Abstract
Metabolite changes in Nile tilapia in response to carbonate alkalinity stress were investigated by transferring the fish directly from freshwater into different carbonate alkaline water. Levels of plasma pH/HCO3− concentration, the mRNA and protein expression of two carbonic anhydrases (CAhz and CAIV), and two HCO3− transporters (Na+/HCO3− cotransporter and Cl−/HCO3– exchanger) in the gill, kidney, and intestine were determined using a pH meter, UV spectrophotometer, quantitative real-time PCR, and western blotting within 192 h of exposure. Plasma pH showed an “up-peak-down” variation, whereas HCO3− concentration decreased at first and then increased in all alkaline water groups. The overall mRNA expression was regulated in an alkalinity- and time-dependent manner. Western blot results showed that the Cl−/HCO3− exchanger protein was detected in all tissues examined, whereas the two carbonic anhydrases and Na+/HCO3− cotransporter proteins were only expressed in the gill and kidney. Therefore, the studied carbonic anhydrases and HCO3− transporters are involved in the HCO3− metabolism and transport to maintain acid–base balance in Nile tilapia under carbonate alkalinity stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Alkali–saline water accounts for a large proportion of the water resources in the world. Highly alkaline water (AW) is an extreme type of alkali–saline water that greatly limits the survival, growth, and reproduction of aquatic organisms because of its high carbonate alkalinity, high pH value, and complex ion systems (such as HCO3− and CO32−) (Parra & Baldisserotto, 2007). Nile tilapia Oreochromis niloticus (Linnaeus, 1758) is one of the most cultured fish in the world. Its tolerance to carbonate alkalinity is higher than other freshwater (FW) fishes. O. niloticus can adapt to water with carbonate alkalinity as high as 40 mmol/l (Zhao et al., 2020). Thus, Nile tilapia is an excellent model for investigating the mechanisms of alkaline adaptation.
Earlier studies have shown that osmoregulation, acid–base regulation, and ammonia metabolism regulation are important physiological mechanisms and adaptation strategies used by fish in extremely alkaline environments (Wilkie & Wood, 1996). Recently, omics data revealed that the ability of the fish to adapt to high carbonate alkalinity involves the MAPK signaling pathway (related to regulating osmotic pressure), signaling by platelet-derived growth factor, ammonia excretion, and inflammation and immune responses in O. niloticus (Zhao et al., 2020), Amur ide Leuciscus waleckii (Dybowski, 1869) (Wang et al., 2021), and crucian carp Carassius auratus (Linnaeus, 1758) (Liu et al., 2022). HCO3− metabolism and transport are processes that can directly affect osmoregulation and acid–base balance. There are many studies on marine teleost fish osmoregulation, showing that solute coupled water absorption by intestinal anion exchange results in CaCO3 precipitation and then a reduction in osmotic pressure (Grosell, 2006, 2011). However, studies on HCO3− metabolism and transport in the adaptation and survival of FW fish in alkali–saline water have only been conducted with a few species, including Magadi tilapias Alcolapia grahami (Boulenger, 1912) (Pierre et al., 2000), Lahontan cutthroat trout Oncorhynchus henshawi (Gill & Jordan, 1878) (Wilkie et al., 1994), Rainbow trout Oncorhynchus mykiss (Walbaum, 1792) (Goss et al., 1992), and Amur ide (Chang et al., 2021). It is generally considered that the Cl−/HCO3− or HCO3−/CO32− transport system is activated to mediate acid–base balance in an alkali–saline environment.
Carbonic anhydrases (CAs), SLC4, and SLC26 gene superfamilies are associated with HCO3− metabolism and transport (Georgalis et al., 2006; Kurita et al., 2008). CAs are a large family of zinc metalloenzymes that catalyze the reversible reactions of CO2 and water to HCO3− and H+ (or vice versa). These molecules play a crucial role in systemic acid–base regulation in fish (Georgalis et al., 2006). In general, membrane-bound CA (CAIV) that are in direct contact with the plasma HCO3−, will convert HCO3− to CO2, which diffuses across the gill epithelium into the water. Cytosol CA (CAII), known as CAhz in teleosts, hydrolyzes CO2 and supplies the HCO3− and H+ that are used as counter-ions for Na+/H+ and Cl−/HCO3− ionic exchangers (Sender et al., 1999). In our previous study, the mRNA expression of CAIV in Nile tilapia increased with the increment of carbonate alkalinity (Zhao et al., 2014). However, the role of CAhz in the responses to alkalinity in tilapia remains unclear.
The SLC4 and SLC26 gene superfamilies encode the HCO3− exchangers that directly contribute to HCO3− transport and intracellular pH regulation. SLC4A4 (Na+/HCO3− cotransporter) is involved in the formation of carbonate precipitates in marine teleost fish (Kurita et al., 2008). SLC26A6 (Cl−/HCO3− exchangers) plays an important role in branchial bicarbonate transport in many fish species (Gilmour & Perry, 2009; Boyle et al., 2015; Michael et al., 2016; Ruiz-Jarabo et al., 2017; Chang et al., 2021). In naked carp Gymnocypris przewalskii (Kessler, 1876), an alkali–saline-tolerant species that inhabits Lake Qinghai of China, the SLC26A6 gene is highly expressed in the intestine, whereas the lowest expression is in the gill (Wang et al., 2015). In O. niloticus, the results of immunohistochemistry showed that SLC4A4 and SLC26A6 are expressed in the gill and kidney after 7 days of exposure to AW, but no positive reaction is detected in the intestines (Wang et al., 2016). It is suggested that in different tissues of different fish species, the function of these genes in response to alkalinity is different. A protein expression analysis or time-course study is needed to understand and explain this issue.
To clarify the changes in the pH and HCO3− and especially the expression pattern of CAs and HCO3− transporters under carbonate alkalinity stress, we measured the plasma pH, HCO3− concentration, and the mRNA and protein expressions of CAhz, CAIV, SLC4A4, and SLC26A6 in different tissues (i.e., gill, kidney, and intestine) of O. niloticus under carbonate alkalinity stress. In general, the plasma pH/HCO3− concentration and mRNA expressions of CAhz, CAIV, SLC4A4, and SLC26A6 showed similar alkalinity- and time-dependent trends in the gill, kidney, and intestine. The SLC26A6 protein was detected in all tissues, but the CAhz, CAIV, and SLC4A4 proteins were only expressed in the gill and kidney. This information will assist in the understanding of transport and metabolic pathways of HCO3− in fish under carbonate alkalinity stress.
Materials and methods
Fish and experimental conditions
This study was approved by the animal ethics committee of Shanghai Ocean University (approval no. 2018–085). Juvenile O. niloticus (N = 180, approximately half male and half female) were the offspring of the GIFT strain of Nile tilapia (O. niloticus) and were obtained from the Fish Germplasm Station, Shanghai Ocean University. The fish were 15.2 ± 0.6 cm in length and 62.6 ± 4.5 g in weight. The fish were taken back to the laboratory and maintained in recirculated aquariums with FW for two weeks of acclimatization. According to a previous study, tilapia can survive in carbonate AW of 41.6 mmol/l alkalinity (pH 9.42). We have recently conducted a breeding program to generate a new Nile tilapia strain with enhanced saline–alkaline tolerance in China. In the actual breeding process, stress conditions were created using 2 g/l and 4 g/l AW. Thus, two concentrations of AW, 2 g/l (alkalinity = 24.7 mmol/l, pH 8.4) and 4 g/l (alkalinity = 46.8 mmol/l, pH 8.5), were prepared using FW (alkalinity = 0.22 mmol/l, pH 7.2) with sodium bicarbonate (NaHCO3). The alkalinity was determined with acidimetric titrations and expressed in mmol/l. Fish of the same size were directly transferred from FW to different levels of AW. The experimental systems consisted of nine 100 l plastic containers, and each container was stocked with 20 fish with three replicates per treatment. During exposure, the fish were not fed, but no fish died. Approximately, 90% of the solution in each tank was changed daily. Water temperature was maintained at 25.0 ± 1.5 °C, whereas dissolved oxygen was kept at about 8.2 mg/l, NH4+–N at < 0.6 mg/l, and NO2–N at < 0.04 mg/l. Five fish were randomly sampled from each treatment at 0, 3, 6, 12, 24, 48, 72, 96, and 192 h post-transfer.
Plasma pH and HCO3 − concentration
Blood samples were extracted by caudal puncture with a prepared heparinized syringe. Plasma was obtained by centrifugation at 1200×g for 10 min, and the plasma pH and HCO3− concentration were measured instantly using a blood gas analyzer (VetScan I-STA T®1, Union City, CA, USA).
Real-time PCR
The fish were terminally anesthetized with neutralized MS-222 at a concentration of 50 mg/l (Sigma-Aldrich, St.Lou, Missour (MO), USA). Tissues from the fish gill, kidney, and intestine (mid intestine) were immediately dissected after blood extraction. For real-time PCR, the samples were snap-frozen in liquid nitrogen and stored at − 80 °C until use. The total RNA from the tissues was reverse transcribed using PrimeScript™ RT reagent Kit with gDNA Eraser (TaKaRa, Kusatsu, Shiga, Japan). The primer sets of CAhz, CAIV, SLC4A4, and SLC26A6 were designed with Primer Express software version 5.0 in accordance with their cDNA sequences (Table 1).
Real-time PCR was performed using a CFX96 Real-time PCR Detection System (Bio-Rad, Hercules, CA, USA). β-actin was used as the reference gene. Each reaction mixture consisted of 1 µl of cDNA, 7 µl of ddH2O, 10 µl of 2 × SsoAdvanced™ SYBR Green Supermix (Bio-Rad), and 1 µl of each primer set of each gene (10 µM). The real-time PCR was performed as follows: one cycle of 95 °C for 30 s; 40 cycles of 95 °C for 5 s; and 59.4–60.0 °C for 30 s. The data were then analyzed by CFX Manager software version 2.1 (Bio-Rad), and the 2−∆∆CT method was applied to analyze the expression.
Western blot analysis
Proteins from the gill, kidney, and intestine were extracted after samples were lysed in RIPA buffer (Beyotime, China) containing 1 mM phenylmethylsulfonyl fluoride and protease inhibitor (Roche Diagnostics, USA) at a ratio of 10 mg tissue/50 µl RIPA buffer. The mixture was centrifuged for 10 min at 10,000×g at 4 °C. The supernatant was collected and transferred to a fresh tube. The protein concentration was determined using the bicinchoninic acid method. The protein was diluted to 10 mg/ml and stored at − 80 °C. Protein samples were loaded on a 12% SDS-polyacrylamide gel (Solarbio, Beijing, China) and then transferred to a polyvinylidene difluoride (PVDF) membrane (Solarbio, Beijing, China). After blocking for 1 h with 5% bovine serum albumin, the membrane was incubated overnight at 4 °C with antibodies against CAII (Abcam, Cambridge, UK, ab124687, polyclonal antibody, 1:1500 dilution), CAIV (Abcam, ab85225, polyclonal antibody, 1:700 dilution), SLC4A4 (Abcam, ab187511, polyclonal antibody, 1:1000 dilution), and SLC26A6 (Abcam, ab217269, polyclonal antibody, 1:300 dilution). The primary antibody against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the loading control (1:2000 dilution). The membrane was then incubated with an HRP-conjugated secondary antibody at room temperature for another 2 h. The signal of the band was detected using SuperSignal West Pico chemiluminescent substrate (Thermo Fisher Scientific, Carlsbad, CA, USA). The relative quantities of proteins were determined using Image J and normalized against the loading control.
PVDF membranes were blocked in PBS containing 5% nonfat milk for 1 h at room temperature and were then incubated with primary antibodies against PLIN2 (1:1,000; A6276; ABclonal Biotech Co., Ltd.) and GAPDH (1:3,000; BM3876; Wuhan Boster Biological Technology, Ltd., Wuhan, China) overnight at 4 °C. Then, the membranes were incubated with secondary antibodies (Abcam, ab97230, 1:3000 dilution) for 2 h at room temperature. Finally, the proteins were visualized using ChemiDoc-XRS + (Bio-Rad). Each antibody measured from same tissue within 0–192 h sampling points was on the same gel.
Data analysis
The mRNA and protein expression data were compared by ANOVA with the Duncan multiple range test pair-wise method using SPSS 22.0 software (the significant level was set to P < 0.05). Values were expressed as the means and standard deviation, unless otherwise stated.
Results
Plasma pH in response to carbonate alkalinity
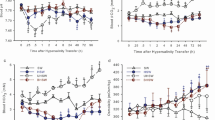
In the FW group, the plasma pH was relatively stable and was maintained at 7.37–7.42 during the 192 h experiment (Fig. 1A). In the 2 and 4 g/l AW groups, the plasma pH increased and peaked (7.60 ± 0.029 and 7.68 ± 0.035) at 24 h post-transfer (P < 0.05).
Plasma pH (A) and HCO3− concentration (B) in Oreochromis niloticus transferred from freshwater to alkaline water (2 g/l and 4 g/l). The values are presented as the means ± standard deviation (*P < 0.05). Five fish were randomly sampled for each treatment per sampling point. The values below the abscissa represent the sampling point (the same below)
HCO3 − concentration in response to carbonate alkalinity
In the FW group, the plasma HCO3− concentration was also relatively stable and maintained at 6.2–6.6 mmol/l during the 192 h experiment (Fig. 1B). In the 2 and 4 g/l AW groups, the plasma HCO3− concentrations decreased slowly at first and then increased gradually, reaching 6.6 ± 0.33 mmol/l and 6.8 ± 0.17 mmol/l at 192 h post-transfer, respectively (P < 0.05).
mRNA expression of CAhz, CAIV, SLC4A4, and SLC26A6 in response to carbonate alkalinity
All primer pairs showed a correlation coefficient (R2) higher than 0.980. The amplification efficiency varied from 91.89 to 105.43%. Therefore, all primer pairs were well designed in the RT-qPCR experiments. The reference gene was stable throughout the experiment.
Alkalinity stress resulted in a marked increment in the mRNA levels of CAhz, CAIV, SLC4A4, and SLC26A6 in an alkalinity concentration-dependent manner in the three tissues. With increasing carbonate alkalinity (FW, AW 2 g/l, and AW 4 g/l), the mRNA expression of CAhz, CAIV, SLC4A4, and SLC26A6 was significantly upregulated in the gill and kidney after 12 h of stress (Figs. 2 and 3), whereas significant changes in these genes were found in the intestine after 24 h of stress (Fig. 4).
The mRNA expressions of CAhz, CAIV, SLC4A4, and SLC26A6 in the gill of Oreochromis niloticus transferred from freshwater to alkaline water (2 g/l and 4 g/l). The values are presented as the means ± standard deviation. **and *Significance at P < 0.01 and P < 0.05 levels, respectively. Five fish were randomly sampled for each treatment per sampling point
The overall mRNA expressions of CAhz, CAIV, SLC4A4, and SLC26A6 in the examined tissues showed an “up-peak-down” variation during 192 h of stress. In the gill, the highest expression levels of the CAhz, CAIV, SLC4A4, and SLC26A6 genes occurred at 24, 24, 48, and 24 h post-transfer, respectively. No significant changes in the genes were detected within the first 6 h (P > 0.05) (Fig. 2). In the kidney, the highest levels of CAhz, CAIV, SLC4A4, and SLC26A6 occurred at 24, 24, 12, and 24 h post-transfer, respectively (Fig. 3). In the intestine, the highest levels occurred at 48, 48, 24, and 72 h post-transfer, respectively. No significant changes were detected within the first 12 h (P > 0.05; Fig. 4).
Protein expressions of CAhz, CAIV, SLC4A4, and SLC26A6 in response to carbonate alkalinity
Protein bands corresponding to antibodies against CAhz, CAIV, SLC4A4, and SLC26A6 were obtained at 29, 35, 121, and 106 kDa, as expected. Levels of CAhz, CAIV, SLC4A4, and SLC26A6 protein expression in the tissues (gill, kidney, and intestine) of fish transferred from FW to AW (4 g/l) were analyzed. To compare the expression patterns of four proteins in same tissue, gel photos of four different antibodies from same tissue are combined in a single image. In the gill, the protein expressions of CAhz, CAIV, SLC4A4, and SLC26A6 were downregulated at the beginning (0–6 h). The expression was upregulated and reached maximum levels at 24, 24, 48, and 24 h, respectively. Then, the proteins decreased again and finally stabilized (Fig. 5). The variation in the levels of CAhz, CAIV, SLC4A4, and SLC26A6 protein expression in the kidney was similar to that in the gill. These proteins increased significantly with the highest level at 24, 24, 12, and 24 h, respectively (Fig. 6).
Protein expressions of CAhz, CAIV, SLC4A4, and SLC26A6 in the gill of Oreochromis niloticus transferred from freshwater to alkaline water (4 g/l). In western blot analysis, 50 µg of protein was loaded in each lane. The values are presented as the mean ± standard deviation. The different lowercase letters indicate significant differences (P < 0.05). Five fish were randomly sampled for each treatment per sampling point
Although the mRNAs of CAhz, CAIV, and SLC4A4 mRNA were detected in the intestine by using RT-PCR, these proteins were not detected in the intestine. The SLC26A6 protein was detected at 6 h post-transfer, reached a peak at 72 h, and continued until 192 h (Fig. 7).
Discussion
It is generally accepted that the caudal puncture of fish removed from the water will cause blood acidosis. That is, when fish gills leave the water (becoming hypoxic), it will lead to an increase in lactic acid (from anaerobic respiration) and carbon dioxide (due to gill collapse), which together reduce the blood pH. Therefore, the actual plasma pH should be higher than the value measured in this study. Water with high carbonate alkalinity can cause different degrees of damage to the tissues and organs of the fish (Galat et al., 1985; Li & Chen, 2008). Previous studies have shown that when fish are exposed to alkaline (high pH) water, which is in equilibrium with atmospheric air (and therefore atmospheric CO2), the effect on blood acid–base status causes respiratory alkalosis. Respiratory alkalosis is a rapid rise in blood pH that is caused by a rapid change in pCO2 in the water, particularly, in the boundary layer next to the gills. The movement of CO2 across the gills is very rapid due to the high permeability of the gills to gases compared to ions (Brauner et al., 2019). In this study, the fish plasma pH concentration increased in the AW groups and showed an “up-peak-down” variation trend. Plasma HCO3− decreased slowly at first and then increased gradually in the AW groups. It is suggested that the fish respond to initial respiratory alkalosis by a gradual reduction in plasma bicarbonate over time (by slower gill ion exchange processes), which helps to bring blood pH back down to normal.
CA is widely distributed in various tissues and organs. The conversion of excessive HCO3− into CO2 by CAIV catalysis could be used as an alternative rapid pathway to reduce the HCO3− concentration absorbed from HCO3− AW. CAII catalyzes the reversible conversion of CO2 and water to HCO3− and H+, whereas anion exchanger 1 (AE1) transports HCO3− in exchange for Cl− reversibly across the plasma membrane (Perry et al., 2003; Georgalis et al., 2006). The SLC4A4 (Na+/HCO3−) cotransporter is a basolateral Na+-dependent bicarbonate transporter that plays important roles in intracellular pH regulation and transepithelial HCO3− movement. In terrestrial vertebrates, this transporter usually mediates the coupled movement of sodium and bicarbonate ions across the plasma membrane with a stoichiometry of 3 HCO3− per Na+ (Romero et al., 2004). SLC26A6 is the predominant apical membrane Cl−/HCO3− exchanger and is widely expressed in various epithelial tissues, including renal tubular epithelial and intestinal epithelial membranes (Mount & Romero, 2004; Alper & Sharma, 2013; Boyle et al., 2015). The SLC26 anion channel family can encode and transport various anions, including Cl−, HCO3−, and SO42−. The expression of SLC26A6 in the luminal membrane of epithelial tissue is involved in the absorption of 1 Cl− and secretion of 2 HCO3− (Mount & Romero, 2004; Alper & Sharma, 2013; Boyle et al., 2015). In this study, the mRNA expressions of CAhz, CAIV, SLC4A4, and SLC26A6 changed in alkalinity- and time-dependent manners in response to the alkaline stress in the gill, kidney, and intestine of tilapia. The protein expression of these genes showed a “down-up-peak-down-stable” pattern in the gill and kidney. This expression pattern was consistent with the trend of the plasma pH level and HCO3− concentration. Thus, these four genes are involved in the response to alkalinity stress to maintain the acid–base and ion homeostasis in tilapia. Protein expression of SLC4A4 precedes mRNA induction (or the downregulation of protein at the beginning) could be explained by the consumption of existing proteins. In addition, the expression of CAhz and CAIV reached a peak value at the same time in the same tissues. By contrast, there was always a difference between SLC26A6 and SLC4A4 in the time the peak values were reached. The time of the highest expression level was always later in the intestine than in the gill and kidney. These results suggest apparent differences in the timing of the response in the different tissues in relation to the same gene. The differential expression between SLC26A6 and SLC4A4 may be explained by their distinct functions or distributions. Further study is needed to reveal the corresponding mechanisms. In addition, pH is also increased along with salinity, especially from FW (pH is approximately 7) to seawater (pH is over 8). In an experiment that transferred tilapia from FW to seawater, it was found that the expression of SLC9A3 and carbon metabolism-related genes changed significantly, and was different from that under alkalinity stress (Xu et al., 2015). It was speculated that the effects of two environmental stresses on pH and carbonate/bicarbonate in tilapia were different, and the corresponding adaptation mechanisms were also different.
The regulation of alkalinity tolerance in fish has been well studied (Chang et al., 2021; Evans et al., 2005; Liu et al., 2022; Grosell, 2011; Taylor et al., 2010; Zhao et al., 2020). The gill is the main organ responsible for osmotic adjustment, acid–base regulation, and excretion through the exchange of substances between the fish and the external environment (Tang et al., 2010). In our previous studies, the gill structure changed correspondingly during adaptation to alkaline conditions (Wang et al., 2016). The fish kidney can transport HCO3− to the blood through kidney reabsorption, which plays a very important role in acid–base balance (Goss & Perry, 1994; Georgalis et al., 2006). The intestines may also regulate body homeostasis by absorbing water and excreting HCO3− through various ion transporters in the intestinal cell membrane (Grosell & Taylor, 2007). In this study, western blot results showed that the CAhz, CAIV, SLC4A4, and SLC26A6 proteins were expressed in the gill and kidney (0–192 h), whereas the CAhz, CAIV, and SLC4A4 proteins were not detected in the intestine (0–192 h). SLC26A6 was detected in the intestine at a relatively low level (12–192 h). Hence, we considered that the intestinal tract could also play a role in osmotic regulation and acid–base homeostasis in tilapia, but its effect may not be as obvious as in the gill and kidney. The absence of protein is because RT-PCR technology is more sensitive than western blot technology, which requires a certain abundance of the associated protein in the tissue for detection. In addition, further studies of comparing the effects of different environment stress caused by different ion components (such as NaCl, Na2SO4 and NaHCO3) on fish would help us to find key pathway or ion channel for acid–base regulation under certain stress.
Conclusion
In summary, stress caused by carbonate alkalinity could increase the plasma pH in O. niloticus. During the early stage of stress, the plasma bicarbonate concentration decreases, which helps to return the plasma pH value to normal. CAhz, CAIV, SLC4, and SLC26 contribute to the regulation of homeostasis through HCO3− metabolism or transport in tilapia.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Alper, S. L. & A. K. Sharma, 2013. The SLC26 gene family of anion transporters and channels. Molecular Aspects of Medicine 34: 494–515. https://doi.org/10.1016/j.mam.2012.07.009.
Boyle, D., A. M. Clifford, E. Orr, D. Chamot & G. G. Goss, 2015. Mechanisms of Cl− uptake in rainbow trout: Cloning and expression of slc26a6, a prospective Cl−/HCO3− exchanger. Comparative Biochemistry and Physiology Part A Molecular and Integrative Physiology 180: 43–50. https://doi.org/10.1016/j.cbpa.2014.11.001.
Brauner, C. J., R. B. Shartau, C. Damsgaard, A. J. Esbaugh, R. W. Wilson & M. Grosell, 2019. 3-Acid–base physiology and CO2 homeostasis: regulation and compensation in response to elevated environmental CO2. Fish Physiology 37: 69–132. https://doi.org/10.1016/bs.fp.2019.08.003.
Chang, Y. M., X. F. Zhao, H. J. Liew, B. Sun, S. Y. Wang, L. Luo, L. M. Zhang & L. Q. Liang, 2021. Effects of bicarbonate stress on serum ions and gill transporters in alkali and freshwater forms of amur ide (Leuciscus waleckii). Frontiers in Physiology 12: 676096. https://doi.org/10.3389/fphys.2021.676096.
Evans, D. H., P. M. Piermarini & K. P. Choe, 2005. The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid–base regulation, and excretion of nitrogen waste. Physiological Reviews 85: 97–177. https://doi.org/10.1152/physrev.00050.2003.
Galat, D. L., G. Post, T. J. Keefe & G. R. Bouck, 1985. Histological changes in the gill, kidney and liver of Lahontan cutthroat trout, Salmo clarki henshawi, living in lakes of different salinity-alkalinity. Journal of Fish Biology 27: 533–552. https://doi.org/10.1111/j.1095-8649.1985.tb03200.x.
Georgalis, T., S. F. Perry & K. M. Gilmour, 2006. The role of branchial carbonic anhydrase in acid–base regulation in rainbow trout (Oncorhynchus mykiss). Journal of Experimental Biology 209: 518. https://doi.org/10.1242/jeb.02018.
Gilmour, K. M. & S. F. Perry, 2009. Carbonic anhydrase and acid–base regulation in fish. Journal of Experimental Biology 212: 1647–1661. https://doi.org/10.1242/jeb.029181.
Goss, G. G. & S. F. Perry, 1994. Different mechanisms of acid–base regulation in rainbow trout (Oncorhynchus mykiss) and american eel (Anguilla rostrata) during NaHCO3 infusion. Physiological Zoology 67: 381–406. https://doi.org/10.1086/physzool.67.2.30163854.
Goss, G. G., S. F. Perry, C. M. Wood & P. Laurent, 1992. Mechanisms of ion and acid–base regulation at the gills of freshwater fish. The Journal of Experimental Zoology 263: 143–159. https://doi.org/10.1002/jez.1402630205.
Grosell, M., 2006. Intestinal anion exchange in marine fish osmoregulation. The Journal of Experimental Biology 209: 2813–2827. https://doi.org/10.1242/jeb.02345.
Grosell, M., 2010. 4-The role of the gastrointestinal tract in salt and water balance. Fish Physiology 30: 135–164. https://doi.org/10.1016/S1546-5098(10)03004-9.
Grosell, M., 2011. Intestinal anion exchange in marine teleosts is involved in osmoregulation and contributes to the oceanic inorganic carbon cycle. Acta Physiologica 202: 421–434. https://doi.org/10.1111/j.1748-1716.2010.02241.x.
Grosell, M. & J. R. Taylor, 2007. Intestinal anion exchange in teleost water balance. Comparative Biochemistry and Physiology Part a, Molecular and Integrative Physiology 148: 14–22. https://doi.org/10.1016/j.cbpa.2006.10.017.
Kurita, Y., T. Nakada, A. Kato, H. Doi, A. C. Mistry, M. H. Chang & S. Hirose, 2008. Identification of intestinal bicarbonate transporters involved in formation of carbonate precipitates to stimulate water absorption in marine teleost fish. American Journal of Physiology Regulatory Integrative and Comparative Physiology 294: R1402. https://doi.org/10.1152/ajpregu.00759.2007.
Li, C. C. & J. C. Chen, 2008. The immune response of white shrimp Litopenaeus vannamei and its susceptibility to Vibrio alginolyticus under low and high pH stress. Fish and Shellfish Immunology 25: 701–709. https://doi.org/10.1016/j.fsi.2008.01.007.
Liu, Y., M. Yao, S. Li, X. Wei, L. Ding, S. Han, P. Wang, B. Lv, Z. Chen & Y. Sun, 2022. Integrated application of multi-omics approach and biochemical assays provides insights into physiological responses to saline-alkaline stress in the gills of crucian carp (Carassius auratus). Science of the Total Environment 822: 153622. https://doi.org/10.1016/j.scitotenv.2022.153622.
Michael, K., C. M. Kreiss, M. Y. Hu, N. Koschnick, U. Bickmeyer, S. Dupont, H. O. Pörtner & M. Lucassen, 2016. Adjustments of molecular key components of branchial ion and pH regulation in Atlantic cod (Gadus morhua) in response to ocean acidification and warming. Comparative Biochemistry and Physiology Part b, Biochemistry and Molecular Biology 193: 33–46. https://doi.org/10.1016/j.cbpb.2015.12.006.
Mount, D. B. & M. F. Romero, 2004. The SLC26 gene family of multifunctional anion exchangers. Pflügers Archiv European Journal of Physiology 447: 710–721. https://doi.org/10.1007/s00424-003-1090-3.
Parra, J. G. & B. Baldisserotto, 2007. Effect of Water pH and Hardness on Survival and Growth of Freshwater Teleosts. In Kapoor, B. (ed), Fish Osmoregulation Science Publishers, New York: 135–150.
Perry, S. F., M. Furimsky, M. Bayaa, T. Georgalis, A. Shahsavarani, J. G. Nickerson & T. W. Moon, 2003. Integrated responses of Na+/HCO3- cotransporters and V-type H+-ATPases in the fish gill and kidney during respiratory acidosis. Biochimica Et Biophysica Acta (BBA) Biomembranes 1618: 175–184. https://doi.org/10.1016/j.bbamem.2003.09.015.
Pierre, L., P. W. Michael, C. Claudine & M. W. Chris, 2000. The effect of highly alkaline water (pH 9.5) on the morphology and morphometry of chloride cells and pavement cells in the gills of the freshwater rainbow trout: Relationship to ionic transport and ammonia excretion. Canadian Journal of Zoology 78: 307–319. https://doi.org/10.1139/z99-207.
Romero, M. F. & W. F. Boron, 1999. Electrogenic Na+/HCO3− cotransporters: cloning and physiology. Annual Review of Physiology 61: 699–723. https://doi.org/10.1146/annurev.physiol.61.1.699.
Romero, M. F., C. M. Fulton & W. F. Boron, 2004. The SLC4 family of HCO3- transporters. Pflügers Archiv: European Journal of Physiology 447: 495–509. https://doi.org/10.1007/s00424-003-1180-2.
Ruiz-Jarabo, I., S. F. Gregório, P. Gaetano, F. Trischitta & J. Fuentes, 2017. High rates of intestinal bicarbonate secretion in seawater tilapia (Oreochromis mossambicus). Comparative Biochemistry and Physiology Part a, Molecular and Integrative Physiology 207: 57–64. https://doi.org/10.1016/j.cbpa.2017.02.022.
Sender, S., K. Böttcher, Y. Cetin & G. Gros, 1999. Carbonic anhydrase in the gills of seawater- and freshwater-acclimated flounders Platichthys flesus: purification, characterization, and immunohistochemical localization. Journal of Histochemistry and Cytochemistry 47: 43–50. https://doi.org/10.1177/002215549904700105.
Tang, C. H., L. Y. Hwang & T. H. Lee, 2010. Chloride channel ClC-3 in gills of the euryhaline teleost, Tetraodon nigroviridis: expression, localization and the possible role of chloride absorption. Journal of Experimental Biology 213: 683–693. https://doi.org/10.1242/jeb.040212.
Taylor, J. R., E. M. Mager & M. Grosell, 2010. Basolateral NBCe1 plays a rate-limiting role in transepithelial intestinal HCO3- secretion, contributing to marine fish osmoregulation. Journal of Experimental Biology 213: 459–468. https://doi.org/10.1242/jeb.029363.
Wang, P., Q. F. Lai, Z. L. Yao, K. Zhou, T. T. Lin & H. Wang, 2015. Differential expressions of genes related to HCO3- secretion in the intestine of Gymnocypris przewalskiii during saline-alkaline water transfer. Marine Fisheries 37: 341–348. https://doi.org/10.13233/j.cnki.mar.fish.2015.04.007 (in Chinese).
Wang, Y., J. L. Zhao, J. W. Wu, T. Jeerawat, M. M. Yue & Y. Zhao, 2016. Effects of alkalinity on morphology of gill ionocytes and HCO3- transporters in gill, kidney and intestine of nile tilapia (Oreochromis niloticus). Chinese Journal of Zoology 51: 1027–1037. https://doi.org/10.13859/j.cjz.201606011 (in Chinese).
Wang, S., Y. Kuang, L. Liang, B. Sun, X. Zhao, L. Zhang & Y. Chang, 2021. Resequencing and SNP discovery of Amur ide (Leuciscus waleckii) provides insights into local adaptations to extreme environments. Scientific Reports 11: 5064. https://doi.org/10.1038/s41598-021-84652-5.
Wilkie, M. P. & C. M. Wood, 1996. The adaptations of fish to extremely alkaline environments. Comparative Biochemistry and Physiology Part b: Biochemistry and Molecular Biology 113: 665–673. https://doi.org/10.1016/0305-0491(95)02092-6.
Wilkie, M. P., P. A. Wright, G. K. Iwama & C. M. Wood, 1994. The physiological adaptations of the lahontan cutthroat trout (Oncorhynchus clarki henshawi) following transfer from well water to the highly alkaline waters of Pyramid Lake, Nevada (pH 9.4). Physiological Zoology 67: 355–380. https://doi.org/10.1086/physzool.67.2.30163853.
Xu, Z. X., L. Gan, T. Y. Li, C. Xu, K. Chen, X. D. Wang, J. G. Qin, L. Q. Chen & E. Li, 2015. Transcriptome profiling and molecularpathway analysis of genes in association with salinity adaptation in Nile Tilapia Oreochromis niloticus. PLoS ONE 10: e0136506. https://doi.org/10.1371/journal.pone.0136506.
Zhao, L. H., J. L. Zhao, J. Thammaratsuntorn & Y. Zhao, 2014. Effects of salinity-alkalinity on serum osmolality, ion concentration and mRNA expression of ion transport enzymes of Oreochromis niloticus. Journal of Fisheries of China 38: 1696–1704 (in Chinese).
Zhao, Y., C. S. Zhang, H. T. Zhou, L. Y. Song, J. Wang & J. L. Zhao, 2020. Transcriptome changes for Nile tilapia (Oreochromis niloticus) in response to alkalinity stress. Comparative Biochemistry and Physiology Part d, Genomics and Proteomics 33: 100651. https://doi.org/10.1016/j.cbd.2019.100651.
Acknowledgements
This research was supported by the National Key R&D Program of China (Grant No. 2020YFD0900400), the China Agriculture Research System of MOF and MARA (Grant No. CARS-46), and the National Natural Science Foundation of China (Grant No. 31602128). We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript. I certify that this manuscript is original and has not been published elsewhere in any form or language and will not be submitted elsewhere for publication while being considered by Hydrobiologia. In addition, the study is not split into several parts to increase the number of submissions and submitted to various journals or one journal over time. No data have been fabricated or manipulated (including images) to support our conclusions. No data, text, or theories by others are presented as if they were our own. Proper acknowledgments to other works have been given.
Funding
This research was supported by the National Key R&D Program of China (Grant No. 2020YFD0900400), the China Agriculture Research System of MOF and MARA (Grant No. CARS-46), and the National Natural Science Foundation of China (Grant No. 31602128).
Author information
Authors and Affiliations
Contributions
The submission has been received explicitly from all co-authors. Authors whose names appear on the submission have contributed sufficiently to the scientific work and therefore share collective responsibility and accountability for the results.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that no competing financial interests are associated with the work submitted for publication. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted. The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the animal ethics committee of Shanghai Ocean University (approval no. 2018–085).
Research involving human rights
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: S. Koblmüller, R. C. Albertson, M. J. Genner, K. M. Sefc & T. Takahashi / Advances in Cichlid Research V: Behavior, Ecology and Evolutionary Biology
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Y., Wang, Y., Zhang, C. et al. Variation in pH, HCO3−, carbonic anhydrases, and HCO3− transporters in Nile tilapia during carbonate alkalinity stress. Hydrobiologia 850, 2447–2459 (2023). https://doi.org/10.1007/s10750-022-05020-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-05020-6