Abstract
Aquatic decapod crustaceans live in a highly variable and constantly changing environment, permanently challenging their physiological homeostasis. One of the key processes considered ensuring physiological performance and function is the maintenance of acid–base balance. This chapter aims to provide a comprehensive overview of the challenges for aquatic decapod crustaceans’ acid–base homeostasis, as well as the current knowledge regarding the respective mechanisms for acid–base regulation. Like many other marine organisms including fish and cephalopods, aquatic decapod crustaceans are capable of counteracting an acidosis or alkalosis of their bodily fluids mainly by modulating their hemolymph bicarbonate levels in order to buffer the pH. In addition, they adjust the excretion of acid and/or base equivalents, respectively. It is evident that ion transport mechanisms at the level of the gill epithelium contribute substantially to these acid–base regulatory processes, including the modulation of gene (mRNA) expression levels of distinct gill epithelial transcripts like carbonic anhydrase, Rhesus-like protein, Na+/K+-ATPase, V-ATPase and Cl−/HCO3 −-exchanger. As a result of recently generated data mainly from gill perfusion experiments, a novel hypothetical working model for branchial acid–base regulation is put forward. It ties in general ion as well as ammonia regulatory mechanisms and accounts for the obvious linkage between these three processes.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Summary
Aquatic decapod crustaceans live in a highly variable and constantly changing environment, permanently challenging their physiological homeostasis. One of the key processes considered ensuring physiological performance and function is the maintenance of acid–base balance. This chapter aims to provide a comprehensive overview of the challenges for aquatic decapod crustaceans’ acid–base homeostasis, as well as the current knowledge regarding the respective mechanisms for acid–base regulation. Like many other marine organisms including fish and cephalopods, aquatic decapod crustaceans are capable of counteracting an acidosis or alkalosis of their bodily fluids mainly by modulating their hemolymph bicarbonate levels in order to buffer the pH. In addition, they adjust the excretion of acid and/or base equivalents, respectively. It is evident that ion transport mechanisms at the level of the gill epithelium contribute substantially to these acid–base regulatory processes, including the modulation of gene (mRNA) expression levels of distinct gill epithelial transcripts like carbonic anhydrase, Rhesus-like protein, Na+/K+-ATPase, V-(H+)-ATPase and Cl−/HCO3 −-exchanger. As a result of recently generated data mainly from gill perfusion experiments, a novel hypothetical working model for branchial acid–base regulation is put forward. It ties in general ion as well as ammonia regulatory mechanisms and accounts for the obvious linkage between these three processes.
6.2 The Importance of Acid–Base Homeostasis in Aquatic Decapod Crustaceans
Maintaining acid–base balance is fundamental for all living organisms, including decapod crustaceans (Henry and Wheatly 1992). Only slight disturbances in the concentration of acid–base equivalents resulting in shifts of pH in the intra- or extracellular fluids may impair properties of essential proteins and their regulation (i.e. enzymes, Somero 1986; respiratory proteins, Riggs 1988; Truchot 1975a) and ultimately lead to a disruption of basic physiological functions. Consequently, securing whole animal acid–base homeostasis not only includes the maintenance of a constant intra- and extracellular pH but also needs mechanisms in place for its re-adjustments after an acid–base disturbance. Factors to disrupt acid–base homeostasis in decapod crustaceans might include a variety of intrinsic as well extrinsic parameters like the internal acid load due to exercise (e.g. Booth et al. 1984; Rose et al. 1998), shifts in CaCO3 handling during the moulting process (e.g. Mangum et al. 1985) and fluctuations of environmental parameters like salinity (e.g. Whiteley et al. 2001), temperature (e.g. McMahon et al. 1978; Whiteley and Taylor 1990), pO2/pCO2 (e.g. De Fur et al. 1983; Urbina et al. 2013) and ammonia (e.g. Cheng et al. 2013; Martin et al. 2011). The regulation of acid–base balance in aquatic decapod crustaceans therefore is a complex interaction of physiological and biochemical processes including respiratory gas exchange, ion regulation and overall adjustments of metabolism (Henry and Wheatly 1992).
Most studies on acid–base regulation in decapod crustaceans to date concentrate on whole animal extracellular acid–base status and its responses upon disturbances (7 see Sect. 6.3). Few studies investigated acid–base regulation on a cellular level, and therefore, little is known about direct trans-branchial transport of acid–base equivalents in decapod crustaceans (7 see Sect. 6.4). While most available acid–base related data has been collected on brachyuran hyper-regulating crabs (e.g. the green crab Carcinus maenas, the blue crab Callinectes sapidus and the Chinese mitten crab Eriocheir sinensis), this chapter aims to provide a broader review of the currently available literature on acid–base regulation also in the other major members of decapod crustaceans, namely, prawns (in this chapter referred to as synonym for penaeoid and sergestoid shrimp), Anomura, shrimp (caridean shrimp), lobster and crayfish (De Grave et al. 2009; . Fig. 6.1).
Overview of the different subgroups of decapod crustaceans discussed in this chapter. The nomenclature follows De Grave et al. (2009). To avoid confusion, members of the suborder Dendrobranchiata are referred to as “prawns” throughout the text, while “shrimps” solely refers to the infraorder Caridae
6.2.1 Tissues Involved in Acid–Base Regulation
In crustaceans, anisosmotic extracellular regulation (AER), or the osmotic and ionic buffering of the extracellular fluid in order to maintain (acid–base) homeostasis, is believed to be mainly driven by the gills, antennal glands and gut (McNamara and Faria 2012). A tissue-specific inventory of epithelial membrane transporters then translates the changes of extracellular adjustments into the cell to ensure the intracellular maintenance of acid–base balance (Freire et al. 2008).
Gills
Similar to fish and cephalopods (7 see Chap. 11), the majority of the acid–base-relevant ion regulatory apparatus of decapod crustaceans is located in their gill epithelia (Henry et al. 2012; Larsen et al. 2014, and references therein). Not only are the gills involved in respiratory and acid–base physiology, but they are the major organs also for ion regulation and ammonia excretion, therefore linking all of these regulatory processes (Freire et al. 2008; Henry et al. 2012).
All decapod crustaceans possess paired gills that are covered by a fine chitinous cuticle, lined by a single-layered epithelium and attached to a basal lamina (Freire et al. 2008). Depending on taxa, the number of paired gills, their location of attachment and the arrangement of the gill lamellae (phyllobranchiate, trichobranchiate, dendrobranchiate, as indicated in . Fig. 6.1) vary substantially, providing more or less gill surface amplification for ion and gas exchange processes between the external (environment) and the internal medium (hemolymph). For further details, the reader is referred to the extensive descriptions by Taylor and Taylor (1992).
According to their different life strategies (i.e. primary habitat/habitat changes), gill epithelia of decapod crustaceans exhibit specific characteristics that can vary even within the respective sub-/infraorder or (super) family. Acid–base status and regulation in decapod crustaceans have been shown to be linked to external salinity and NaCl regulation (i.e. in the freshwater crayfish Astacus leptodactylus (Ehrenfeld 1974), C. sapidus (Henry and Cameron 1982) and E. sinensis (Whiteley et al. 2001), and therefore the tightness of the gill epithelium consequently might also affect the animals’ capability for acid–base regulation. While the gill epithelia of strong hyper-regulators like E. sinensis (Weihrauch et al. 1999) and freshwater crayfish (Wheatly and Gannon 1995) represent a tight epithelium (conductance for ions <5 mS cm−2), the epithelium of weak hyper-regulators like C. maenas (Weihrauch et al. 1999) and the American lobster Homarus americanus (Lucu and Towle 2010) is much leakier and allows for increased intercellular transport of ions (conductance 40–60 mS cm−2). Gills of osmoconforming crustaceans like M. magister (Hunter and Rudy 1975) or Cancer pagurus (Weihrauch et al. 1999) in contrast are highly permeable for ions (conductance > 200 mS cm−2), and therefore these species are very limited in their capability to osmoregulate (Larsen et al. 2014).
Furthermore, specializations of gill epithelia can be seen at the ultrastructural level. Of the five different cell types found in decapod crustacean gill epithelia (thin cells, thick cells, flange cells, attenuated cells and pilaster cells; Freire et al. 2008), thin cells are generally believed to be associated with respiratory epithelia due to their low height (1–5 μM), a lack of extensive membrane folding and low number of mitochondria. Consequently, they have been considered to play an increased role in acid–base regulation rather than osmoregulation (Freire et al. 2008). Thin cells are found in all gills of osmoconforming crabs as well as the most anterior four to six pairs of gills of hyper-regulating crabs like C. maenas (Compere et al. 1989), C. sapidus (Copeland and Fitzjarrell 1968) and E. sinensis (Barra et al. 1983). Some thin cells were also observed in the gill epithelium of lobsters (Haond et al. 1998). In some hyper-regulating crabs like C. maenas (Compere et al. 1989) and C. sapidus (Copeland and Fitzjarrell 1968), thin cells are found to surround thick cells (also called ionocytes due to their supposed major role in ion transport) in the most posterior (osmoregulatory) pairs of gills, therefore indicating that acid–base regulatory properties might not be solely associated with the anterior gills in these species. To date, however, the direct site for acid–base regulation in euryhaline Brachyuran gills has not been confirmed, while osmoregulation has been demonstrated to be associated predominantly with the posterior gills (Henry et al. 2012) and ammonia with both anterior and posterior gills (Weihrauch et al. 1999; 7 see Chap. 1).
The gill epithelia of lobsters (Haond et al. 1998), prawns (Bouaricha et al. 1994), shrimp (Freire and McNamara 1995) and freshwater crayfish (Morse et al. 1970) on the other hand are more homogeneous and possess so-called flange cells that exhibit features of both thin and thick cells of crabs and are therefore believed to incorporate both respiratory/acid–base and ion regulatory functions (Freire et al. 2008).
Even though most pilaster cells (resembling thin or thick cell criteria depending on their localization in the gill, Compere et al. 1989) in the epithelia of crabs and crayfish seem to inherit a mainly stabilizing function for the intra-lamellar septum, they are the exclusive sites for the vacuolar-type H+-ATPase (V-(H+)-ATPase) in E. sinensis, indicating an additional role for these cells in acid–base regulation in this species (Freire et al. 2008).
Antennal Glands
Situated at the anterior end of the body at the base of the eyestalks, the paired antennal glands are mainly involved in the production (ultrafiltration) and ionic regulation of the urine to maintain extracellular water balance (Larsen et al. 2014). Therefore, they can be regarded as analogues of the nephron of the vertebrate kidney, the major acid–base regulatory organ in mammals and other terrestrial vertebrates (Weiner and Verlander 2013). Even though urine [Na+], [K+] and [Cl−] are adjusted upon disturbance of (acid–base) homeostasis, antennal glands are believed to rather contribute to the regulation of divalent cations like Ca2+ and Mg2+ based on the respective clearance ratios (Wheatly 1985).
Only a few studies have investigated the role of antennal glands in acid–base regulation in decapod crustaceans. In freshwater-acclimated blue crabs C. sapidus, net urinary acid–base and ammonia efflux were negligible and did not change significantly when animals were exposed to hypercapnia (2 % pCO2; Cameron and Batterton 1978a). In the Dungeness crab Metacarcinus magister acclimated to dilute salinity (~20 ppt), the increase in antennal gland-mediated HCO3 − efflux resulted in an increased alkalinization of the urine, but was accompanied by an increase in HCO3 − reabsorption over time, likely to assist in HCO3 − accumulation in the hemolymph (Wheatly 1985). However, in respect to the overall base output in response to dilute salinity acclimation, the antennal gland of M. magister contributed to only 10 % at best (Wheatly 1985). Also in the freshwater-acclimated euryhaline crayfish Pacifastacus leniusculus exposed to hyperoxia, an initial extracellular acidosis resulted in an increase in HCO3 − reabsorption from the urine to buffer hemolymph pH, but in parallel, an acidification of the urine was observed mainly due to increased ammonia (NH4 +) excretion (Wheatly and Toop 1989). Similar to the observations of hyposaline-acclimated M. magister, however, net H+ efflux accounted for only 10 % of the whole animal response in this crayfish. Interestingly, antennal glands of P. leniusculus show a significantly higher activity of carbonic anhydrase (CA), the enzyme involved in the hydration of CO2 to form H2CO3 and subsequently dissociate to H+ and HCO3 −, compared to the gills (Wheatly and Henry 1987). When acclimated to hypersaline conditions, however, CA activity was progressively reduced with increased salinity (up to 80 % at ~25 ppt).
In conclusion, the existing data suggest an overall negligible involvement of antennal glands in acid–base regulation in decapod crustaceans.
Gut and Gut Diverticula
Besides the respective adjustment of urine flow, gut-mediated fluid absorption and secretion of digestive fluid have been shown to be ion dependent in both hypo- and hyper-regulating crustaceans and likely help in the regulation of the hemolymph composition (Mantel and Farmer 1983). Accordingly, crustacean gut epithelia have been shown to possess Na+/K+-ATPase that in addition to their function in ion regulation might also promote the uptake of nutrients (Chu 1987; Chung and Lin 2006; Mantel and Farmer 1983). Even though being directly exposed to the environment and showing evidence for the capability to take up/excrete small ions like Na+, Cl−, K+, Ca2+ and SO4 − (Ahearn 1978; Mantel and Farmer 1983), a potential role of the gut in acid–base regulation has not been investigated to date.
In addition to the gut, the presence of an electrogenic, likely apically situated 2Na+:1H+-exchanger in the hepatopancreas of lobster and freshwater prawns (Ahearn et al. 1990, 1994) would provide an important key player for acid–base regulation in this tissue. Similar as for the gut, however, a direct involvement for the hepatopancreas in maintaining acid–base homeostasis has not been investigated to date. Clearly, future studies need to be performed in order to characterize the potential roles of gut and hepatopancreas in crustacean acid–base regulation.
6.2.2 Preadaptation Through Life Strategies?
The estimated 14,000 species of aquatic decapod crustaceans can be found in nearly all water bodies of the world. As described earlier, the capability of inhabiting specific water bodies mainly depends on the crustaceans’ regulatory capacity due to the characteristics of the gill epithelium as well as the general permeability of their carapace. Acid–base regulatory capabilities are therefore likely correlated with life history, genetic predisposition and physiological plasticity (Melzner et al. 2009). As metazoans with a relatively high metabolic rate and level of activity, a high capacity to adjust body fluid pH (also dependent on the relatively large fluid volume) and relatively little expressed calcified structures compared to other marine calcifiers like corals, echinoderms and molluscs (decapod), crustaceans are believed to cope better with changes in their environment than other marine invertebrates (Wittmann and Pörtner 2013).
The following sections are intended to roughly characterize the basic life strategies for key species of the major decapod crustacean groups that will be discussed in more detail concerning their acid–base regulatory capabilities in the subsequent chapters.
Prawns
While most Dendrobranchiata (Penaeidae) are euryhaline hyper-/hypo-osmoregulators and found in the marine environment (e.g. (Marsu)Penaeus japonicus (Cheng et al. 2013); (Lito)Penaeus vannamei (Liu et al. 2015)), some species of Sergestidae are found in freshwater. Many species inhabit deep (offshore) waters, while most Penaeidae are mainly found in shallow inshore tropical and subtropical waters and estuaries. Some prawn species are known to bury in mud substrates during the daytime (Tavares and Martin 2010), challenging their acid–base homeostasis as described below.
Anomura
While king crabs (Lithodidea) like the southern king crab Lithodes santolla are subtidal species that can be found between 5 and 700 m depth in temperate waters (Urbina et al. 2013), hermit crabs (Paguroidea) like Pagurus bernhardus (De la Haye et al. 2011) and Porcelain crabs (Galatheoidea) like Petrolisthes cinctipes (Carter et al. 2013) are commonly found in the intertidal zone, potentially being trapped in rocky tide pools experiencing large spatial and temporal changes in abiotic parameters as discussed below.
Crabs
With over 6700 species in 93 families, brachyuran crabs constitute to ca. 50 % of all decapod crustaceans (Ng et al. 2008). Accordingly, all imaginable life strategies and habitat uses are exhibited by this infraorder, including terrestrial species. Some of the most thoroughly investigated crabs are the osmoconforming Dungeness crab M. magister, the weak hyper-osmoregulating green crab C. maenas and the closely related blue crab C. sapidus, as well as the strong hyper-osmoregulating Chinese mitten crab E. sinensis. Like prawns and Anomura, they are oftentimes trapped in tide pools (Truchot and Duhamel-Jouve 1980) and some species bury in the sediment (Bellwood 2002).
Shrimp
Besides brachyuran crabs (palaemonid), shrimps are the most diverse of the decapod groups with a great inter- and intraspecific variability in osmoregulatory capabilities. While most species can be found in freshwater and are strong hyper-regulators (i.e. genus Macrobrachium), some species inhabit estuarine (brackish) and even marine waters (i.e. genus Palaemon, Palaemonetes) and are hyper-/hypo-osmoregulators (Freire et al. 2003; McNamara and Faria 2012). Some shrimp species are associated with the intertidal zones and therefore more shallow waters (i.e. Crangon crangon, Almut and Bamber 2013) or are amphidromous and occupy those habitats during their early life stages (e.g. Pandalus borealis, Hammer and Pedersen 2013; Macrobrachium olfersii, Freire and McNamara 1995; McNamara and Lima 1997). Other shrimps are deep-water dwellers (e.g. P. borealis, Hammer and Pedersen 2013).
Lobster
For the longest time, lobsters, especially the commercially important lobsters of the genus Homarus and the Norway lobster Nephros norvegicus, have traditionally been considered to be osmoconforming, stenohaline (salinity > 25 ppt) and limited to coastal and offshore habitats down to 700 m depth (Chapman 1980; Cooper and Uzmann 1980; Dall 1970). More recent research, however, identified them to be present also in estuarine and intertidal regions where they experience short-term fluctuation in salinity and other abiotic parameters (Charmantier et al. 2001). Additionally, the reproduction of lobsters seems to be dependent on higher water temperatures that lead to the animals’ migration into different habitats and consequently, their exposure to different environmental conditions that potentially challenge acid–base homeostasis (Charmantier et al. 2001).
Crayfish
Crayfish belong to the only decapod superfamily that is almost entirely found in freshwater (Reynolds et al. 2013). However, many crayfish species depend on the connection to the ocean in order to breed and therefore have a limited capability to osmo- and ion-regulate (Pequeux 1995). A remarkable number of crayfish build complex burrows in which they spend most of their life (Crandall and Buhay 2008; Guiasu 2002). Others are defined as stream or lake/pond/large river dwellers or are obligated cave dwellers (Crandall and Buhay 2008). Due to the very different chemistry of freshwater (low total osmolarity of ~4 mOsm/mainly CaCO3 vs. 1000 mOsm/mainly NaCl in marine environments), ion regulation in crayfish is challenged and they maintain a lower, yet still hyper-osmotic extracellular ionic concentration and a lower carapace permeability for ions and water compared to marine decapod crustaceans (Wheatly and Gannon 1995).
6.2.3 Challenging Acid–Base Homeostasis
There are several factors that can challenge the acid–base regulatory machinery of animals. As (opportunistic) omnivores, all decapod crustaceans experience regular internal acid loads due to the catabolism of proteins and the resulting build-up of extracellular ammonia (mostly NH4 + at physiological pH, Weiner and Verlander 2013) that can affect extra- as well as intracellular acid–base homeostasis (Larsen et al. 2014). The response of decapod crustaceans upon exercise (functional/internal hypoxia) on the other hand results in a build-up of lactate and CO2 in the hemolymph, therefore delivering H+ and challenging extracellular acid–base regulation (Henry et al. 1994; see below).
Besides these intrinsic sources of acid–base disturbances, many of the mainly benthic aquatic decapods experience regular fluctuations of the abiotic parameters pH, pCO2/pO2, salinity and temperature in their surrounding environment. In intertidal zones, estuaries and water bodies like the Baltic Sea with restricted connection to the well-buffered open ocean, naturally recurrent elevated pCO2 (hypercapnia, ~234 Pa vs. normal levels of 39 Pa) and changes in pH (7.5–8.2) and temperature (3.3–18.7 °C), as well as salinity (14.5–21.5 ppt), challenge acid–base homeostasis on a regular basis. Additionally, decapods in these shallow water environments are prone to be trapped in tide pools, where they experience even more drastic changes in all abiotic parameters (Truchot 1988), including extremely low levels of oxygen (hypoxia; Truchot and Duhamel-Jouve 1980). In extreme cases, these tide pools fall dry so that decapods are exposed to air. As mentioned above, many decapod crustacean species hide in burrows and caves or bury in the sediment to avoid predators (Larsen et al. 2014). With only limited water circulation around them, metabolic ammonia builds up around the animals, consequently exposing them to high environmental ammonia (HEA, Weihrauch et al. 2004a, b), another challenging factor for acid–base homeostasis mainly for osmoconforming crabs like M. magister (Martin et al. 2011). Furthermore, pH has been shown to decrease to as low as 6.5 already within the first few centimetres of sand and mud substrates, accompanied by elevated CO2 levels of up to 1,600 Pa (Widdicombe et al. 2011).
Besides these naturally occurring challenges for acid–base homeostasis, global climate change and its impacts on acid–base regulation of decapod crustaceans and other invertebrates have become of greatest concern (Whiteley 2011). On the one hand, the anthropogenic increase of atmospheric pCO2 and its oceanic uptake will result in a decrease of the surface ocean pH of up to 0.3 units by the year 2100 (corresponding to pCO2 of 1000 ppm; IPCC 2013) and up to 1.4 units by the year 2300 (corresponding to a pCO2 of 8000 ppm; Caldeira and Wickett 2005), a process termed ocean acidification (IPCC 2013). Even though crustaceans are predicted to be less sensitive to ocean acidification than other invertebrates, still one third of all investigated species in a current meta-analysis by Wittmann and Pörtner (2013) were negatively affected at an environmental pCO2 of 851 and 1,370 μatm (scenario RCP8.5., Meinshausen et al. 2011). On the other hand, the increase of ocean surface temperature of up to 3 °C by the year 2100 as predicted by the Intergovernmental Panel on Climate Change (Collins et al. 2013) might pose an additional challenge for acid–base homeostasis in decapod crustaceans and even to shifts in whole ecosystem ecology and animal distributions (Walther et al. 2002). As a result, so-called dead zones and oxygen minimum zones (zones of depleted or low oxygen) are markedly increasing due to the anthropogenic pollution and the resulting increase in algal blooms and likely also due to a decrease in water circulation resulting from global warming (Mora et al. 2013). In combination, ocean acidification and warming negatively affected crustacean growth and potentially survival, but had no severe effects on calcification (Harvey et al. 2013).
6.3 Whole Animal Acid–Base Homeostasis and Regulation
6.3.1 Hemolymph Acid–Base Status
In decapod crustaceans, acid–base homeostasis on the whole animal level is described best by the carbonate system characteristics of the extracellular fluid. A disturbance of acid–base homeostasis can be of metabolic (shifts in aerobic/anaerobic metabolism and production of organic acids) or respiratory (shifts in respiratory CO2) origin and leads to a decrease (acidosis) or increase (alkalosis) in hemolymph pH if not compensated for. Typically, these fluctuations are depicted in a Davenport diagram as shown in . Fig. 6.2 (Davenport 1974).
Davenport diagram for disturbances in acid–base homeostasis. (a) Metabolic and (b) respiratory components of acid–base disturbance. Indicated values are fitted roughly to represent an average decapod crustacean as listed in . Tables 6.1, 6.2, 6.3, 6.4, 6.5, 6.6 and 6.7. Diagrams are reproduced according to Davenport (1974). Filled circles, acid–base homeostasis; open circles, status after disturbance. Thin horizontal lines indicate the non-carbonate buffer capacity of the hemolymph. Thin curved lines indicate CO2 isopleths. The grey circle indicates a potential partial compensation by accumulation of HCO3 − (see text for details)
Besides a small contribution of non-carbonate buffers like the respiratory pigments and other hemolymph proteins, adjustments in the hemolymph carbonate speciation allows for the buffering of the extracellular fluid upon an acid–base disturbance, according to following equation:
Due to the low solubility of O2 in water, aquatic animals need to establish a high flow rate of the external medium over their major gas exchange surfaces in order to ensure sufficient oxygenation. Aquatic decapod crustaceans are therefore very restricted in adjusting ventilation rates in order to regulate CO2 flow. With a pK value of 6.2, extracellular CO2 is mainly dissociated into H+ and HCO3 − at average physiological pH. Therefore, acid–base disturbances may be counteracted using primarily ion regulatory mechanisms at the gills (Henry et al. 2012; Truchot 1988).
. Tables 6.1, 6.2, 6.3, 6.4, 6.5, 6.6 and 6.7 give an overview of hemolymph acid–base characteristics of decapod crustaceans under control conditions, as well as changes induced by various stressors as described in 7 Sect. 6.2.3 and below. Due to the vast number of publications on acid–base disturbances, we do not claim for the list to be complete, but rather tried to give representative examples for as many different species as possible. In case multiple studies were available for the same species, we attempted to include the most relevant publication(s) that was (were) comparable to the other studies. Data was partly extracted from graphs and values transformed into the units as depicted in the tables where applicable. If significantly different, values are given for the time point of maximum effect as well as from the end of the incubation period.
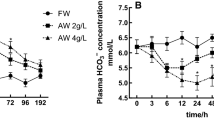
Under control conditions, all decapod crustaceans maintain their hemolymph pH typically between 7.7 and 8.0. Hemolymph pCO2 levels, however, can vary quite substantially between crustacean species and are typically low (ca. 200–500 Pa) due to the almost complete dissociation to H+ and HCO3 − at physiological pH, but high enough likely to enable diffusion out of the body along the gradient between the extracellular fluid and the environment (ca. 40 Pa; Henry et al. 2012; Melzner et al. 2009). Prawns like the Japanese tiger prawn Penaeus japonicus seem to be an exception: These decapod crustaceans have a slightly lower than average hemolymph pH (7.5) and, accordingly, a higher pCO2 (ca. 600 Pa; Cheng et al. 2013). Similar to hemolymph pCO2, levels of hemolymph HCO3 − have also been observed to vary between species and typically average between 4 and 9 mmol L−1. As indicated in . Tables 6.1, 6.2, 6.3, 6.4, 6.5, 6.6 and 6.7, control levels for hemolymph [HCO3 −] as high as 14 mmol L−1 have been observed in some studies, but these values have to be treated with caution as they may indicate that animals were in premoult rather than intermoult stages (7 see Sect. 6.3.2).
Exposure to Air (. Table 6.1)
The primary consequence for most aquatic decapod crustaceans of emerging from water is the collapse of their gills, physically impairing gas exchange processes. Many crabs are therefore retaining branchial water in their gill chambers likely to facilitate CO2 diffusion (Burnett and McMahon 1987). Due to the higher solubility of O2 in air compared to water, however, some decapod crustaceans like Pachygrapsus crassipes (Burnett and McMahon 1987) or C. maenas (Simonik and Henry 2014) are capable of extracting O2 from the air and voluntarily move out of the water to offset acid–base disturbances resulting from other stressors like hypoxia (Wheatly and Taylor 1979).
Generally, exposure to air results in a pronounced respiratory acidosis with a pH drop of 0.1–0.2 units (in crabs) up to 0.5–0.7 units (in Anomura and crayfish), a two- to fivefold increase in hemolymph pCO2 and a significant two- to threefold elevation of hemolymph HCO3 − in all investigated decapod crustaceans (. Table 6.1).
It seems, however, that there are marked species-specific differences in compensating for the experienced acidosis. While some crabs (De Fur and McMahon 1984; Simonik and Henry 2014), lobsters (Taylor and Whiteley 1989; Whiteley and Taylor 1990) and crayfish (Taylor and Wheatly 1981) seem to switch partly to anaerobic metabolism and therefore experience an additional metabolic acidosis with a pronounced increase in hemolymph lactate levels, other crabs like Eurytium albidigitum (Luquet and Ansaldo 1997) and Pachygrapsus crassipes (Burnett and McMahon 1987) seem to undergo a metabolic depression (E. albidigitum) or maintain or even increase their aerobic metabolism (P. crassipes). Additionally, some crab species were observed to increase their strong ion difference (SID) likely via ion exchange processes at the gill in response to emersion (Burnett and McMahon 1987; Luquet and Ansaldo 1997; Truchot 1979), which is interpreted to help offset the experienced acidosis (Stewart 1978). Consequently, C. maenas (Truchot 1975b), S. serrata (Varley and Greenaway 1992), H. gammarus (Whiteley and Taylor 1990), A. pallipes (Taylor and Wheatly 1981), P. crassipes and H. nudus (Burnett and McMahon 1987) are capable of fully compensating for the pH drop resulting from the experienced acidosis, while E. albidigitum is not (Burnett and McMahon 1987).
Hyper-/Hypoxia (. Table 6.2)
Due to its low solubility in water compared to air, oxygen has to be considered one of the limiting factors in the aquatic environment (Dejours 1975). Hence, only subtle changes in water pO2 result in immediate alterations of ventilation rates in aquatic decapod crustaceans in order to be able to maintain aerobic metabolism (Jouve-Duhamel and Truchot 1983; Truchot 1988). Consequently, hyperventilation as observed in moderate hypoxic conditions simultaneously leads to an increase in branchial CO2 excretion and therefore respiratory alkalosis (elevated pH and lower HCO3 −), while reduced ventilation in a (moderate) hyperoxic environment ultimately leads to accumulation of hemolymph pCO2 and hence a respiratory acidosis (lower pH and higher HCO3 −), as can be seen in . Table 6.2. In shrimp and C. maenas, the increase in hemolymph lactate during moderate hypoxia and anoxia, respectively, indicates that in these decapod crustaceans, a metabolic component seems to be present that might explain the reduced levels of total carbon/HCO3 −, but did not affect the actual increase in hemolymph pH (Taylor and Spicer 1991). Interestingly, when the same shrimp species P. elegans and P. serratus were exposed to a severe hypoxia (<2 Pa), lactate levels stayed constant and total carbon was not affected (Taylor and Spicer 1991).
Hypercapnia (. Table 6.3)
In contrast to hypoxia, exposure to elevated environmental pCO2 does not drive a ventilation response in decapod crustaceans due to its very similar solubility in air and water (Henry et al. 2012; Jouve-Duhamel and Truchot 1983). Nonetheless, exposure to hypercapnia leads to a respiratory acidosis marked by a rapid drop in hemolymph pH of up to 0.4 units and substantial increases in pCO2 (two- to fourfold) in all investigated decapod crustaceans (. Table 6.3). Elevated extracellular pCO2 is believed to be maintained in order to ensure an outwardly directed CO2 gradient for the diffusion-based excretion of metabolic CO2 (Melzner et al. 2009). Most decapod crustaceans are capable of fully compensating for the respiratory acidosis by accumulating HCO3 − in their hemolymph to buffer excess protons, likely via active ion regulatory processes at the gill. While some species are capable of maintaining or even increasing their metabolic rate in response to hypercapnia (C. maenas, Appelhans pers. communication), others experience a metabolic depression (e.g. M. magister, Hans et al. 2014; P. borealis, Hammer and Pedersen 2013). Interestingly, green crabs C. maenas that are acclimated to full-strength seawater (32–35 ppt; Truchot 1975c; Fehsenfeld and Weihrauch 2016a) seemed to accumulate more CO2 in their hemolymph than brackish-water acclimated specimen (Appelhans et al. 2012; Fehsenfeld and Weihrauch 2013). In contrast to brackish-water crabs, the resulting respiratory acidosis in the seawater-acclimated crabs was not fully compensated for after 24 h, and hemolymph pH decreased. This example indicates that acid–base and osmoregulation might indeed be closely linked in this species.
Temperature (. Table 6.4)
It has been shown for poikilotherm animals such as decapod crustaceans that temperature correlates inversely with hemolymph pH in order to maintain extracellular H+/OH− ratios to ensure a constant net charge of proteins (Howell et al. 1973; Truchot 1973). In parallel, hemolymph pCO2 seems to generally stay constant/increase only slightly with increasing temperature, while [HCO3 -] and/or total carbon (CT) decreases more drastically. The authors of the respective studies (Cameron and Batterton 1978b; Truchot 1973) attributed the changes in CT to active regulation of HCO3 − via ion exchanges at the gills in order to compensate for the acidosis, rather than solution of the carapace or passive processes alone (Henry et al. 2012).
Salinity (. Table 6.5)
Generally, acclimation to low salinity results in a metabolic alkalosis in all investigated decapod crustaceans, characterized by an increase in hemolymph pH at relatively stable pCO2 and a significant increase in [HCO3 −]. Conversely, when freshwater crayfish (Wheatly and McMahon 1982), freshwater E. sinensis (Truchot 1992) or brackish-water acclimated C. maenas (Truchot 1981) were acclimated to full-strength seawater, they developed a metabolic acidosis characterized by a decrease in hemolymph pH and HCO3 −. In this case, however, an additional slight respiratory alkalosis (decrease in pCO2) was observed in parallel, likely compensating for the respiratory acidosis. Throughout the time course of different salinity acclimations, species can exhibit specific alterations to this general pattern. An initial respiratory acidosis, for example, was observed in C. maenas upon acclimation to dilute salinity before switching into the expected metabolic alkalosis (Truchot 1981), and in E. sinensis a transient respiratory acidosis marked by a spontaneous drop in pH was only present at days 6 (Truchot 1992).
While Henry and Cameron (1982) attributed the observed increase in hemolymph [HCO3 -] following acclimation of C. sapidus to dilute salinity to the additionally observed change in the strong ion difference (SID), no equivalent observation was made in brackish-water acclimated E. sinensis in the study by Whiteley et al. (2001). In contrast, Truchot (1981, 1992) suggested metabolic adjustments correlated to isosmotic intracellular regulation in the cells to be responsible, resulting in either a measureable efflux of base or acid into the environment. These observations reveal the complex nature of acid–base disturbances upon different salinity acclimations, and consequently, the reasons for the observed metabolic alkalosis and acidosis are not yet fully understood.
Exercise (. Table 6.6)
Hemolymph lactate levels are typically held lower than 1 mmol L−1 and negligible in undisturbed decapod crustaceans, but can increase more than tenfold in crabs that experience a metabolic acidosis due to exercise (forced movement). Furthermore, the experienced acidosis is characterized by an immediate drop in pH of up to 0.4 units and a twofold increase in hemolymph pCO2, therefore also resembling characteristics of a respiratory acidosis. Interestingly, exercised lobsters seem to be able to avoid anaerobic metabolism during exercise for the most part and experience primarily a respiratory acidosis without the substantial rises in hemolymph lactate observed in other crustaceans (Rose et al. 1998).
In M. magister (McDonald et al. 1979), C. maenas and C. sapidus (Booth et al. 1984), the proton concentration in the hemolymph was observed to be lower than could be expected from the accumulated lactate, given that both are produced in equimolar quantities during glycolysis (Hochachka and Mommsen 1983). Due to the observed drastic increase in ammonia excretion, Booth et al. (1984) concluded that at least part of the protons are excreted as NH4 + via ion exchange processes at the gill epithelium.
Combined Stressors (. Table 6.7)
As is clear by the previous sections, extracellular acid–base regulation in response to environmental disturbances can be quite complex despite some common principles. While the discussed studies isolated one stressor at a time and investigated its effects on the respective species’ acid–base characteristics, environments are rarely that “simple” and a combination of simultaneous stressors seems much more likely, especially in tide pools (Truchot 1988) or in the face of ongoing global climate change (IPCC 2013).
For example, when combined with an increase in water temperature, hypercapnia resulted in a respiratory alkalosis in N. puber that was not observed when crabs were exposed to either one of the stressors alone (Rastrick et al. 2014). Even though the increase in HCO3 − upon hypercapnia and high-temperature acclimation rendered the crabs more resistant to short periods of subsequent emersion, they still experienced the same magnitude of acidosis, and recovery from these stressors was significantly attenuated. In a different example, prior acclimation of green crabs to hypercapnia enabled them to avoid an uncompensated hypercapnic acidosis that was induced by low environmental alkalinity in normocapnic-acclimated animals (Truchot 1984). In C. productus, the respiratory acidosis usually observed following exposure to hyperoxia was not observed in crabs that were first exposed to air (De Fur et al. 1980). Finally, P. joyneri exposed to a combination of elevated temperature and hypercapnia no longer experienced an acidosis as to be expected by studies on other decapod crustaceans, but exhibited an alkalosis (Dissanayake and Ishimatsu 2011).
Even though studies on combined environmental stressors are rare, the existing data indicates alarming differences in the acid–base responses of decapod crustaceans in comparison to single-stressor studies. Therefore, it would be desirable for future research to focus on a more holistic and realistic approach.
6.3.2 Calcification, CaCO3 and Moulting
Interestingly, as one of the most important physiological processes, growth in crustaceans is closely linked to the whole animal acid–base status and regulation. Due to their hard and inflexible exoskeleton, decapod crustaceans depend on a series of moults in order to grow. During the different pre-, post- and intermoult stages that compose the complex moult cycle (Mangum et al. 1985), the connectives between the living tissue and the extracellular cuticle are loosened and water uptake ensures the shedding of the old and the expansion of the new carapace. The exoskeleton contains the majority of the organismal CaCO3 that is mobilized during the moult in order to soften this structure and either excreted into the environment or stored in gastroliths for the new exoskeleton (Ahearn et al. 2004). Generally, decapod crustaceans experience a pronounced premoult alkalosis (increase in hemolymph HCO3 −) in order to compensate for a concomitant acidosis of mainly metabolic origin (increase in hemolymph lactate) after successful exuviation (Mangum et al. 1985). Interestingly, this HCO3 − seems not to originate from mobilization of the exoskeletal stores. Even though an early study by Robertson (1960) detected a seemingly HCO3 −-correlated increase in hemolymph [Ca2+] and [Mg2+] in premoult C. maenas, later studies on the blue crab C. sapidus did not detect a change in hemolymph [Ca2+] but observed a decrease in [Cl−] instead, indicating a direct Cl-/HCO3 −-exchange with the environment as the source of the extracellular HCO3 − (Cameron and Wood 1985; Cameron 1978; Henry et al. 1981; Mangum et al. 1985). As a response to air exposure, however, crayfish (Wheatly and Gannon 1995), the anomuran porcelain crabs Petrolisthes laevigatus (Lagos and Cáceres 2008) and Petrolisthes violaceus (Vargas et al. 2010) and subpopulations of the brachyuran crab Cyclograpsus cinereus (Lagos et al. 2014) and N. granulata (Luquet and Ansaldo 1997) were able to mobilize exoskeletal Ca2+/HCO3 − stores in response to the acid–base disturbance.
Interestingly and in contrast to other invertebrate marine calcifiers like mussels (Beniash et al. 2010; Michaelidis et al. 2005) and corals (Langdon et al. 2000; 7 Chap. 7), calcification of the carapace in response to hypercapnia (ocean acidification) seems to increase in the red rock cleaner shrimp Lysmata californica (Taylor et al. 2015), the prawns (Lito)Penaeus occidentalis and P. monodon (Wickins 1984), female Anomuran red king crab Paralithodes camtschaticus (Long et al. 2013), as well as the crab C. sapidus, the lobster H. americanus and the prawn P. plebejus (Ries et al. 2009). The robustness of the crustacean carapace is believed to be due to its increased amount of calcite, the less soluble form of CaCO3 (Taylor et al. 2015), as well as its complete coverage with a relatively thick organic epicuticle (Ries et al. 2009), and the crustaceans’ generally high capability for acid–base regulation. While an increase in calcification might sound advantageous, it potentially has negative effects on the crustaceans’ moulting frequency (Wickins 1984) and crypsis/predator defence (Taylor et al. 2015). The metabolic investment and possible allocation of energy resources due to an increased calcification might also lead to other negative impacts in these crustaceans that might reduce the overall fitness (i.e. metabolic depression and reduced growth; Taylor et al. 2015). An increase in calcification as well as an increase in metabolic costs in premoult was already observed in very early life stages (zoea I larval stage) of the Anomuran red king crab P. camtschaticus (Long et al. 2013) and the brachyuran great spider crab Hyas araneus (Schiffer et al. 2013).
Only few decapod crustaceans like the velvet swimming crab N. puber, however, might also exhibit a dissolution of their exoskeleton in response to high levels of ocean acidification (Spicer et al. 2007). Also in late European lobster larvae, exposure to hypercapnia resulted in significantly lower carapace mass as well as less mineralization in response to hypercapnia (Arnold et al. 2009), as well as a delay in the first moult cycles (Keppel et al. 2012).
6.4 Gill Epithelial Acid–Base Regulation
6.4.1 Gill Epithelial Transporters Involved in Acid–Base Regulation
While the numerous studies on acid–base homeostasis in aquatic decapod crustaceans mainly have focussed on describing the whole animal acid–base status in response to diverse environmental stressors as described above, to date only a few investigations have commented on the actual regulatory mechanisms involved in these processes. These few studies indicate that the high acclimation potential of decapod crustaceans in response to environmental changes can be attributed mainly to ion exchange processes in the gill epithelium (see above). As the major site for osmoregulation and ammonia excretion (reviewed by Henry et al. 2012; Larsen et al. 2014; Weihrauch et al. 2004b), the gills possess many epithelial membrane transporters that are likely also involved in acid–base regulation. Indirect evidence was drawn from the observation that changes in hemolymph acid–base equivalents (H+/HCO3 −) were accompanied by changes in the strong ion difference (Na+/Cl−), when decapod crustaceans were exposed to air and dilute salinity (Burnett and McMahon 1987; Ehrenfeld 1974; Henry and Cameron 1982; Luquet and Ansaldo 1997; Truchot 1979) or when carbonic anhydrase, the enzyme responsible for the conversion of CO2 into H2CO3 and subsequent dissociation to H+ and HCO3 -, was blocked (Burnett et al. 1981; Henry and Cameron 1983; Henry et al. 2003). While the crustacean gill epithelium has been subject to many investigations of membrane transporters involved in osmoregulation and ammonia excretion (for reviews see Henry et al. 2012; Larsen et al. 2014; Weihrauch et al. 2004a, b), hardly anything is known about the respective mechanisms for acid–base regulation. A recent set of gill perfusion experiments on anterior gills of seawater-acclimated C. maenas, however, shed some light on the gill transporter inventory potentially involved in branchial acid–base regulation and its linkage to ammonia regulation (see also section above) in this species (Fehsenfeld and Weihrauch 2016a).
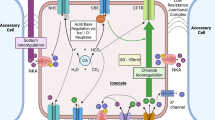
. Figure 6.3a–d represents the current working models for osmoregulation, ammonia excretion and general acid–base regulation, as well as a new, more specific model for acid–base regulation and its overlap/link to ammonia excretion in the model organism C. maenas.
Current working models for branchial ion regulatory processes in decapod crustaceans. (a) Osmoregulation, depicted after Onken et al. (2003); (b) ammonia excretion in the weak hyper-osmoregulator C. maenas, depicted after 7 Chap. 1; and (c) general hypothetical model for acid–base regulation, depicted after Freire et al. (2008). (d) Depicts a recently developed model for acid–base regulation and its linkage with ammonia regulation in C. maenas after Fehsenfeld and Weihrauch (2016a). Potential overlaps in the transporter inventory of all models are basolateral Na+/K+-ATPase, basolateral K+-channels (including a member of the HCN family, Fehsenfeld and Weihrauch 2016b) and potential apical Na+/H+-exchanger. (a, c, d) Also share a potential apical HCO3 -/Cl−-exchanger. Light dashed lines to the left (a–c) and top (d) indicate the cuticle covering the apical membrane. Notice the different orientation of panel D. Blue colour indicates the involvement of the respective transporter only in acid–base, but not in ammonia excretion. Bold and bigger letters indicate proposed major routes for transport of respective acid-base equivalents. Amt ammonia transporter, ATP ATPase, CA carbonic anhydrase, NHE Na+/H+-exchanger, MT microtubule network, Rh Rhesus-like protein
Trans-branchial active NaCl transport in moderate hyper-osmoregulators such as C. maenas (Riestenpatt et al. 1996) and N. granulata (Lucu and Siebers 1987; Onken et al. 2003) is fairly well characterized. As can be seen in . Fig. 6.3a, basolateral Na+/K+-ATPase and Cl−-channels, as well as apical Na+/K+/2Cl−-cotransporter supported by apical and basolateral K+-channels, are key players in this osmoregulatory mechanism. A number of studies also indicated an alternative pathway for NaCl uptake via apical Na+/H+- and HCO3 −/Cl− -exchangers, linked to the actions of a carbonic anhydrase (Henry et al. 2003; Lucu 1990; Onken et al. 2003; Tresguerres et al. 2008), therefore directly linking NaCl transport to the transport of acid–base equivalents. In N. granulata, however, a basolateral Na+/H+-exchanger seems to promote intracellular Na+ uptake in exchange for H+ rather than being situated apically (Tresguerres et al. 2008).
Overlapping with the model for NaCl transport, basolateral Na+/K+-ATPase and Cs+/Ba2+-sensitive K+-channels have also been shown to be involved in ammonia excretion through the gills of C. maenas (Weihrauch et al. 1998, 2004a, b; . Fig. 6.3b). In addition to the general Ba2+-sensitive K+-channels, a ZD7288-sensitive K+-channel of the hyperpolarization activated cyclic nucleotide-gated potassium channel family (HCN) has recently been identified to contribute to NH4 + regulation over the gill epithelium of C. maenas (Fehsenfeld and Weihrauch 2016b).
Additionally, a cytoplasmic V-(H+)-ATPase and a functional microtubule network have been hypothesized to promote ammonia excretion over the apical membrane via NH3 trapping and transport in acidified vesicles in this species (Weihrauch et al. 2002), potentially linking ammonia excretion with acid–base regulation.
In comparison to the models for osmoregulation and ammonia excretion, the hypothetical model for general crustacean acid–base regulation after Freire et al. (2008) as seen in . Fig. 6.3c is much more speculative. When considered in correlation with ammonia excretion as seen in . Fig. 6.3d, however (7 see also Sect. 6.5), potential pathways become more comprehensive. The most significant key player in C. maenas’ branchial acid–base regulation (although not affecting ammonia excretion), as identified in a recent gill perfusion study applying pharmaceuticals to block specific transporters in C. maenas gills, was a potential basolateral Na+/HCO3 −-cotransporter (Fehsenfeld and Weihrauch 2016a). A recently identified basolateral Na+/HCO3 −-exchanger in the squid Sepioteuthis lessoniana (Hu et al. 2014, 7 Chap. 11) has also been postulated to be important for acid–base regulation in the euryhaline crab N. granulata (Tresguerres et al. 2008).
A strictly apical distribution of V-(H+)-ATPase as hypothesized in the model of Freire et al. (2008) and depicted in . Fig. 6.3c has only been identified in freshwater (and terrestrial) crustaceans (Tsai and Lin 2007), including the red crab Dilocarcinus pagei (Weihrauch et al. 2004a, b) and E. sinensis (Onken and Putzenlechner 1995; Tsai and Lin 2007), as well as many freshwater fish (Gilmour and Perry 2009), to generate an electrochemical gradient over the apical membrane to drive Na+ uptake (Larsen et al. 2014; Weihrauch et al. 2001). While an apical V-(H+)-ATPase seems unlikely to be present in sea- and brackish-water acclimated decapod crustaceans for osmoregulatory purposes, an apical presence cannot be excluded to be involved in acid–base regulation. The pharmacological studies by Fehsenfeld and Weihrauch (2016a) on the isolated perfused gill and the immunohistochemical localization (Weihrauch et al. 2001), however, indicated a significant contribution of a cytoplasmic V-(H+)-ATPase that – together with the Rhesus-like protein – has been hypothesized to be involved in ammonia trapping in acidified vesicles as suggested by Weihrauch et al. (2002), therefore promoting the excretion of both, NH3 and H+.
Supporting the findings of the above-mentioned gill perfusion study in C. maenas gills (Fehsenfeld and Weihrauch 2016a), a study by Siebers et al. (1994), identified basolateral Na+/K+-ATPase (NKA) to be involved in branchial acid–base regulation. K+-channels on the other hand provide a backflow of K+ into the hemolymph. As mentioned earlier, both structures provide additional transport of NH4 + due to its similar size and charge compared to K+ (Skou 1960; Lignon 1987; Weihrauch et al. 1998; Choe et al. 2000). Additionally, the HCN-like potassium channel recently identified to be involved in NH4 + movements over the gill epithelium of C. maenas as mentioned above has also been shown to be involved in branchial acid–base regulation in the respective study (Fehsenfeld and Weihrauch 2016b).
In fish, the Na+/K+-ATPase generates the electrochemical gradient over the basolateral membrane that is then the major driving force for the excretion of H+ via apical Na+/H+-exchanger (NHE) in acid excretory epithelial cells (Choe et al. 2005; Edwards et al. 2002). While a potential electrogenic NHE (2Na+/1H+) has been identified to be present in crustacean gills (Shetlar and Towle 1989), an apical localization as suggested by studies on Cancer antennarius and P. cinctipes (Hunter and Kirschner 1986) and C. maenas (Weihrauch et al. 1998) is not clear to date due to the interference of the employed pharmaceuticals (amiloride) with the cuticle (Onken and Riestenpatt 2002; Weihrauch et al. 2002). A basolateral NHE that promotes H+ excretion into the hemolymph, however, has been observed in N. granulata (Tresguerres et al. 2008). An additional cytoplasmic distribution of NHE that is potentially involved in both ammonia and proton excretion via vesicles is supported by phylogenetic analysis of diverse NHEs as conducted by Fehsenfeld and Weihrauch (2016a). Also in gills of N. granulata, Tresguerres et al. (2008) identified an apical Cl−/HCO3 −-exchanger that would provide an apical exit of HCO3 − as indicated in the proposed model (. Fig. 6.3d). In the gills of C. maenas, two isoforms of branchial carbonic anhydrase have been identified, a cytoplasmic and a membrane bound isoform (Boettcher et al. 1990; Serrano and Henry 2008). Supported by findings of the study of Siebers et al. (1994), carbonic anhydrase played a role in branchial acid–base regulation as observed in the inhibitor study by Fehsenfeld and Weihrauch (2016a).
The described epithelial transport processes closely resemble mechanisms that are observed in the mammalian kidney. While the crustacean epithelial ion regulatory mechanisms based on . Fig. 6.3a has been compared to the thick ascending limb of the mammalian kidney in the past (Riestenpatt et al. 1996), the proposed new model for branchial acid–base regulation and its link to ammonia regulation as seen in . Fig. 6.3d additionally resembles features of the mammalian kidney collecting duct (Weiner and Verlander 2013).
6.4.2 Genetic Responses to Acid–Base Disturbance
Two microarray and transcriptomic studies have identified changes in (mRNA) expression levels of gill epithelial transporters upon environmental disturbances that helped identify some of the candidate genes involved in acid–base regulation.
Interestingly, exposure to hypercapnia (400 Pa for 7 days) did not seem to elucidate a typical stress response in posterior gills of osmoregulating green crabs. Applying microarray and quantitative real-time experiments, Fehsenfeld et al. (2011) observed generally only subtle changes in mRNA expression levels among over 4400 genes in C. maenas and did not identify any changes in heat-shock proteins resembling direct indicators for stress. Instead, the data suggested an increased contribution of vesicular membrane transport, indicating that the proposed vesicular transport for active ammonia excretion (Weihrauch et al. 2002; . Fig. 6.3b) might indeed contribute to H+(NH4 +) excretion and therefore acid–base regulation in this species. Additionally, most of the annotated common ion transporters of the gill epithelium were not differentially expressed with the exception of a significant upregulated calcium-activated chloride channel and the downregulated Cl−/HCO3 − exchanger of the SLC 4 family, as well as a downregulated glycosyl-phosphatidylinositol-linked carbonic anhydrase VII. Interestingly, these genes were also affected by acclimation of green crabs to dilute salinity (Towle et al. 2011). One of the most downregulated transcripts in the hypercapnia/microarray study, the hippocampus abundant gene transcript or 1 (initially falsely annotated as a hyperpolarization activated nucleotide-gated potassium channel), has been confirmed to have significantly reduced mRNA expression levels in posterior gill 7 after 7 days of hypercapnia, as well as HEA-acclimation (Fehsenfeld and Weihrauch, unpublished data). A very similar response (downregulation) was observed in HCN as identified in the recent study of Fehsenfeld and Weihrauch (2016b). Both genes are therefore interesting novel candidate genes for further studies in respect to branchial acid–base regulation.
In a different study, changes in branchial mRNA expression levels of a number of important epithelial transporters were monitored by quantitative real-time PCR after acclimation of C. maenas to hypercapnia (Fehsenfeld and Weihrauch 2013). Similar to the results of the microarray study mentioned above, only subtle changes in mRNA expression were observed in individual gills, but the experiments indicated a role for the Rhesus-like protein, Na+-K+-ATPase and glycosyl-phosphatidylinositol-linked carbonic anhydrase VII in branchial acid–base regulation, as well as potentially the Na+/H+-exchanger and anion exchanger.
A different picture is generated in the branchial response of the great spider crab Hyas araneus upon exposure to different levels of environmental pCO2 combined with varying temperatures (Harms et al. 2014). While Na+/K+-ATPase was upregulated following hypercapnia alone and hypercapnia in combination with temperature, mRNA levels of V-(H+)-ATPase and carbonic anhydrase were only significantly elevated upon moderate and severe hypercapnia (Harms et al. 2014). Additionally, changes in genes involved in metabolism indicated an enhanced aerobic metabolism in response to moderate hypercapnia, while severe hypercapnia induced a metabolic depression. Specifically, decreased trehalose metabolism of the gills seems to be a common response of hypercapnia as well as temperature acclimation in H. araneus.
Similar to the response of C. maenas (Fehsenfeld et al. 2011), analysis of the Gene Ontology terms (GO-terms) indicated a restructuring of the gill epithelium and/or the cytoskeleton upon hypercapnia in H. araneus (Harms et al. 2014), a phenomenon that can also be observed upon acclimation to dilute salinity in posterior gills of C. maenas (Compere et al. 1989). In contrast to C. maenas, however, gill epithelia of H. araneus seem to undergo a pronounced stress response that includes the elevation of genes involved in intracellular oxidative stress defence, including a number of peroxidases.
6.5 Linking Acid–Base to Ammonia Regulation
Even though ammonia excretion in decapod crustaceans has been the focus of many studies, the potential importance of ammonia regulatory patterns in respect to acid–base regulation has not been acknowledged to date. Generally, ammonia exists in a pH-dependent equilibrium between the weak base NH3 and its acidic form NH4 +. With a pKa of 9.15, most ammonia is present as NH4 + at physiological pH (Weiner and Verlander 2013). Due to its physical properties, ammonia (and therefore ammonia excretion) might therefore very well contribute to acid–base homeostasis as an additional hemolymph buffer beside the carbonate system. Being the primary waste product of protein catabolism, NH3/NH4 + levels are ultimately linked to the overall metabolic rate of the organism. As mentioned earlier, metabolic rates of decapod crustaceans are individually adjusted when experiencing external stressors that simultaneously also affect acid–base homeostasis. In response to hypoxia, for example, metabolism and hemolymph ammonia decreased significantly in N. norvegicus (Hagerman et al. 1990). A similar response was seen in M. magister when exposed to hypercapnia and included also a significant decrease in ammonia excretion rates (Hans et al. 2014). In C. maenas on the other hand, hemolymph ammonia as well as whole animal ammonia excretion increased significantly upon exposure to hypercapnia in both full-strength seawater and brackish-water acclimated specimen (Fehsenfeld and Weihrauch 2013; Fehsenfeld and Weihrauch 2016a). Interestingly, NH4 + excretion by individual gills of brackish-water acclimated C. maenas closely mirrored their H+ excretion, indicating that acid excretion over the gill epithelium was mainly accomplished by NH4 + excretion (Fehsenfeld and Weihrauch 2013). Blocking basolateral V-(H+)-ATPase, Na+/K+-ATPase and general K+-channels (Fehsenfeld and Weihrauch 2016a), as well as the recently identified transporter HCN (Fehsenfeld and Weihrauch 2016b) by transporter-specific pharmaceuticals, simultaneously affected branchial NH3/NH4 + excretion as well as the excretion of acid–base equivalents in this decapod crustacean.
Furthermore, as an identified key player in branchial ammonia excretion in crustaceans (Weihrauch et al. 2004b; Martin et al. 2011), Rhesus proteins have recently been strongly suggested to not only mediate NH3 but to also act as CO2 channels in human red blood cells (Endeward et al. 2008; Kustu and Inwood 2006; Musa-Aziz et al. 2009; Soupene et al. 2002, 2004) and fish gills (Perry et al. 2010). Interestingly, this protein is significantly downregulated in C. maenas in anterior gill 4 in response to hypercapnia (Fehsenfeld and Weihrauch 2013), as well as in posterior gills in response to both hypercapnia and high environmental ammonia (HEA; Fehsenfeld, pers. communication), but is significantly upregulated in HEA-acclimated M. magister (Martin et al. 2011), clearly indicating its role in acid–base regulation and providing a link to ammonia regulation in these decapod crustaceans.
6.6 Conclusion
Thanks to the numerous descriptive studies, we presently have a very good understanding of how various environmental factors influence acid–base homeostasis in aquatic decapod crustaceans. Accomplished mainly via adjustments of extracellular HCO3 − concentrations and the correlated excretion of acid- and/or base equivalents, possibly connected to changes in the strong ion difference, crustaceans are capable of efficiently counteracting acid–base disturbances. Even though the collected data support a major role for the gill epithelium and the respective ion exchange processes in this organ in acid–base homeostasis, information on the actual mechanisms contributing its regulation are sparse. Studies on gill epithelial transporters involved in branchial acid–base regulation, however, deliver strong indications for a close link between ion and especially ammonia and acid–base regulation in the crustacean gill. The proposed mechanisms therefore resemble closely what is observed in the mammalian kidney, specifically in the thick ascending limb and the collecting duct. Future work needs to verify the localization of most of the respective proposed transporters in the crustacean gill epithelium and possibly other organs.
References
Ahearn GA (1978) Allosteric cotransport of sodium, chloride, and calcium by the intestine of freshwater prawns. J Membr Biol 42:281–300
Ahearn GA, Franco P, Clay LP (1990) Electrogenic 2 Na+/1 H+ exchange in crustaceans. J Membr Biol 116:215–226
Ahearn GA, Zhuang Z, Duerr J, Pennington V (1994) Role of the invertebrate electrogenic 2Na+/1H+ antiporter in monovalent and divalent cation transport. J Exp Biol 196:319–335
Ahearn GA, Mandal PK, Mandal A (2004) Calcium regulation in crustaceans during the molt cycle: a review and update. Comp Biochem Physiol A 137:247–257
Almut G, Bamber S (2013) Behavioural responses of Crangon crangon (Crustacea, Decapoda) to reduced seawater pH following simulated leakage from sub-sea geological storage. J Environ Prot 4:61–67
Appelhans YS, Thomsen J, Pansch C et al (2012) Sour times: Seawater acidification effects on growth, feeding behaviour and acid–base status of Asterias rubens and Carcinus maenas. Mar Ecol Prog Ser 459:85–98
Arnold KE, Findlay HS, Spicer JI et al (2009) Effect of CO2-related acidification on aspects of the larval development of the European lobster, Homarus gammarus (L.). Biogeosciences 6:1747–1754
Barra J-A, Pequeux A, Humbert W (1983) A morphological study on gills of a crab acclimated to fresh water. Tissue Cell 15:583–596
Bellwood O (2002) The occurrence, mechanics and significance of burying behaviour in crabs (Crustacea: Brachyura). J Nat Hist 36:1223–1238
Beniash E, Ivanina A, Lieb NS et al (2010) Elevated level of carbon dioxide affects metabolism and shell formation in oysters Crassostrea virginica. Mar Ecol Prog Ser 419:95–108
Boettcher K, Siebers D, Becker W (1990) Localization of carbonic anhydrase in the gills of Carcinus maenas. Comp Biochem Physiol B 96:243–246
Booth CE, McMahon BR, De Fur PL, Wilkes PRH (1984) Acid–base regulation during exercise and recovery in the blue crab, Callinectes sapidus. Respir Physiol 58:359–376
Bouaricha N, Charmantier-Daures M, Thuet P et al (1994) Ontogeny of osmoregulatory structures in the shrimp Penaeus japonicus (Crustacea, Decapoda). Biol Bull 186:29–40
Burnett LE, Johansen K (1981) The role of branchial ventilation in hemolymph acid–base changes in the shore crab Carcinus maenas during hypoxia. J Comp Physiol 141:489–494
Burnett LE, McMahon BR (1987) Gas Exchange, hemolymph acid–base status, and the role of branchial water stores during air exposure in three littoral crab species. Physiol Zool 60:27–36
Burnett LE, Woodson PB, Rietow M, Vilicich VC (1981) Crab gill intra-epithelial carbonic anhydrase plays a major role in haemolymph CO2 and chloride ion regulation. J Exp Biol 92:243–254
Caldeira K, Wickett ME (2005) Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J Geophys Res 110:1–12
Cameron JN (1978) Effects of hypercapnia on blood acid–base status, NaCl fluxes, and trans-gill potential in freshwater blue crabs, Callinectes sapidus. J Comp Physiol B 123:137–141
Cameron JN, Batterton CV (1978a) Antennal gland function in the freshwater blue crab, Callinectes sapidus: water, electrolyte, acid–base and ammonia excretion. J Comp Physiol B 123:143–148
Cameron JN, Batterton CV (1978b) Temperature and blood acid–base status in the blue crab, Callinectes sapidus. Respirin Physiol 35:101–110
Cameron JN, Wood CM (1985) Apparent H+ excretion and CO2 dynamics accompanying carapace mineralization in the blue crab (Callinectes sapidus) following moulting. J Exp Biol 114:181–196
Carter HA, Ceballos-Osuna L, Miller NA, Stillman JH (2013) Effects of ocean acidification on early life-history stages of the intertidal porcelain crab Petrolisthes cinctipes. J Exp Biol 216:1405–1411
Chapman CJ (1980) Ecology of juvenile and adult Nephrops. In: Cobb JS, Phillips BF (eds) The biology and management of lobsters. Academic Press, Inc., New York, p 1980
Charmantier G, Haond C, Lignot J-H, Charmantier-Daures M (2001) Ecophysiological adaptation to salinity throughout a life cycle: a review in homarid lobsters. J Exp Biol 204:967–977
Cheng SY, Shieh LW, Chen JC (2013) Changes in hemolymph oxyhemocyanin, acid–base balance, and electrolytes in Marsupenaeus japonicus under combined ammonia and nitrite stress. Aquat Toxicol 130–131:132–138
Choe H, Sackin H, Palmer LG (2000) Permeation properties of inward-rectifier potassium channels and their molecular determinants. J Gen Physiol 115:391–404
Choe KP, Kato A, Hirose S et al (2005) NHE3 in an ancestral vertebrate: primary sequence, distribution, localization, and function in gills. Am J Physiol Regul Integr Comp Physiol 289:R1520–R1534
Chu KH (1987) Sodium transport across the perfused midgut and hindgut of the blue crab, Callinectes sapidus: The possible role of the gut in crustacean osmoregulation. Comp Biochem Physiol A 87:21–25
Chung KF, Lin HC (2006) Osmoregulation and Na, K-ATPase expression in osmoregulatory organs of Scylla paramamosain. Comp Biochem Physiol A 144:48–57
Collins M, Knutti R, Dufresne J-L et al (2013) Long-term climate change: projections, commitments and irreversibility. In: Stocker TF, Qin D, Plattner G-K et al (eds) Climate change 2013: the physical scienc basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel of climate change. Cambridge University Press, Cambridge/New York
Compere P, Wanson S, Pequeux A et al (1989) Ultrastructural changes in the gill epithelium of the green crab Carcinus maenas in relation to the external salinity. Tissue Cell 21:299–318
Cooper RA, Uzmann JR (1980) Ecology of juvenile and adult Homarus. In: Cobb JS, Phillips BF (eds) The biology and management of lobsters. Academic Press, Inc., New York, pp 97–142
Copeland DE, Fitzjarrell AT (1968) The salt absorbing cells in the gills of the blue crab (Callinectes sapidus Rathbun) with notes on modified mitochondria. Zeitschrift fuer Zellforsch und Mikroskopische Anat 92:1–22
Crandall KA, Buhay JE (2008) Global diversity of crayfish (Astacidae, Cambaridae, and Parastacidae – Decapoda) in freshwater. Hydrobiologia 595:295–301
Dall W (1970) Osrnoregulation in the lobster Homarus americanus. J Fish Res Board Can 27:1123–1130
Davenport HW (1974) The ABC of acid base chemistry: The elements of physiological blood gas chemistry for medical students and physicians, 6th edn. The University of Chicago Press, Chicago
De Fur PL, McMahon BR (1984) Physiological compensation to short-term air exposure in Red rock crabs, Cancer productus Randall, from littoral and sublittoral habitats. II. Acid–base balance. Physiol Zool 57:151–160
De Fur PL, Wilkes PRH, McMahon BR (1980) Non-equilibrium acid–base status in C. productus: role of exoskeletal carbonate buffers. Respir Physiol 42:247–261
De Fur PL, McMahon BR, Booth CE (1983) Analysis of hemolymph oxygen levled and acid–base status during emersion “in situ” in the red rock crab, Cancer productus. Biol Bull 165:582–590
De Grave S, Pentcheff ND, Ahyong ST et al (2009) A classification of living and fossil genera of decapod crustaceans. Raffles Bull Zool Suppl Ser 21:1–109
De la Haye KL, Spicer JI, Widdicombe S, Briffa M (2011) Reduced sea water pH disrupts resource assessment and decision making in the hermit crab Pagurus bernhardus. Anim Behav 82:495–501
Dejours P (1975) Principles of comparative respiratory physiology. Elsevier North Holland, New York
Dejours P, Beekenkamp H (1977) Crayfish respiration as a function of water oxygenation. Respir Physiol 30:241–251
Dissanayake A, Ishimatsu A (2011) Synergistic effects of elevated CO2 and temperature on the metabolic scope and activity in a shallow-water coastal decapod (Metapenaeus joyneri; Crustacea: Penaeidae). ICES J Mar Sci 68:1147–1154
Dissanayake A, Clough R, Spicer JI, Jones MB (2010) Effects of hypercapnia on acid–base balance and osmo-/iono-regulation in prawns (Decapoda: Palaemonidae). Aquat Biol 11:27–36
Edwards SL, Donald JA, Toop T et al (2002) Immunolocalisation of sodium/proton exchanger-like proteins in the gills of elasmobranchs. Comp Biochem Physiol A 131:257–265
Ehrenfeld J (1974) Aspects of ionic transport mechanisms in crayfish Astacus leptodactylus. J Exp Biol 61:57–70
Endeward V, Cartron J-P, Ripoche P, Gros G (2008) RhAG protein of the Rhesus complex is a CO2 channel in the human red cell membrane. FASEB J 22:64–73
Fehsenfeld S, Weihrauch D (2013) Differential acid–base regulation in various gills of the green crab Carcinus maenas: effects of elevated environmental pCO2. Comp Biochem Physiol A 164:54–65
Fehsenfeld S, Weihrauch D (2016a) Mechanisms of acid–base regulation in seawater-acclimated green crabs (Carcinus maenas). Can J Zool 94:95–107
Fehsenfeld S, Weihrauch D (2016b) The role of an ancestral hyperpolarization-activated cyclic nucleotide-gated K+ channel in branchial acid–base regulation in the green crab, Carcinus maenas. J Exp Biol 219:1–10. doi:10.1017/CBO9781107415324.004
Fehsenfeld S, Kiko R, Appelhans Y et al (2011) Effects of elevated seawater pCO2 on gene expression patterns in the gills of the green crab, Carcinus maenas. BMC Genomics 12:488
Freire CA, McNamara JC (1995) Fine structure of the gills of the fresh-water shrimp Macrobrachium olfersii (Decapoda): effect of acclimation to high salinity medium and evidence for involvement of the lamellar septum in ion uptake. J Crustac Biol 15:103–116
Freire CA, Cavassin F, Rodrigues EN et al (2003) Adaptive patterns of osmotic and ionic regulation, and the invasion of fresh water by the palaemonid shrimps. Comp Biochem Physiol A 136:771–778
Freire CA, Onken H, McNamara J (2008) A structure-function analysis of ion transport in crustacean gills and excretory organs. Comp Biochem Physiol A 151:272–304
Gilmour K, Perry S (2009) Carbonic anhydrase and acid–base regulation in fish. J Exp Biol 212:1647–1661
Guiasu RC (2002) Cambarus. In: Holdich DM (ed) Biology of Freshwater Crayfish. Blackwell Science Ltd, Oxford, pp 609–664
Hagerman L, Sondergaard T, Weile K et al (1990) Aspects of blood physiology and ammonia excretion in Nephrops norvegicus nuder hypoxia. Comp Biochem Physiol A 97:51–55
Hamilton NM, Houlihan DF (1992) Respiratory and circulatory adjustments during aquatic treadmill exercise in the European shore crab Carcinus maenas. J Exp Biol 162:37–54
Hammer KM, Pedersen SA (2013) Deep-water prawn Pandalus borealis displays a relatively high pH regulatory capacity in response to CO2-induced acidosis. Mar Ecol Prog Ser 492:139–151
Hans S, Fehsenfeld S, Treberg JR, Weihrauch D (2014) Acid–base regulation in the Dungeness crab (Metacarcinus magister). Mar Biol 161:1179–7793
Haond C, Flik G, Charmantier G (1998) Confocal laser scanning and electron microscopical studies on osmoregulatory epithelia in the branchial cavity of the lobster Homarus gammarus. J Exp Biol 201:1817–1833
Harms L, Frickenhaus S, Schiffer M et al (2014) Gene expression profiling in gills of the great spider crab Hyas araneus in response to ocean acidification and warming. BMC Genomics 15:789
Harvey BP, Gwynn-Jones D, Moore PJ (2013) Meta-analysis reveals complex marine biological responses to the interactive effects of ocean acidification and warming. Ecol Evol 3:1016–1030
Henry RP, Cameron JN (1982) Acid–base balance in Callinectes sapidus during acclimation from high to low salinity. J Exp Biol 101:255–264
Henry RP, Cameron JN (1983) The role of carbonic anhydrase in respiration, ion regulation and acid–base balance in the aquatic crab Callinectes sapidus and the terrestrial crab Gecarcinus lateralis. J Exp Biol 103:205–223
Henry RP, Wheatly MG (1992) Interaction of respiration, ion regulation, and acid–base balance in the everyday life of aquatic crustaceans. Am Zool 32:407–416
Henry RP, Kormanik GA, Smatresk NJ, Cameron JN (1981) The role of CaCO3 dissolution as a source of HCO3 − for the buffering of hypercapnic acidosis in aquatic and terrestrial decapod crustaceans. J Exp Biol 94:269–274
Henry RP, Booth CE, Lallier FH, Walsh PJ (1994) Post-exercise lactate production and metabolism in three species of aquatic and terrestrial decapod crustaceans. J Exp Biol 186:215–234
Henry RP, Gehnrich S, Weihrauch D, Towle DW (2003) Salinity-mediated carbonic anhydrase induction in the gills of the euryhaline green crab, Carcinus maenas. Comp Biochem Physiol A 136:243–258
Henry RP, Lucu Č, Onken H, Weihrauch D (2012) Multiple functions of the crustacean gill: Osmotic/ionic regulation, acid–base balance, ammonia excretion, and bioaccumulation of toxic metals. Front Physiol 3:1–33
Hill AD, Taylor AC, Strang RHC (1991) Physiological and metabolic responses of the shore crab Carcinus maenas (L.) during environmental anoxia and subsequent recovery. J Exp Mar Bio Ecol 150:31–50
Hochachka PW, Mommsen TP (1983) Protons and anaerobiosis. Science 219:1391–1397
Howell BJ, Rahn H, Goodfellow D, Herreid C (1973) Acid–base regulation and temperature in selected invertebrates as a function of temperature. Integr Comp Biol 13:557–563
Hu MY, Guh Y-J, Stumpp M et al (2014) Branchial NH4 +-dependent acid–base transport mechanisms and energy metabolism of squid (Sepioteuthis lessoniana) affected by seawater acidification. Front Zool 11:55
Hunter KC, Kirschner LB (1986) Sodium absorption coupled to ammonia excretion in osmoconforming marine invertebrates. Am J Physiol 251:R957–R962
Hunter KC, Rudy PPJ (1975) Osmotic and ionic regulation in the Dungeness crab, Cancer magister dana. Comp Biochem Physiol A 51A:439–447
IPCC (2013) Summary for policymakers. In: Stocke TF, Qin D, Plattner GK et al (eds) Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge/New York
Jouve-Duhamel A, Truchot J-P (1983) Ventilation in the shore crab Carcinus maenas (L.) as a function of ambient oxygen and carbon dioxide: field and laboratory studies. J Exp Mar Bio Ecol 70:281–296
Keppel EA, Scrosati RS, Courtenay SC (2012) Ocean acidification decreases growth and development in American lobster (Homarus americanus) larvae. J Northwest Atl Fish Sci 44:61–66
Kustu S, Inwood W (2006) Biological gas channels for NH3 and CO2: evidence that Rh (Rhesus) proteins are CO2 channels. Transfus Clin Biol 13:103–110
Lagos ME, Cáceres CW (2008) Como afecta la exposición aérea el equilibrio ácido base de organismos móviles del intermareal: Petrolisthes laevigatus (Guérin, 1835) (Decapoda: Porcellanidae), como caso de estudio. Rev Biol Mar Oceanogr 43:591–598
Lagos M, Cáceres CW, Lardies MA (2014) Geographic variation in acid–base balance of the intertidal crustacean Cyclograpsus cinereus (Decapoda, Grapsidae) during air exposure. J Mar Biol Assoc U K 94:159–165
Langdon C, Takahashi T, Sweeney C et al (2000) Effect of calcium carbonated saturation state on the calcification rate of an experimental coral reef. Global Biogeochem Cycles 14:639–654
Larsen EH, Deaton LE, Onken H et al (2014) Osmoregulation and excretion. Compr Physiol 4:405–573
Lignon JM (1987) Ionic permeabilities of the isolated gill cuticle of the shore crab Carcinus maenas. J Exp Biol 131:159–174
Liu M, Liu S, Hu Y, Pan L (2015) Cloning and expression analysis of two carbonic anhydrase genes in white shrimp Litopenaeus vannamei, induced by pH and salinity stresses. Aquaculture. doi:10.1016/j.aquaculture.2015.04.038
Long CW, Swiney KM, Foy RJ (2013) Effects of ocean acidification on the embryos and larvae of red king crab, Paralithodes camtschaticus. Mar Pollut Bull 69:38–47
Lucu Č (1990) Ionic regulatory mechanisms in crustacean gill epithelia. Comp Biochem Physiol A 97:297–306
Lucu Č, Siebers D (1987) Linkage of Cl− fluxes with ouabain sensitive Na/K exchange through Carcinus gill epithelia. Comp Biochem Physiol A 87:807–811
Luquet CM, Ansaldo M (1997) Acid–base balance and ionic regulation during emersion in the estuarine intertidal crab Chasmagnathus granulata Dana (Decapoda Grapsidae). Comp Biochem Physiol A 117:407–410
Mangum CP, McMahon BR, De Fur PL, Wheatly MG (1985) Gas exchange, acid–base balance, and the oxygen supply to the tissues during a molt of the blue crab Callinectes sapidus. J Crustac Biol 5:188–206
Mantel LH, Farmer LL (1983) Osmotic and ionic regulation. In: Mantel LH (ed) The Biology of Crustacea. Academic Press, Inc., New York, pp 53–161
Martin M, Fehsenfeld S, Sourial MM, Weihrauch D (2011) Effects of high environmental ammonia on branchial ammonia excretion rates and tissue Rh-protein mRNA expression levels in seawater acclimated Dungeness crab Metacarcinus magister. Comp Biochem Physiol A 160:267–277
McDonald DG, McMahon BR, Wood CM (1979) An analysis of acid–base disturbances in the haemolymph following strenuous activity in the Dungeness crab, Cancer magister. J Exp Biol 79:47–58
McMahon BR, Sinclair F, Hassall CD et al (1978) Ventilation and control of acid–base status during temperature acclimation in the crab, Cancer magister. J Comp Physiol B 128:109–116
McNamara JC, Faria SC (2012) Evolution of osmoregulatory patterns and gill ion transport mechanisms in the decapod Crustacea: a review. J Comp Physiol B 182:997–1014
McNamara JC, Lima AG (1997) The route of ion and water movements across the gill epithelium of the freshwater shrimp Macrobrachium olfersii (Decapoda, Palaemonidae): evidence from ultrastructural changes induced by acclimation to saline media. Biol Bull 192:321–331
Meinshausen M, Smith SJ, Calvin K et al (2011) The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Clim Change 109:213–241
Melzner F, Gutowska M, Langenbuch M et al (2009) Physiological basis for high CO2 tolerance in marine ectothermic animals: pre-adaptation through lifestyle and ontogeny? Biogeosciences 6:2313–2331
Michaelidis B, Ouzounis C, Paleras A, Pörtner HO (2005) Effects of long-term moderate hypercapnia on acid–base balance and growth rate in marine mussels Mytilus galloprovincialis. Mar Ecol Prog Ser 293:109–118
Mora C, Wei CL, Rollo A et al (2013) Biotic and human vulnerability to projected changes in ocean biogeochemistry over the 21st century. PLoS Biol 11, e1001682
Morse HC, Harris PJ, Dornfeld EJ (1970) Pacifastacus leniusculus: Fine structure of arthrobranch with reference to active ion uptake. Trans Am Microsc Soc 89:12–27
Musa-Aziz R, Chen L-M, Pelletier MF, Boron WF (2009) Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc Natl Acad Sci U S A 106:5406–5411
Ng PKL, Davie PJF, Guinot D (2008) Systema brachyurorum : part I. An annotated checklist of extant brachyuran crabs of the world. Raffles Bull Zool 17:1–286
Onken H, Putzenlechner M (1995) A V-ATPase drives active, electrogenic and Na+-independent Cl− absorption across the gills of Eriocheir sinensis. J Exp Biol 198:767–774
Onken H, Riestenpatt S (2002) Ion transport across posterior gills of hyperosmoregulating shore crabs (Carcinus maenas): amiloride blocks the cuticular Na+ conductance and induces current-noise. J Exp Biol 205:523–531
Onken H, Tresguerres M, Luquet CM (2003) Active NaCl absorption across posterior gills of hyperosmoregulating Chasmagnathus granulatus. J Exp Biol 206:1017–1023
Pane EF, Barry JP (2007) Extracellular acid–base regulation during short- term hypercapnia is effective in a shallow water crab, but ineffective in a deep- sea crab. Mar Ecol Prog Ser 334:1–9
Pequeux A (1995) Osmotic regulation in crustaceans. J Crustac Biol 15:1–60
Perry SF, Braun MH, Noland M et al (2010) Do zebrafish Rh proteins act as dual ammonia-CO2 channels? J Exp Zool Part A 313(A):618–621
Rastrick SPS, Calosi P, Calder-Potts R et al (2014) Living in warmer, more acidic oceans retards physiological recovery from tidal emersion in the velvet swimming crab, Necora puber. J Exp Biol 217:2499–2508
Reynolds J, Souty-Grosset C, Richardson A (2013) Ecological roles of crayfish in freshwater and terrestrial habitats. Freshw Crayfish 19:197–218
Ries JB, Cohen AL, McCorkle DC (2009) Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37:1131–1134
Riestenpatt S, Onken H, Siebers D (1996) Active absorption of Na+ and Cl− across the gill epithelium of the shore crab Carcinus maenas: voltage-clamp and ion-flux studies. J Exp Biol 199:1545–1554
Riggs AF (1988) The Bohr effect. Annu Rev Physiol 50:181–204
Robertson JD (1960) Ionic regulation in the crab Carcinus maenas (L.) in relation to the moulting cycle. Comp Biochem Physiol 1:183–212
Rose RA, Wilkens JL, Walker RL (1998) The effects of walking on heart rate, ventilation rate and acid–base status in the lobster Homarus americanus. J Exp Biol 201:2601–2608
Schiffer M, Harms L, Pörtner HO et al (2013) Tolerance of Hyas araneus zoea I larvae to elevated seawater PCO2 despite elevated metabolic costs. Mar Biol 160:1943–1953
Serrano L, Henry R (2008) Differential expression and induction of two carbonic anhydrase isoforms in the gills of the euryhaline green crab, Carcinus maenas, in response to low salinity. Comp Biochem Physiol D 3:186–193
Shetlar RE, Towle DW (1989) Electrogenic sodium-proton exchange in membrane vesicles from crab (Carcinus maenas) gill. Am J Physiol 257:R924–R931
Siebers D, Lucu Č, Böttcher K, Jürss K (1994) Regulation of pH in the isolated perfused gills of the shore crab Carcinus maenas. J Comp Physiol B 164:16–22
Simonik E, Henry RP (2014) Physiological responses to emersion in the intertidal green crab, Carcinus maenas (L.). Mar Freshw Behav Physiol 47:101–115
Skou JC (1960) Further investigations on a Mg++ +Na+-activated adenosinetriphosphatase, possible related to the active, linked transport of Na+ and K+ across the nerve membrane. Biochim Biophys Acta 42:6–23
Small D, Calosi P, White D et al (2010) Impact of medium-term exposure to CO2 enriched seawater on the physiological functions of the velvet swimming crab Necora puber. Aquat Biol 10:11–21
Somero GN (1986) Protons, osmolytes, and fitness of internal milieu for protein function. Am J Physiol 251:R197–R213
Soupene E, King N, Feild E et al (2002) Rhesus expression in a green alga is regulated by CO2. Proc Natl Acad Sci U S A 99:7769–7773
Soupene E, Inwood W, Kustu S (2004) Lack of the Rhesus protein Rh1 impairs growth of the green alga Chlamydomonas reinhardtii at high CO2. Proc Natl Acad Sci U S A 101:7787–7792
Spicer JI, Raffo A, Widdicombe S (2007) Influence of CO2-related seawater acidification on extracellular acid–base balance in the velvet swimming crab Necora puber. Mar Biol 151:1117–1125
Stewart PA (1978) Independent and dependent variables of acid–base control. Respir Physiol 33:9–26
Tavares C, Martin JW (2010) Suborder dendrobranchiata bate, 1888. In: Schram FR, von Vaupel Klein JC, Forest J, Charmantier-Daures M (eds) Eucarida: Euphausiacea, Amphionidacea, and Decapoda (partim.). Treatise on zoology – anatomy, taxonomy, biology – The Crustacea. Koninklijke Brill NV, Leiden, pp 99–164
Taylor AC, Spicer JI (1991) Acid–base disturbances in the haemolymph of the prawns, Palaemon elegans (Rathke) and P. serratus (Pennant) (Crustacea: Decapoda) during exposure to hypoxia. Comp Biochem Physiol A 98:445–452
Taylor HH, Taylor EW (1992) Gills and lungs: The exchange of gases and ions. In: Harrison FW, Humes AG (eds) Microscopid anatomy of invertebrates, decapod Crustacea. Wiley-Liss, New York, pp 203–293
Taylor EW, Wheatly MG (1981) The effect of long-term aerial exposure on heart rate, ventilation respiratory gas exchange and acid–base status in the crayfish Austropotamobius pallipes. J Exp Biol 92:109–124
Taylor EW, Whiteley NM (1989) Oxygen transport and acid–base balance in the haemolymph of the lobster, Homarus gammarus, during aerial exposure and resubmersion. J Exp Biol 144:417–436
Taylor JRA, Gilleard JM, Allen MC, Deheyn DD (2015) Effects of CO2-induced pH reduction on the exoskeleton structure and biophotonic properties of the shrimp Lysmata californica. Nat Sci Rep 5:10608
Towle D, Henry R, Terwilliger N (2011) Microarray-detected changes in gene expression in gills of green crabs (Carcinus maenas) upon dilution of environmental salinity. Comp Biochem Physiol D 6:115–125
Tresguerres M, Parks S, Sabatini S et al (2008) Regulation of ion transport by pH and [HCO3 −] in isolated gills of the crab Neohelice (Chasmagnathus) granulata. Am J Physiol Regul Integr Comp Physiol 294:R1033–R1043
Truchot J-P (1973) Temperature and acid–base regulation in the shore crab Carcinus maenas (L.). Respir Physiol 17:11–20
Truchot J-P (1975a) Factors controlling the in vitro and in vivo oxygen affinity of the hemocyanin in the crab Carcinus maenas (L.). Respir Physiol 24:173–189
Truchot J-P (1975b) Blood acid–base changes during experimental emersion and reimmersion ot the intertidal crab Carcinus maenas (L.). Respir Physiol 23:351–360
Truchot J-P (1975c) Action de l’hypercapnie sur l’etat acide-base du sang chez le crabe Carcinus maenas (L.) (Crustace’, De’capode). Comptes Rendus l’Académie des Sci 280:311–314
Truchot J-P (1979) Mechanisms of the compensation of blood respiratory acid–base disturbances in the shore crab, Carcinus maenas (L.). J Exp Biol 210:407–416
Truchot J-P (1981) The effect of water salinity and acid–base state on the blood acid–base balance in the euryhaline crab, Carcinus maenas (L.). Comp Biochem Physiol A 68:555–561
Truchot J-P (1984) Water carbonate alkalinity as a determinant of hemolymph acid–base balance in the shore crab, Carcinus maenas: a study at two different ambient PCO2 and PO2 levels. J Comp Physiol B 154:601–606
Truchot J-P (1988) Problems of acid–base balance in rapidly changing intertidal environments. Am Zool 28:55–64
Truchot J-P (1992) Acid–base changes on transfer between sea- and freshwater in the Chinese crab, Eriocheir sinensis. Respir Physiol 87:419–427
Truchot J-P, Duhamel-Jouve A (1980) Oxygen and carbon dioxide in the marine intertidal environment: diurnal and tidal changes in rockpools. Respir Physiol 39:241–254
Tsai J-R, Lin H-C (2007) V-type H + -ATPase and Na+, K + -ATPase in the gills of 13 euryhaline crabs during salinity acclimation. J Exp Biol 210:620–627
Urbina MA, Paschke K, Gebauer P et al (2013) Physiological responses of the southern king crab, Lithodes santolla (Decapoda: Lithodidae), to aerial exposure. Comp Biochem Physiol A 166:538–545
Vargas M, Lagos ME, Contreras DA, Caceres CW (2010) Área de estructuras respiratorias y su efecto en la regulación del equilibrio ácido-base en dos especies de cangrejos porcelánidos intermareales, Petrolisthes laevigatus y Petrolisthes violaceus. Rev Biol Mar Oceanogr 45:245–253
Varley DG, Greenaway P (1992) The effect of emersion on haemolymph acid–base balance and oxygen levels in Scylla serrata Forskal (Brachyura: Portunidae). J Exp Mar Bio Ecol 163:1–12
Walther G-R, Post E, Convey P et al (2002) Ecological responses to recent climate change. Nature 416:389–395
Weihrauch D, Becker W, Postel U et al (1998) Active excretion of ammonia across the gills of the shore crab Carcinus maenas and its relation to osmoregulatory ion uptake. Comp Biochem Physiol B 168:364–376
Weihrauch D, Becker W, Postel U et al (1999) Potential of active excretion of ammonia in three different haline species of crabs. J Comp Physiol B 169:25–37
Weihrauch D, Ziegler A, Siebers D, Towle DW (2001) Molecular characterization of V-type H+-ATPase (B-subunit) in gills of euryhaline crabs and its physiological role in osmoregulatory ion uptake. J Exp Biol 204:25–37
Weihrauch D, Ziegler A, Siebers D, Towle DW (2002) Active ammonia excretion across the gills of the green shore crab Carcinus maenas: participation of Na+/K+-ATPase, V-type H+-ATPase and functional microtubules. J Exp Biol 205:2765–2775
Weihrauch D, McNamara JC, Towle DW, Onken H (2004a) Ion-motive ATPases and active, transbranchial NaCl uptake in the red freshwater crab, Dilocarcinus pagei (Decapoda, Trichodactylidae). J Exp Biol 207:4623–4631
Weihrauch D, Morris S, Towle DW (2004b) Ammonia excretion in aquatic and terrestrial crabs. J Exp Biol 207:4491–4504
Weiner ID, Verlander JW (2013) Renal ammonia metabolism and transport. Comp Physiol 3:201–220
Wheatly MG (1985) The role of the antennal gland in ion and acid–base regulation during hyposaline exposure of the Dungeness crab Cancer magister (Dana). J Comp Physiol B 155:445–454
Wheatly MG, Gannon AT (1995) Ion regulation in crayfish: freshwater adaptations and the problem of molting. Am J Zool 35:49–59
Wheatly MG, Henry RP (1987) Branchial and antennal gland Na+/K+-dependent ATPase and carbonic anhydrase activity during salinity acclimation of the euryhaline crayfish Pacifastacus liniusculus. J Exp Biol 133:73–86
Wheatly MG, McMahon BR (1982) Responses to hypersaline exposure in the euryhaline crayfish Pacifastacus leniusculus. J Exp Biol 99:425–445
Wheatly MG, Taylor EW (1979) Oxygen levels, acid–base status and heart rate during emersion of the shore crab Carcinus maenas (L.) into air. J Comp Physiol B 311:305–311
Wheatly MG, Taylor EW (1981) The effect of progressive hypoxia on heart rate, ventilation, respiratory gas exchange and acid–base status in the crayfish Austropotamobius pallipes. J Exp Biol 92:109–124
Wheatly MG, Toop T (1989) Physiological responses of the crayfish Pacifastacus leniusculus to environmental hyperoxia. J Exp Biol 143:53–70
Wheatly MG, Toop T, Morrison RT, Yow LC (1991) Physiological responses of the crayfish Pacifasticus leniusculus (Dana) to environmental hyperoxia. III. Intracellular acid–base balance. Physiol Zool 64:323–343
Whiteley NM (2011) Physiological and ecological responses of crustaceans to ocean acidification. Mar Ecol Prog Ser 430:257–271
Whiteley NM, Taylor EW (1990) The acid–base concequences of aerial exposure in the lobster, Homarus gammarus (L.) at 10 and 20C. J Therm Biol 15:47–56
Whiteley NM, Scott JL, Breeze SJ, McCann L (2001) Effects of water salinity on acid–base balance in decapod crustaceans. J Exp Biol 204:1003–1011
Wickins JF (1984) The effect of hypercapnic sea water on growth and mineralization in penaied prawns. Aquaculture 41:37–48
Widdicombe S, Spicer JI, Kitidis V (2011) Effects of ocean acidification on sediment fauna. In: Gattuso J-P, Hansson L (eds) Ocean Acidification. Oxford University Press, New York, pp 176–191
Wilkes PRH, McMahon BR (1982) Effect of maintained hypoxic exposure on the crayfish Orconectes rusticus. I. ventilatory, acid–base and cardiovascular adjustments. J Exp Biol 98:139–149
Wittmann AC, Pörtner H-O (2013) Sensitivities of extant animal taxa to ocean acidification. Nat Clim Chang 3:995–1001
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Fehsenfeld, S., Weihrauch, D. (2017). Acid–Base Regulation in Aquatic Decapod Crustaceans. In: Weihrauch, D., O’Donnell, M. (eds) Acid-Base Balance and Nitrogen Excretion in Invertebrates. Springer, Cham. https://doi.org/10.1007/978-3-319-39617-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-39617-0_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-39615-6
Online ISBN: 978-3-319-39617-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)