although further study is needed to clarify the effective dose curve of CpG ODN on late allergic rhinitis. In addition, although we have reported previously that IFN-γ expressing plasmid DNA reduced allergic lower airway inflammation [55], therapeutic application of CpG ODN in the late eosinophilic rhinitis may be advantageous, since it is safe, cheap, stable, easy to make and effective by different routes of administration [56].
References
Strachan, D. P. 1989. Hay fever, hygiene and hosehold size. BMJ 299:1259–1260.
Von Hertzen, L. C., and T. Haahtela. 2004. Asthma and atopy–the price of affluence? Allergy 59:124–137.
Galli, S. J. 1997. Complexity and redundancy in the pathogenesis of asthma: Reassessing the roles of mast cells and T cells. J. Exp. Med. 186:343–347.
O’Byrne, P. M., J. Dolovich, and F. E. Hargreave. 1987. Late asthmatic responses. Am. Rev. Respir. Dis. 136:740–751.
Lei, H.-Y., K.-J. Huang, C.-L. Shen, and J.-L. Huang. 1989. An antigen-specific hypersensitivity which does not fit into traditional classification of hypersensitivity. J. Immunol. 143:432–438.
Corrigan, C. J., Q. Hamid, J. North, J. Barkans, R. Moqbel, S. Durham, V. Gemou-Engesaeth, and A. B. Kay. 1995. Peripheral blood CD4 but not CD8 T-lymphocytes in patients with exacerbation of asthma transcribe and translate messenger RNA encoding cytokines which prolong eosinophil survival in the context of a Th2-type pattern: Effect of glucocorticoid therapy. Am. J. Respir. Cell Mol. Biol. 12:567–578.
Kosgren, M., J. S. Erjefalt, O. Korsgren, F. Sundler, and C. G. Persson. 1997. Allergic eosinophil-rich inflammation develops in lungs and airways of B cell-deficient mice. J. Exp. Med. 185:885–893.
Takeda, K., E. Hamelmann, A. Joetham, L. D. Shultz, G. L. Larsen, C. G. Irvin, and E. W. Gelfand. 1997. Development of eosinophilic airway inflammation and airway hyperresponsiveness in mast cell-deficient mice. J. Exp. Med. 186:449–454.
Hayashi, T., Y. Adachi, K. Hasegawa, and M. Morimoto. 2003. Less sensitivity for late airway inflammation in males than females in BALB/c mice. Scand. J. Immunol. 57:562–567.
Hayashi, T., A. Ishii, S. Nakai, and K. Hasegawa. 2004. Ultrastructure of goblet-cell metaplasia from clara cell in the allergic asthmatic airway inflammation in a mouse model of asthma in vivo. Virchows Arch. 444:66–73.
Roche, W. R., J. H. Williams, R. Beasley, and S. T. Holgate. 1989. Subepithelial fibrosis in the bronchi of asthmatics. Lancet 1:520–524.
Klimek, L., and G. Eggers. 1997. Olfactory dysfunction in allergic rhinitis is related to nasal eosinophilic inflammation. J. Allergy Clin. Immunol. 100:158–164.
Moll, B., L. Klimek, G. Eggers, and W. Mann. 1998. Comparison of olfactory function in patients with seasonal and perennial allergic rhinitis. Allergy 53:297–301.
Apter, A. J., J. F. Gent, and M. E. Frank. 1999. Fluctuating olfactory sensitivity and distorted odor perception in allergic rhinitis. Arch. Otolaryngol. Head Neck Surg. 125:1005–1010.
Baraniuk, J. N. 2001. Mechanisms of allergic rhinitis. Curr. Allergy Asthma Rep. 1:207–217.
Varney, V. A., M. R. Jacobson, R. M. Sudderic, D. S. Robinson, A. M. Irani, B. Schwartz, I. Macskay, A. B. Kay, and S. R. Durham. 1992. Immunohistology of the nasal mucosa following allergen-induced rhinitis. Identification of activated T lymphocytes, eosinophils, and neutrophils. Am. Rev. Respir. Dis. 146:170–176.
Coyle, A. J., G. Le Gros, C. Bertrand, S. Tsuyuki, C. H. Heusser, M. Kope, and G. P. Anderson. 1995. Interleukin-4 is required for the induction of lung Th2 mucosal immunity. Am. J. Respir. Cell Mol. Biol. 13:54–59.
Kaneko, M., Y. Hitoshi, K. Takatsu, and S. Matsumoto. 1991. Role of interleukin-5 in local accumulation of eosinophils in mouse allergic peritonitis. Int. Arch. Allergy Appl. Immunol. 96:41–45.
Zhu, Z., R. J. Horner, Z. Wang, Q. Chen, G. P. Geba, J. Wang, Y. Zhang, and J. A. Elias. 1999. Pulmonary expression of interleukin-13 cause inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities and eotaxin production. J. Clin. Invest. 103:779–788.
Klinman, D. M., A. K. Yi, S. L. Beaucage, J. Comover, and A. M. Krieg. 1996. CpG motifs present in bacterial DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon γ. Proc. Natl. Acad. Sci. U. S. A. 93:2879–2883.
Pisetsky, D. S. 1996. Immune activation by bacterial DNA: A new genetic code. A review. Immunity 5:303–310.
Jacob, T., P. S. Walker, A. M. Krieg., M. C. Udey, and J. C. Vogel. 1998. Activation of cutaneous dendritic cells by CpG-containing oligodeoxynucleotides: a role for dendritic cells in the augmentation of Th1 responses by immunostimulatory DNA. J. Immunol. 161:3042–3049.
Sparwasser, T., E. S. Koch, R. M. Vabulas, K. Heeg, G. B. Lipfpord, J. W. Ellwart, and H. Wagner. 1998. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur. J. Immunol. 28:2045–2054.
Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. Toll-like receptor recognizes bacterial DNA. Nature 408:740–745.
Kranzer, K., M. Bauer, G. B. Lipford, K. Heeg, H. Wagner, and R. Lang. 2000. CpG-oligodeoxynucleotides enhance T-cell receptor-triggered interferon-gamma production and up-regulation of CD69 via induction of antigen-presenting cell-derived interferon type and interleukin-12. Immunology 99:170–178.
Mosmann, T. R., and R. L. Coffman. 1989. Th1 and Th2 cells: different patterns of lymphokine secretion leads to different functional patterns. Annu. Rev. Immunol. 7:145–173.
Broide, D., J. Schwarze, H. Tighe, T. Gifford, M. D. Nguyen, S. Malek, J. van Uden, O. Martin, E. W. Gelfand, and E. Raz. 1998. Immunostimulatory DNA sequences inhibit IL-5, eosinophilic inflammation, and airway hyperresponsiveness in mice. J. Immunol. 161:7054–7062.
Sur, S., L. S. Wild, B. K. Choudhury, N. Sur, R. Alam, and D. M. Klinman. 1999. Long term prevention of allergic lung inflammation in a mouse model of asthma by CpG Oligodeoxynucleotides. J. Immunol. 162:6284–6293.
Serebrisky, D., A. A. Teper, C.-K. Huang, S.-Y. Lee, T. F. Zhang, B. H. Schofield, M. Kattan, H. A. Sampson, and X.-M. Li. 2000. CpG oligodeoxynucleotides can reverse Th2-associated allergic airway responses and alter the B.7/B7.2 expression in a murine model of asthma. J. Immunol. 165:5906–5912.
Shirota, H., K. Sano, T. Kikuchi, G. Tamura, and K. Shirota. 2000. Regulation of murine airway eosinophilia and Th2 cells by antigen-conjugated CpG oligodeoxynucleotides as a novel antigen-specific immunomodulator. J. Immunol. 164:5575–5582.
Kline, J. N., K. Kitagaki, T. R. Businga, and V. V. Jain. 2002. Treatment of established asthma in a murine model using CpG olygodeoxynucleotides. Am. J. Physiol. Lung Cell Mol. Physiol. 283:170–179.
Ikeda, R. K., J. Nayar, J. Y. Cho, M. Miller, M. Rodriguez, E. Raz, and D. H. Broide. 2003. Resolution of airway inflammation following ovalubumin inhaltation: Comparison of ISS DNA and corticosteroids. Am. J. Respir. Cell Mol. Biol. 28:655–663.
Hessel, E. M., M. Chu, J. O. Lizcano, B. Chang, N. Herman, S. A. Kell, M. Willskarp, and R. L. Coffman. 2005. Immunostimulatory oligonucleotides block allergic airway inflammation and IgE-mediated cytokine induction. J. Exp. Med. 202:1563–1573.
Hussain, I., V. Jain, K. Kitagaki, T. R. Businga, P. O’Shaughnessy, and J. N. Kline. 2002. Modulation of murine allergic rhinosinusitis by CpG oligodeoxynucleotides. Laryngoscope 112:1819–1826.
Rhee, C.-S., L. Libet, D. Chisholm, K. Takabayashi, S. Baird, T. D. Bigby, C. H. Lee, A. A. Horner, and E. Raz. 2004. Allergen-independent immunostimulatory sequence oligodeoxynucleotide therapy attenuates experimental allergic rhinitis. Immunology 113:106–113.
Hasegawa, K., and T. Hayashi. 2003. CpG oligodeoxynucleotides accelerate the development of lupus nephritis during preactive phase in NZB × NZWF1 mice. Lupus 12:1–8.
Harris, N., R. Peach, J. Naemura, P. S. Linsley, L. G. Gros, and F. Ronchese. 1997. CD80 costimulation is essential for the induction of airway eosinophilia. J. Exp. Med. 185:177–182.
Hussain, I., D. Randolph, S. L. Brody, S.-K. Song, A. Hsu, A. M. Kahn, D. D. Chaplin, and D. L. Hamilos. 2001. Induction, distribution and modulation of upper airway allergic inflammation in mice. Clin. Exp. Allergy 31:1048–1059.
Farraj, A. K., J. R. Harkema, and N. E. Kaminski. 2004. Allergic rhinitis induced by intranasal sensitization and challenge with trimellitic anhydride but not with dinitrochorobenzen or oxazolone in A/J mice. Toxicol. Sci. 79:315–325.
Yamada, T., S. Kataoka, K. Ogasawara, R. Ishimitsu, K. Hashiguchi, T. Suzuki, and H. Kawauchi. 2005. Mucosal immunity of nasopharynx: An experimental study in TCR-transgenic (OVA23-3) mice. Rhinology 43:190–198.
Hayashi, T., K. Hasegawa, Y. Sasaki, and T. Onodera. 2006. Elimination of CD4+ CD25+ T cell enhances Reo-2-triggered and CpG oligodeoxynucleotides-induced prolonged autoimmune insulitis in DBA/1 mice. Scand. J. Immunol. 163:116–124.
Rutenfranz, I., and H. Kirchner. 1988. Pharmacokinetics of recombinant murine-γ interferon in mice. J. Interf. Res. 8:573–580.
Hart, T. K., R. M. Cook, P. Zia-Amirhosseni, E. Minthorn, T. S. Sellers, B. E. Maleeff, S. Eustis, L. M. Schwartz, P. Tsui, E. R. Appelbaum, E. C. Martiu, P. J. Bugelski, and D. J. Herzyk. 2001. Preclinical efficacy and safety of mepolizumab (SB-240563), a humanized monoclonal antibody to IL-5, in cynomolgus monkeys. J. Allergy Clin. Immunol. 108:250–257.
Seminario, M.-C., and G. J. Gleich. 1994. The role of eosinophils in the pathogenesis of asthma. Curr. Opin. Immunol. 6:860–864.
Salib, R. J., A. Drake-Lee, and P. H. Howarth. 2003. Allergic rhinitis: past, present and the future. Clin. Otalaryngol. Allied Sci. 28:291–303.
Takamura, H. 1994. Immunohistochemical study of inferior turbinate of nasal allergy with reference to eosinophil. J. Otolaryngol. Jpn. 97:61–66.
Wihl, J. A., and N. Mygind. 1997. Studies on the allergen-challenged human nasal mucosa. Acta Oto-laryngol. 84:281–286.
Karlsson, G., and U. Pipkorn. 1989. Natural allergen exposure does not influence the density of goblet cells in the nasal mucosa of patients with seasonal allergic rhinitis. ORL J. Otorhinolaryngol. Relat. Spec. 51:171–174.
Berger, G., A. Morz, Z. Marom, and D. Ophir. 1999. Inferior turbinate goblet cell secretion in patients with perennial allergic and nonallergic rhinitis. Am. J. Rhinol. 13:473–477.
Tyner, J. W., E. Y. Kim, K. Ide, M. R. Pelletier, W. T. Roswit, J. D. Morton, J. T. Battaile, A. C. Patel, G. A. Patterson, M. Castro, M. S. Spoor, Y. You, S. L. Brody, and M. J. Horzman. 2006. Blocking airway mucus metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals. J. Clin. Invest. 116:309–321.
Shimizu, T., H. Hirano, Y. Majima, and Y. Sakakura. 2000. A mechanism of antigen-induced mucus production in nasal epithelium of sensitized rats. A comparison with lipopolysaccharide induced mucus production. Am. J. Respir. Crit. Care Med. 161:1648–1654.
Miyahara, S., N. Miyahara, S. Matsubara, K. Takeda, T. Koya, and E. W. Gelfand. 2006. IL-13 is essential to the late-phase response in allergic rhinitis. J. Allergy Clin. Immunol. 118:1110–1116.
Nelson, H. S. 2000. The use of standardized extracts in allergen immunotherapy. J. Allergy Clin. Immunol. 106:41–45.
Durham, S. R., S. M. Walker, E. M. Varga, M. R. Jacobson, F. O’Brien, W. Noble, S. J. Till, Q. A. Hamid, and K. T. Nouri-Aria. 1999. Long-term clinical efficacy of grass-pollen immunotherapy. N. Engl. J. Med. 104:1258–1264.
Hayashi, T., K. Maeda, K. Hasegawa, S. Nakai, T. Hamachi, and H. Iwata. 2002. Systemic administration of interferon-γ-expressing plasmid reduces late allergic bronchitis in a mouse model of asthma. Int. J. Exp. Pathol. 83:81–86.
Jahn-Schmid, B., U. Wiedermann, B. Bohle, A. Rapa, D. Kraft, and C. Ebner. 1999. Oligodeoxynucleotides containing CpG motifs modulate the allergic TH2 response of BALB/c mice to Bet vl, the major birch pollene allergy. J. Allergy Clin. Immunol. 104:1015–1023.
Acknowledgements
This study was supported in part by grant-in-aid of the ministry of Education, Science, Sports and Culture of Japan (No.17658142). Thanks are due to Dr. KL Bui for reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors wish it to be known, in their opinion, Toshiharu Hayashi and Keiko Hasegawa contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hayashi, T., Hasegawa, K. & Sasaki, Y. Systemic Administration of Olygodeoxynucleotides with CpG Motifs at Priming Phase Reduces Local Th2 Response and Late Allergic Rhinitis in BALB/c Mice. Inflammation 31, 47–56 (2008). https://doi.org/10.1007/s10753-007-9048-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-007-9048-9