Abstract
MHTP [2-methoxy-4-(7-methoxy-1,2,3,4-tetrahydroisoquinolin-1-yl) phenol], a synthetic isoquinolinic alkaloid, presented anti-inflammatory activity in several experimental models of acute inflammation as lipopolysaccharide (LPS)-induced acute lung injury and phlogistic agent-induced edema and presented low preclinical toxicity. The aim of this study was to determine the MHTP effect on ovalbumin (OVA)-induced pulmonary allergic inflammation. In other to realize this study, female BALFB/c mice were sensitized and challenged with OVA (OVA group) and treated with MHTP (MHTP group) by nasal instillation. Inflammatory, allergic, and immunomodulatory parameters such as migration of inflammatory cells to the lung tissue, pulmonary histological analysis, serum level of IgE-allergen specific, cytokine secretion, and lung T cell population characterization were analyzed and the data were considered statistically significant with p < 0.05. OVA-sensitized and OVA-challenged and MHTP (5.0 mg/kg)-treated mice presented reduction on total leukocyte migration into the bronchoalveolar lavage (BALF) dependent of lymphocyte and eosinophil migration (p < 0.001 and p < 0.01) as compared with the OVA group. Flow cytometric analysis showed that MHTP treatment decreased the percentage of granulocytes (p < 0.001) into the BALF and lung tissue histological analyzes demonstrated that the MHTP treatment decreased leukocyte migration and mucus production. In addition, treatment with MHTP decreased the number of CD3+CD4+ T cells independently of CD8+ T cell reduction into the BALF. The treatment also reduced significantly (p < 0.05) the serum level of IgE-OVA specific followed by reduction of IL-4, IL-13, and IL-17 production. Surprisingly, the MHTP treatment increased significantly (p < 0.05) the IFN-γ production in the BALF of these animals. Therefore, the results presented here showed that MHTP treatment, by nasal instillation, in a mouse model of OVA-induced pulmonary allergy has anti-allergic and immunomodulatory effects dependent on a Th1-skewed cytokine production that ameliorate the pulmonary allergic inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Pulmonary allergic inflammation comprehends several diseases among asthma, bronchitis, and emphysema that affects millions of people all over the world [1, 2]. These diseases promote airway inflammation, airway hyperresponsiveness (AHR), mucus hyper production, and sometimes high serum levels of IgE-allergen specific, and in some asthmatic individuals, it is hard to control the disease [3].
Asthma is a disease worldwide and affects about 7–13% of the world population [1, 4]. In asthma, the pulmonary inflammatory process develops to nonspecific stimuli, in which epithelial cells and innate and adaptive immune systems act together to cause airway hyperreactivity (AHR), overproduction of mucus, remodeling of various areas of the lung, and narrowing of the airways, causing shortness of breath, wheezing, and chest tightness [2, 3]. When the stimulus is an allergen, elevated serum levels of IgE-allergen specific and TH2/type 2 (“type 2 high”) pulmonary inflammatory phenotype are observed. This type 2 phenotype is characterized by an intense adaptive immune response with production of type 2 cytokines IL-4, IL-5, and IL-13 (TH2 profile) [5, 6]; periostin [4]; fractional exhaled nitric oxide (FeNO); and eosinophilia [4]. The type 2 cytokines mediate most of the asthma features, for instance, IL-5 plays a key role in eosinophilia process toward the airways, IL-4 and IL-13 are essential for the synthesis of IgE-allergen specific, and IL-13 contributes to the AHR, mucus production, and airway fibrosis [6,7,8].
Despite type 2 phenotype predominates in lung tissue of allergic individuals as well as animals from mouse model of asthma, immunomodulation toward a TH1 profile with production of type 1 cytokines, mainly IFN-γ, ameliorates the pulmonary inflammatory process and clinical discomfort caused by the disease [9]. IFN-γ negatively regulates IL-4 production preventing the production of IgE-allergen specific and proliferation, activation, and transmigration of IL-5-induced eosinophils to the lung tissue. Additionally to TH1 IFN-γ producer, CD8+ T cells are responsible for the production of this regulatory type 1 cytokine into the lung of allergic individuals [10]; therefore, CD8+ T cells are found in controlled asthmatic individuals [9,10,11]. Thus, pharmacological drugs that induce a beneficial immunomodulatory profile in pulmonary allergic diseases and induce low side effects are extremely desirable.
Toward this argument, there are some plant compounds such as alkaloids that have been tested in pre-clinical trials that presented anti-asthmatic and anti-inflammatory effects [12, 13]. Indeed, warifteine, an isoquinolinic alkaloid, decreased IL-13 production into the bronchoalveolar fluid, cysteinyl leukotriene production by eosinophils, and eosinophil tissue infiltration as well as diminished the AHR in OVA-induced pulmonary inflammation (mouse model of asthma) [13, 14]. In addition, the natural methylated form of warifteine, methylwarifteine, reduced the number of CD3+ T cells and eosinophil-like cells into the bronchoalveolar space of ovalbumin-sensitized mice [15].
Therefore, we analyzed, in this study, a synthetic isoquinolinic alkaloid named 2-methoxy-4-(7-methoxy-1,2,3,4-tetrahydroisoquinolin-1-yl) phenol (MHTP) (Fig. 1) [16]. Its chemical structure is similar to that of the synthetic isoquinolinic alkaloids (S)-1-(a-naphthylmethyl)-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinolinic (CKD712) [17] and 1-naphthylethyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinolinic (THI52) that inhibited TNF-α and iNOS gene expressions demonstrating anti-inflammatory activity in lipopolysaccharide-activated macrophages [18]. MHTP has been the subject of studies, in our laboratory, since its synthesis [16]. We demonstrated that the alkaloid presented low toxicity, without genotoxicity, and oral treatment with MHTP showed an anti-edematogenic effect on carrageenan-induced paw edema by inhibiting prostaglandine E2 (PGE2) action independently of mast cell degranulation. The alkaloid also inhibited leukocyte migration into the peritoneal cavity in the peritonitis experimental model. In lipopolysaccharide-stimulated macrophage, MHTP decreased the production of inflammatory cytokines IL-1β and IL-6 and nitric oxide (NO). Moreover, the authors demonstrated that the alkaloid was effective in decreasing inflammatory process on lipopolysaccharide-induced acute lung injury (ALI) [16]. Nonetheless, yet no studies have been performed to elucidate its activity on mouse model of asthma.
Thereby, taken all the data together, the goal of the present study was to extend the knowledge of MHTP effect by analyzing the nasal instillation of MHTP on OVA-induced pulmonary inflammation in mice and propose the MHTP as a prototype therapeutic drug to be tested on clinical trial.
METHODS
Animals
Isogenic female BALFB/c mice (6–8-week-old), weighing between 20 and 25 g, and female Wistar rats, weighing on an average 150 g, were used in the experiments. The animals were kept in cages at a temperature of 25 ± 2 °C, 12/12-h light-dark cycle with free access to water and a controlled diet, based on pellets, throughout the trial period. The animals were from Prof. Dr. Thomas George Animal Supplies of the Federal University of Paraíba, João Pessoa, PB, Brazil. The manipulation of the animals was performed according to animal care guide [19]. The Committee on Ethics in Animal Use (CEUA/UFPB) approved the experimental procedures under certificate no. 3105/14. Euthanasia and anesthesia were performed by intramuscular administration (i.m.) of anesthetic solution containing 29 mg/mL ketamine and 1.91 mg/mL xilasin in saline solution (NaCl 0.9%).
MHTP Treatment by Nasal Instillation Route
Animal groups (n = 6) were divided in (1) basal group (non-ovalbumin (non-OVA)-sensitized and non-OVA-challenged animals), (2) OVA group (OVA-sensitized and OVA-challenged animals), and (3) M 2.5 or M 5 groups (OVA-sensitized and OVA-challenged animals and treated with 2.5 or 5.0 mg/kg/40 μL of MHTP, respectively) and Dex group (OVA-sensitized and OVA-challenged animals and treated with 2.0 mg/kg/40 μL of dexamethasone). The MHTP suspension, at 5 mg/kg, was prepared using the concentration of 3.125 mg/mL of MHTP in 100 μL of 1 N nydrochloric acid (HCl) and 500 μL of saline. The pH was adjusted to 7 with 1 N sodium hydroxide (NaOH) and the volume completed to 1000 μL. Ovalbumin and dexamethasone were purchased from Sigma and Achê®, respectively. The MHTP was synthetized by Prof. Dr. Luís Cezar Rodrigues [16]. Treatments occurred 1 h before each OVA challenged, totalizing four treatments per animal.
Ovalbumin-Induced Pulmonary Allergic Inflammation
Female BALB/c mice were sensitized, intraperitoneally (ip, 250 μL/animal), with a suspension of OVA grade V (50 μg/mL, Sigma Chemical, St. Louis, MO) and Al(OH)3 (10 mg/mL, Vetec, Rio de Janeiro, RJ) on the 1st and 12th days of the experimental protocol. The animals were challenged with aerosolized OVA grade II (5% in 0.9% saline) from 19th to 22th days for 30 min in a closed chamber [20]. One hour before each OVA challenge, animals were treated by nasal instillation (n.i.) with MHTP (2.5 or 5 mg/kg), Dex (2 mg/kg), or saline. Twenty-four hours after the last OVA challenge, animals were euthanized with anesthetic solution, and the tracheas were isolated to obtain the bronchoalveolar lavage fluid (BALF). The total cell counts were performed in a Neubauer chamber, using the Turk solution in an optic microscope (× 40 objective). For differential cell counts, BALF samples were centrifuged at 1500 rpm, at 4 °C for 5 min, and cytospin slides were stained with hematoxylin and eosin. Differential cell counts were performed by light microscopy. Each slide was analyzed until the count of 100 cells using oil immersion objective (Fig. 2).
Pulmonary allergic inflammatory model. Representative scheme of ovalbumin (OVA)-sensitized, OVA-challenged, and treated mice [20]. OVA, ovalbumine; i.p., intraperitoneally; i.n., intranasal; BALF, broncoalveolar lavage fluid; IgE, immunoglobulin E; H&E, hematoxylin-eosin; and PAS, periodic acid-Schiff.
Cell Population Analyses by Flow Cytometric Methodology
Heterogeneous cell population on the BALF was analyzed by flow cytometric methodology where the forward-scattered light (FSC) and side-scattered light (SSC) measurements allowed to distinguish them by cell size (diffraction of light) and granularity (light diffusion), respectively. Lymphocytes is identified as FSClo/SSClo and cells with granularity, such as granulocytes (eosinophils), as SSChi [21]. In order to analyze these cell populations, the BALF was obtained from all different animal groups and the FSC and SSC parameters were applied. In addition, the CD3, CD4, and CD8 labeling were used to analyze CD3+ T (total T cell population), CD4+ T (helper), and CD8+ T (cytotoxic) cells, respectively. For these analyzes, 5 × 105 cells/mL were incubated with anti-mouse CD3-FITC, CD4-FITC, and CD8-PE conjugated (R&D Systems, USA) for 30 min at 4 °C. Cells were washed twice, resuspended in cold PBS to obtain a cell suspension. About 10,000 events were analyzed using a Becton Dickinson FACScan, and the data were analyzed using Flowing 2.5.1 software.
Histological Study of Pulmonary Tissue
Lungs were inflated through the heart with saline and the largest lobe of the left lung was removed and fixed in 10% buffered formalin for 24 h at room temperature. The fixed tissues were embedded in paraffin and tissue sections, 5 mm thick, were fixed to microscope slides and deparafinized. The slides were stained with hematoxylin and eosin (H&E) or with periodic acid-Schiff (PAS) and analyzed under light microscopy (Motic BA 410). Digital photographs were captured by the camera Moticam 5.0 MP. Histology analysis was performed with the KS300 software. For the morphological analysis of sections stained with H&E, a score was scored from 0 to 6, where the sum of the parameters was quantified, according to the lesion evidenced. The lesions evidenced were vasodilatation (present [1] or absent [0]), maintenance of bronchiolar integrity (present [1] or absent [0]), and alveolar obstruction (present [1] or absent [0]). Finally, as part of the score, cell migration was evaluated, where zero (0) means absence of migration, (1) few cells 0–9 cells per field, (2) more than 10 cells per field, and (3) formation of perivascular and peribronchiolar cell rings, and was evaluated in five fields of each blade, double blind [20].
Measurement of Cytokines and Serum Level of IgE-OVA Specific
Broncoalveolar lavages (BAL), from different animal groups, were analyzed for the production of IL-4, IL-13 (TH2 profile), IL-17 (TH17 profile), and IFN-γ (TH1 profile) by ELISA according to the manufacturer’s instructions (eBioscience, San Diego, CA). The OVA-specific IgE titer was determined using the passive cutaneous anaphylaxis (PCA) test using Wistar rats [15].
Statistical Analyses
The data were analyzed by GraphPad Prism© version 5.0 software (GraphPad Software, San Diego, CA, USA). Results were expressed as mean ± standard error of the mean (e.p.m.) and statistically analyzed by one-way ANOVA followed by the Tukey test, where values of p < 0.05 were considered significant.
RESULTS
Effect of MHTP on Inflammatory Cell Recruitment into the Bronchoalveolar Lavage of OVA-Sensitized and OVA-Challenged Mice
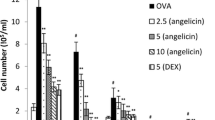
OVA-sensitized and OVA-challenged mice (OVA group) presented a marked recruitment of inflammatory cells to the bronchoalveolar lavage (BALF) as compared with basal group (no OVA-sensitized and OVA-challenged mice) (Fig. 3a). The influx of cells was represented by eosinophils (Fig. 3b) and lymphocytes (Fig. 3c). The highest dose of MHTP (5.0 mg/kg) (MHTP group) as well as dexamethasone (2.0 mg/kg) was able to inhibit the inflammatory cell migration mainly eosinophil (p < 0.01) and lymphocyte (p < 0.001) influx into the bronchoalveolar space as compared with the OVA group.
Effect of nasal instillation of MHTP on cell recruitment into the bronchoalveolar fluid (BALF). Ovalbumin (OVA)-sensitized and OVA-challenged mice (n = 6 animals per group) were treated with MHTP (M 2.5 or M 5.0 mg/kg), saline (basal), or dexamethasone (Dex 2.0 mg/kg) 1 h before each OVA challenge. On day 23, animals were euthanized and BALF of all groups were obtained to count the total and the differential cells. a Total cells. b Eosinophils. c Lymphocytes. Data are presented as mean ± e.p.m. One-way ANOVA followed by the Tukey test was used for statistical analyses. **p < 0.01 and ***p < 0.001, statistically significance when compared to the OVA group.
Effect of MHTP on Eosinophil Recruitment into Bronchoalveolar Lavage of OVA-Sensitized and OVA-Challenged Mice
The eosinophil is one of the biomarkers of pulmonary allergic inflammatory process; thus, we analyzed by flow cytometry the effect of MHTP treatment on OVA-sensitized and OVA-challenged mice. Figure 4a, b shows that the treatment decreased, approximately threefold the number of granulocytes (eosinophils) into the BALF as compared with the OVA group (8.04 ± 1.09 vs 25.38 ± 2.5, p < 0.001) corroborating with previous results. Therefore, these preliminary data indicate that the alkaloid shows an effective effect on decreasing the inflammatory cell migration mainly granulocyte cells, into allergic inflamed lung.
Effect of nasal instillation of MHTP on the percentage of airway granulocytes cells. The cell granularity was analyzed according to forward scatter parameters by flow cytometry. Panel a and bars figure (b) show the percentage of granulocyte cells into the BALF. Data are presented as mean ± e.p.m. One-way ANOVA followed by the Tukey test was used for statistical analyses. ***p < 0.001, statistically significant when compared to the OVA group (n = 6 animals per group).
Effect of MHTP on Histological Aspects of Lung Tissue of OVA-Sensitized and OVA-Challenged Mice
To confirm the data obtained from the BALF of MHTP-treated OVA-sensitized and OVA-challenged mice, we analyzed the presence of inflammatory cells into lung tissue by histological analyzes. Lung tissue sections of animals from the OVA group presented a massive cell infiltration into the pulmonary peribronchiolar and perivascular regions (blue arrow), vessel dilatation (green arrow), loss of bronchiolar epithelial integrity (orange arrow), and alveolar clogging (red arrow) (Fig. 5a) as compared to the basal group. On the other hand, lung tissues of animals from MHTP (5.0 mg/kg) or dexamethasone groups presented drastically reduction of cellular infiltration into the peribronchiolar and perivascular regions (p < 0.001) as well as the other parameters when compared with the OVA group (Fig. 5a, b). Another lung inflammatory parameter in OVA-induced pulmonary inflammation is the intense production of mucus by goblet cells. The OVA group showed mucus production by goblet cells in the primary, terminal, and respiratory bronchioles (yellow arrow) (Fig. 5a) as compared with the basal group. On the other hand, on MHTP or dexamethasone groups, decreasing of mucus production in the terminal and respiratory bronchioles was observed (Fig. 5c).
Photomicrograph, inflammatory scores, and mucus index of lung sections stained with hematoxylin and eosin (H&E) at × 20 or periodic-acid Schiff (PAS) at × 40. a The inflammatory parameters observed were: migration of cells in the perivascular and peribronchiolar space (blue arrow), vasodilatation (green arrow), bronchiolar epithelial integrity (orange arrowhead), alveolar clogging (red arrow head), and mucus production by the unicellular exocrine glands (goblet cells) in the primary, terminal, and respiratory bronchioles (yellow arrow). b Inflammatory scores. c Mucus index. Data are presented as mean ± e.p.m. One-way ANOVA followed by the Tukey test was used for statistical analyses. ***p < 0.001, statistically significance when compared to the OVA group (n = 6 animals per group).
Effect of MHTP on T Cell Recruitment into the Lung Space of OVA-Sensitized and OVA-Challenged Mice
MHTP treatment on OVA-sensitized and OVA-challenged mice reduced CD3+ T cell number (p < 0.05) on the inflamed lung tissue as compared with the OVA group (Fig. 6a, b). The reduction of this cell population is dependent of CD4+ T cells (p < 0.001) (Fig. 6c, d) but independent of CD8+ T cells (Fig. 6c, e).
Effect of nasal instillation of MHTP on the percentage of airway CD3+ CD4+ and CD8+ T cells. The CD3, CD4 and CD8 expressions were analyzed by flow cytometry technique using anti-CD3, anti-CD4 and/or anti-CD8 antibodies. Panel a and bars graph (b) percentage of CD3+, panel c and bars graph (d) percentage of CD4+, and panel c and bars graph (e) percentage of CD8+ in the BALF. Data are presented as mean ± e.p.m. One-way ANOVA followed by the Tukey test was used for statistical analyses. *p < 0.05, **p < 0.01, and ***p < 0.001, statistically significant when compared to the OVA group (n = 6 animals per group).
Effect of MHTP on the Serum Titer of IgE-OVA Specific of OVA-Sensitized and OVA-Challenged Mice
Ovalbumin is an allergen that induces, in the BALFB/c mice, the production of high amount of IgE-allergen specific. Therefore, as we can observe in Fig. 7a, sera from animals of the OVA group presented increase serum titer of IgE-OVA specific (1:2.752) as compared with sera from the basal group. However, MHTP (5.0 mg/kg) or dexamethasone (2.0 mg/kg) treatments significantly decreased the serum titers IgE-OVA specific in OVA-sensitized animals (1:848 and 1:277, p < 0.05 and p < 0.01, respectively) (Fig. 7a), indicating a systemic effect of MHTP nasal instillation by downregulating the production of this immunoglobulin.
Effect of nasal instillation of MHTP on serum level of IgE-OVA specific and on T cell cytokine production. OVA-sensitized and OVA-challenged mice (n = 6 per group) were treated with the MHTP (M 5.0 mg/kg), saline (basal), or dexamethasone (Dex 2.0 mg/kg) 1 h before each OVA challenge. On the 23rd day, animals were euthanized and sera were used to measure a IgE-OVA-specific titer and the BALF to measure the cytokine concentrations b IL-4, c IL-13, d IL-17, and e IFN-γ. Data are presented as mean ± e.p.m. One-way ANOVA followed by the Tukey test was used for statistical analyses. *p < 0.05, **p < 0.01, and ***p < 0.001, statistically significance when compared to the OVA group (n = 6 animals per group).
Effect of MHTP on T Cell Cytokine Production of OVA-Sensitized and OVA-Challenged Mice
The lung TH2 profile is detected in the OVA-induced pulmonary inflammation. Therefore, we analyzed the presence of type 2 cytokine profile in the BALF of animals from different groups. BALF from the OVA group presented high levels of IL-4 (p < 0.001) (Fig. 7b), IL-13 (p < 0.001) (Fig. 7c), and IL-17 (p < 0.001) (Fig. 7d), but not IFN-γ (Fig. 7e). Animals treated with MHTP (5.0 mg/kg) or dexamethasone presented significant reduction of IL-4, IL-13, and IL-17 (p < 0.01). Surprisingly, MHTP treatment induced an increase of IFN-γ production into the BALF of these animals (p < 0.05), indicating an immunomodulatory effect toward TH1 profile.
DISCUSSION
The predominant characteristic of pulmonary allergic inflammation, as asthma, is a type 2 immune response with CD3+CD4+ T cells producing type 2 cytokines such as IL-4, IL-13, and IL-5 (TH2 profile) into the blood and the bronchoalveolar fluid. These cytokines (IL-5, mainly) are associated with the recruitment and activation of eosinophils to the lung tissue [6, 8, 22]. In addition, IL-4 and IL-13 are also responsible for the production of another biomarker molecule in the pulmonary allergic inflammation that is IgE-allergen specific presents into the serum and bond to mast and basophil receptors [3,4,5]. Therefore, regulation of type 2 immune response into the lungs of these individuals as well as decreasing of serum level of IgE-allergen specific is associated with the control of the clinical symptoms of the disease.
The classical pharmacotherapies to manage the pulmonary allergic inflammatory process are based on oral or inhaled administration of short-acting antihistamine therapy and short-acting or long-acting β2 agonists and/or steroids. Nonetheless, the long-term treatment with these drugs promotes severe side effects and contributes to diminish the desirable effects [23, 24]. These side effects, per si, justify the increasing study in natural, synthetic, or semi-synthetic molecules, which may present an immunomodulatory activity diminishing the side effects on allergic patients [24].
Hence, the present study analyzed the effect of nasal instillation of a synthetic alkaloid named MHTP (2-methoxy-4-(7-methoxy-1,2,3,4-tetrahydroisoquinolin-1-yl) phenol) in an experimental model of OVA-sensitized and OVA-challenged BALFB/c mice, a classical mouse model of asthma.
The synthesis methodology used to synthesize the isoquinolinic alkaloid was the condensation method described by Pictet-Spengler and yielded 93.45% of pure molecule [25]. This methodology is simple due to involving a few steps and two low-cost reagents: 4-methoxyphenylethylamine and vanillin. In addition to the low-cost reagents, the rapid synthesis time of MHTP is very favorable to a possible large-scale production of this alkaloid [16].
Comparative study showed that the chemical structure of MHTP is similar to that of the synthetic isoquinolinic alkaloids (S)-1-(a-naphthylmethyl)-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinolinic (CKD712) and 1-naphthylethyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinolinic (THI52) which demonstrated anti-inflammatory activity by inhibiting TNF-α and iNOS gene expressions [17, 18].
The nasal route chosen to administer the MHTP in OVA-sensitized and OVA-challenged BALFB/c mice was due to this pathway been the best one required to treat asthmatic patients during the crises. The main advantages of this route of administration are (1) prevention of first-pass hepatic metabolism which, in many cases, forms toxic or inactive metabolites of the drug; (2) reduction of the systemic absorption of the drug, where up to 70% of the drug remains in the airways; and (3) rapid onset of action of the drug at the inflammatory site, since the availability of the drug will be increased locally by providing its rapid cellular interaction and consequent desired effect [26, 27].
Although the intranasal route presents all the benefits described above, studies on the anti-inflammatory and immunomodulatory effects of molecules using this route in mouse model of asthma are still scarce. Therefore, molecules that have a potential immunomodulatory effect of type 2 immune response, characteristic of asthma, administered intranasally are considered promising in the context of specific therapy.
The OVA-sensitized and OVA-challenged animals nasally treated with MHTP presented reduction of inflammatory cell migration (mainly granulocytes and CD3+CD4+ T cells) to the bronchoalveolar lavage (BALF) and to the peribronchiolar and perivascular space of the lung tissue. Studies carried out with other isoquinolinic alkaloids such as warifteine, curine, matrine, chelidonine, and R8 (vasicine analogue) in the same mouse model of asthma demonstrated that oral administration of these alkaloids decreased the migration of eosinophils into the BALF and lung tissue [12,13,14, 28,29,30,31]. However, in a unique study where it was used, the nasal inhalation of a methylated form of warifteine observed a strong decreasing of eosinophils and CD3+ T cell migration, independently of CD8+ T cells, in the BALF of OVA-sensitized and OVA-challenged mice as well as diminishing of mucus production [15]. Chelidonine, another isoquinolinic alkaloid, but orally administered in mouse model of asthma, decreased the CD3+CD4+ T cell population in the BALF of animals [31]. All these data together demonstrated an important anti-inflammatory effect of the isoquinolinic nucleus in alkaloid molecules by inhibiting eosinophil and CD3+CD4+ T cell (TH2 profile) migration to the inflamed tissue and hence reduction of lung destruction independently of the route used.
The accumulation of activated eosinophils in the airways generates proinflammatory chemokines and cytolytic enzymes, including eosinophilic cationic protein (ECP) and main basic protein (MBP), which cause rupture of the integrity of the epithelium directly causing airway hyperreactivity [32]. Eosinophils also contribute to airway remodeling [33]. This airway remodeling is a consequence of increased deposition of extracellular matrix, increased subepithelial mesenchymal cells, and, mainly, increased smooth muscle mass of the airway, which is the main determinant of airflow obstruction. Eosinophil-derived TGF-β activates fibroblast and release of matrix proteins, causing thickening of the reticular basement membrane associating eosinophilic inflammation in asthma [32]. Therefore, decreasing eosinophil to the inflamed lung tissue ameliorates the airway compliance.
Another important cell population into the inflamed lung tissue is the CD3+CD4+ T cell, with a TH2 profile, that is responsible for orchestrate the entire pathophysiology of the pulmonary allergic inflammation in human and mouse models due to the production of type 2 cytokines IL-4, IL-5, and IL13 [2, 7]. Therefore, in this study, it was demonstrated that MHTP induced a decreasing of this cell population into the lung tissue of OVA-sensitized and OVA-challenged BALFB/c mice, suggesting an immunomodulatory activity of the alkaloid. Indeed, it was also observed decreasing of IL-4 and IL-13 production and hence decreasing of serum level of IgE-OVA specific, indicating clearly the modulation of the type 2 immune response. In addition, the alkaloid also decreased the mucus production by the goblet cells correlating with decreasing of IL-13, a cytokine that promotes hyperproduction of mucus, hyperplasia, hypertrophy, and metaplasia of these cells [6].
Several isoquinolinic alkaloids presented immunomodulatory activity by decreasing the production of IgE-OVA specific in mouse model of asthma and of rhinitis [12, 15, 29,30,31, 34]. By preventing the production of IgE-OVA specific by alkaloids, the entire mast cell and basophil sensitization process is compromised, meaning decreasing of cell degranulation and release of inflammatory mediators [35]. Inflammatory mediators such as the classical cytokines IL-4, main maintainer of the polarization and maintenance of TH2 [29,30,31], and IL-13 involved in the mucus production by goblet cells are diminished by this event [12, 14, 15, 29,30,31]. The MHTP treatment also reduced the IL-17 production, a cytokine produced mainly by TH17 profile. This cytokine is abundant in the lung of corticoid-resistant allergic asthmatic patients [36, 37]. However, another isoquinolinic alkaloid, chelidonine, also prevented the production of this cytokine in the mouse model of asthma [31]. The relevance of this data in this experimental model of asthma is under investigation.
An important effect of the MHTP intranasal treatment on OVA-sensitized and OVA-challenged mice was the induction of IFN-γ production into the BALF of these animals. A described and well-understood way to control the type 2 immune response in asthma is to drive to a type 1 and/or regulatory immune response. Thus, the immune regulatory process usually occurs with the production of regulatory cytokines such as IFN-γ and/or IL-10 both coming from TH1 cells and Treg cells, respectively [3, 38].
IFN-γ is a classical TH1 cytokine profile but also is produced, in a high amount, by CD8+ T cells [3, 9, 10]. Among its functions, INF-γ inhibits the synthesis of IL-4 by decreasing the GATA3 activation, a transcription factor for TH2 profile, hence controlling the presence of type 2 profile [11]. Indeed, in this study, we demonstrated the immunomodulatory profile of MHTP besides controlling the type 2 profile induced the IFN-γ production dependent of CD8+ T cells.
In view of all the issues described above, the relevance of this work is singular, due to a synthetic isoquinolinic alkaloid (MHTP) presented, by nasal instillation, immunomodulatory activity by inhibiting the type 2 immune response dependent of the IFN-γ production in a mouse model of asthma. These data encourage us to persuade the study with this molecule with the prospect of being a pharmacological prototype to control airway inflammatory process.
CONCLUSION
In summary, the results presented in this study infer that MHTP, a synthetic isoquinolinic alkaloid, has immunomodulatory effect on a mouse model of asthma. Thus, MHTP, administered by nasal inhalation, downregulated the type 2 immune response by decreasing the inflammatory cell migration as eosinophil and CD3+CD4+ T cells to the inflamed lung tissue as well as type 2 cytokines IL-4 and IL-13, serum level of IgE-allergen specific dependent of IFN-γ production, and maintenance of CD8+ T cells on the tissue. These preliminary results encourage us to infer that this molecule may be a prototype for an alternative therapeutic protocol in pulmonary allergic inflammation.
References
GINA. 2017. Pocket guide for asthma management and prevention. GloBALF Initiative for Asthma file:///C: 1–29.
Holgate, Stephen T., Sally Wenzel, Dirkje S. Postma, Scott T. Weiss, Harald Renz, and Peter D. Sly. 2015. Asthma. Nature Reviews Disease Primers 1. Nature Publishing Group: 15025. https://doi.org/10.1038/nrdp.2015.25.
Lambrecht, Bart N., and Hamida Hammad. 2015. The immunology of asthma. Nature Immunology 16: 45–56. https://doi.org/10.1038/ni.3049.
Tabatabaian, Farnaz, and Dennis K. Ledford. 2018. Omalizumab for severe asthma: Toward personalized treatment based on biomarker profile and clinical history. Journal of Asthma and Allergy 11. Dove Press: 53–61. https://doi.org/10.2147/JAA.S107982.
Gould, Hannah J., and Brian J. Sutton. 2008. IgE in allergy and asthma today. Nature Reviews Immunology 8: 205–217. https://doi.org/10.1038/nri2273.
Gour, Naina, and Marsha Wills-Karp. 2015. IL-4 and IL-13 signaling in allergic airway disease. Cytokine 75: 68–78. https://doi.org/10.1016/j.cyto.2015.05.014.
Fahy, John V. 2015. Type 2 inflammation in asthma--present in most, absent in many. Nature Reviews. Immunology 15: 57–65. https://doi.org/10.1038/nri3786.
Wynn, Thomas A. 2015. Type 2 cytokines: Mechanisms and therapeutic strategies. Nature Reviews Immunology 15. Nature Publishing Group: 271–282. https://doi.org/10.1038/nri3831.
Szabo, S.J. 2002. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science 295: 338–342. https://doi.org/10.1126/science.1065543.
Biller, H., B. Bade, H. Matthys, W. Luttmann, and J.C. Virchow. 2001. Interferon-gamma secretion of peripheral blood CD8+ T lymphocytes in patients with bronchial asthma: In vitro stimulus determines cytokine production. Clinical and Experimental Immunology 126. Wiley-Blackwell: 199–205. https://doi.org/10.1046/J.1365-2249.2001.01666.X.
Tumes, Damon J., Magdalene Papadopoulos, Yusuke Endo, Atsushi Onodera, Kiyoshi Hirahara, and Toshinori Nakayama. 2017. Epigenetic regulation of T-helper cell differentiation, memory, and plasticity in allergic asthma. Immunological Reviews 278: 8–19. https://doi.org/10.1111/imr.12560.
Ribeiro-Filho, Jaime, Andrea Surrage Calheiros, Adriana Vieira-de-Abreu, Katharinne Ingrid Moraes de Carvalho, Diego da Silva Mendes, Christianne Bandeira Melo, Marco Aurélio Martins, Celidarque da Silva Dias, Márcia Regina Piuvezam, and Patrícia T. Bozza. 2013. Curine inhibits eosinophil activation and airway hyper-responsiveness in a mouse model of allergic asthma. Toxicology and Applied Pharmacology 273: 19–26. https://doi.org/10.1016/j.taap.2013.08.015.
Bezerra-Santos, C.R., F.M.P. BALFestieri, B. Rossi-Bergmann, L.M.T. Peçanha, and M.R. Piuvezam. 2004. Cissampelos sympodialis Eichl. (Menispermaceae): Oral treatment decreases IgE levels and induces a Th1-skewed cytokine production in ovalbumin-sensitized mice. Journal of Ethnopharmacology 95: 191–197. https://doi.org/10.1016/j.jep.2004.06.037.
Bezerra-Santos, Cláudio R., Adriana Vieira-de-Abreu, José Maria Barbosa-Filho, Christianne Bandeira-Melo, Marcia R. Piuvezam, and Patrícia T. Bozza. 2006. Anti-allergic properties of Cissampelos sympodialis and its isolated alkaloid warifteine. International Immunopharmacology 6: 1152–1160. https://doi.org/10.1016/j.intimp.2006.02.007.
Vieira, Giciane C., Josenilson F. De Lima, Regina C.B.Q. De Figueiredo, Sandra R. Mascarenhas, Claudio R. Bezerra-Santos, and Marcia R. Piuvezam. 2013. Inhaled cissampelos sympodialis down-regulates airway allergic reaction by reducing lung CD3+T cells. Phytotherapy Research 27: 916–925. https://doi.org/10.1002/ptr.4791.
de Oliveira, Pacheco, Maria Talita, Theresa Raquel de Oliveira Ramalho, Laércia Karla Liege Paiva Ferreira, Ana Luísa Araújo Lima, Manuela Barbosa Cordeiro, Hermann Ferreira Costa, Luís Cézar Rodrigues, and Marcia Regina Piuvezam. 2015. Synthesis, toxicity study and anti-inflammatory effect of MHTP, a new tetrahydroisoquinolinic alkaloid. Immunopharmacology and Immunotoxicology 37: 400–412. https://doi.org/10.3109/08923973.2015.1070173.
Tsoyi, Konstantin, Hye Jung Kim, Jae Soo Shin, Dal Hyun Kim, Hee Jeong Cho, Sung Sook Lee, Sun Kil Ahn, et al. 2008. HO-1 and JAK-2/STAT-1 signals are involved in preferential inhibition of iNOS over COX-2 gene expression by newly synthesized tetrahydroisoquinolinic alkaloid, CKD712, in cells activated with lipopolysacchride. Cellular Signalling 20: 1839–1847. https://doi.org/10.1016/j.cellsig.2008.06.012.
Kang, Y.J., B.K. Lee, Y.S. Lee, H.G. Seo, M.K. Park, H.J. Kim, H.S. Pyo, et al. 2003. Suppression of tumor necrosis factor-α and inducible nitric oxide synthase gene expression by THI 52, a new synthetic naphthyl-benzylisoquinolinic alkaloid. Biochemical Pharmacology 65: 457–464.
Sherwin, Chris M., Stine B. Christiansen, Ian J. Duncan, Hans W. Erhard, Don C. Lay, Joy A. Mench, Cheryl E. O’Connor, and J. Carol Petherick. 2003. Guidelines for the ethical use of animals in applied ethology studies. Applied Animal Behaviour Science 81: 291–305. https://doi.org/10.1016/S0168-1591(02)00288-5.
Galvão, José Guilherme F.M., Luiz Henrique Agra Cavalcante-Silva, Deyse Cristina M. Carvalho, Laércia Karla D.P. Ferreira, Talissa Mozzini Monteiro, Adriano Francisco Alves, Larissa Adilis M.P. Ferreira, Francisco Allysson A.F. Gadelha, Marcia Regina Piuvezam, and Sandra Rodrigues-Mascarenhas. 2017. Ouabain attenuates ovalbumin-induced airway inflammation. Inflammation Research 66: 1117–1130. https://doi.org/10.1007/s00011-017-1092-9.
Rijt, van, S. Leonie, Harmjan Kuipers, Nanda Vos, Daniëlle Hijdra, Henk C. Hoogsteden, and Bart N. Lambrecht. 2004. A rapid flow cytometric method for determining the cellular composition of bronchoalveolar lavage fluid cells in mouse models of asthma. Journal of Immunological Methods 288: 111–121. https://doi.org/10.1016/j.jim.2004.03.004.
Bisset, Leslie R., and Peter Schmid-Grendelmeier. 2005. Chemokines and their receptors in the pathogenesis of allergic asthma: Progress and perspective. Current Opinion in Pulmonary Medicine 11: 35–42.
GINA. 2017. GloBALF Strategy For Asthma Management and Prevention. GloBALF Initiative for Asthma: http://ginasthma.org/2017-gina-report-gloBALF-strat. doi:https://doi.org/10.1183/09031936.00138707.
Goldstein, Lee H., Corinna Weber-Schöndorfer, and Matitiahu Berkovitch. 2015. Antiasthmatic and cough medication. In Drugs during pregnancy and lactation, 65–74. Elsevier. doi:https://doi.org/10.1016/B978-0-12-408078-2.00004-4.
Stöckigt, Joachim, Andrey P. Antonchick, Wu Fangrui, and Herbert Waldmann. 2011. The Pictet-Spengler reaction in nature and in organic chemistry. Angewandte Chemie - International Edition 50: 8538–8564. https://doi.org/10.1002/anie.201008071.
Leung, James S., David W. Johnson, Arissa J. Sperou, Jennifer Crotts, Erik Saude, Lisa Hartling, and Antonia Stang. 2017. A systematic review of adverse drug events associated with administration of common asthma medications in children. Edited by Imti Choonara. PLOS ONE 12: e0182738. https://doi.org/10.1371/journal.pone.0182738.
Fraga Righetti, Renato, Patricia Angeli da Silva Pigati, Samantha Souza Possa, Anelize Sartori Alves dos Santos, Nathalia Montouro Pinheiro Aless, et al. 2014. New pharmacological targets for asthma drug development. Journal of Allergy & Therapy 05. OMICS International: 1–13. https://doi.org/10.4172/2155-6121.1000170.
Bezerra-santos, Claudio R., Adriana Vieira-de-abreu, Giciane Carvalho, Jaime R. Filho, José Maria Barbosa-filho, Ana Lucia, Marco Aurelio, International Immunopharmacology, et al. 2012. Effectiveness of Cissampelos sympodialis and its isolated alkaloid warifteine in airway hyperreactivity and lung remodeling in a mouse model of asthma. International Immunopharmacology 13. Elsevier B.V.: 148–155. https://doi.org/10.1016/j.intimp.2012.03.014.
Rayees, Sheikh, Ulaganathan MaBALFirajan, Wajid Waheed Bhat, Shafaq Rasool, Rafiq Ahmad Rather, Lipsa Panda, Naresh Kumar Satti, Surrinder Kumar Lattoo, BALFaram Ghosh, and Gurdarshan Singh. 2015. Therapeutic effects of R8, a semi-synthetic analogue of Vasicine, on murine model of allergic airway inflammation via STAT6 inhibition. International Immunopharmacology 26: 246–256. https://doi.org/10.1016/j.intimp.2015.03.035.
Fu, Qiang, Jing Wang, Zhanqing Ma, and Shiping Ma. 2014. Anti-asthmatic effects of matrine in a mouse model of allergic asthma. Fitoterapia 94. Elsevier B.V.: 183–189. https://doi.org/10.1016/j.fitote.2013.12.014.
Kim, Seung Hyung, Jung Hee Hong, and Young Cheol Lee. 2015. Chelidonine, a principal isoquinolinic alkaloid of Chelidonium majus, attenuates eosinophilic airway inflammation by suppressing IL-4 and eotaxin-2 expression in asthmatic mice. Pharmacological Reports 67. Institute of Pharmacology, Polish Academy of Sciences: 1168–1177. https://doi.org/10.1016/j.pharep.2015.04.013.
George, Leena, and Christopher E. Brightling. 2016. Eosinophilic airway inflammation: Role in asthma and chronic obstructive pulmonary disease. Therapeutic Advances in Chronic Disease 7. SAGE Publications: 34–51. https://doi.org/10.1177/2040622315609251.
Kay, A. Barry, Simon Phipps, and Douglas S. Robinson. 2004. A role for eosinophils in airway remodelling in asthma. Trends in Immunology 25: 477–482. https://doi.org/10.1016/j.it.2004.07.006.
Vieira, Giciane C., Francisco A.A.F. Gadelha, Raquel F. Pereira, Laércia K.D.P. Ferreira, José M. Barbosa-filho, Patricia T. Bozza, and Marcia R. Piuvezam. 2018. Warifteine, an alkaloid of Cissampelos sympodialis , modulates allergic profile in a chronic allergic rhinitis model. Revista Brasileira de Farmacognosia 28. Sociedade Brasileira de Farmacognosia: 50–56. https://doi.org/10.1016/j.bjp.2017.10.009.
Palomares, Óscar, Silvia Sánchez-Ramón, Ignacio Dávila, Luis Prieto, Luis Pérez de Llano, Marta Lleonart, Christian Domingo, and Antonio Nieto. 2017. dIverGent: how IgE axis contributes to the continuum of allergic asthma and anti-IgE therapies. International Journal of Molecular Sciences 18: 1–14. https://doi.org/10.3390/ijms18061328.
Peters, Marcus, Stefanie Köhler-Bachmann, Tim Lenz-Habijan, and Albrecht Bufe. 2016. Influence of an allergen-specific Th17 response on remodeling of the airways. American Journal of Respiratory Cell and Molecular Biology 54: 350–358. https://doi.org/10.1165/rcmb.2014-0429OC.
Irvin, Chaoyu, Iram Zafar, James Good, Donald Rollins, Christina Christianson, Magdalena M. Gorska, Richard J. Martin, and Rafeul Alam. 2014. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. Journal of Allergy and Clinical Immunology 134: 1175–1186.e7. https://doi.org/10.1016/j.jaci.2014.05.038.
Keynan, Yoav, Catherine M. Card, Paul J. Mclaren, Magdy R. Dawood, Ken Kasper, and Keith R. Fowke. 2008. The role of regulatory T cells in chronic and acute viral infections. Clinical Infectious Diseases 46: 1046–1052. https://doi.org/10.1086/529379.
Acknowledgments
The Brazilian government sponsored this study by CNPq and CAPES organization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The manipulation of the animals was performed according to animal care guide. The Committee on Ethics in Animal Use (CEUA/UFPB) approved the experimental procedures under certificate no. 3105/14.
Rights and permissions
About this article
Cite this article
Paiva Ferreira, L.K.D., Paiva Ferreira, L.A.M., Alves, A.F. et al. MHTP, 2-Methoxy-4-(7-methoxy-1,2,3,4-tetrahydroisoquinolin-1-yl) phenol, a Synthetic Alkaloid, Induces IFN-γ Production in Murine Model of Ovalbumin-Induced Pulmonary Allergic Inflammation. Inflammation 41, 2116–2128 (2018). https://doi.org/10.1007/s10753-018-0855-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0855-y