Abstract
In an estuary of a neotropical region located in Brazil, Urochloa arrecta occurs only in the area of freshwater. Nonetheless, information about the capacity of this population to invade areas considered inhospitable, i.e., saltwater, is scarce. In this sense, the aim of this study was to evaluate the effect of intermediate (20 ppt) and high (30 ppt) salinity on individuals from a population of U. arrecta located in the freshwater region of an estuarine ecosystem. Analyses of plant biomass, nitrogen, phosphorus, malondialdehyde (MDA) and hydrogen peroxide content were evaluated as possible indicators of tolerance to salt stress. Our results showed that salinity reduced growth and increased oxidative stress. However, under conditions of intermediate salinity, U. arrecta individuals showed a biomass gain greater than 60%, MDA content similar to that in freshwater, and higher nitrogen absorption and assimilation. We conclude that U. arrecta probably presents physiological adjustments that allow its survival at intermediate salinity. Thus, the ability of this species to expand in this area alerts to the importance of studies that seek to adopt policies for the control or management of the species in saline ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Invasive species of aquatic macrophytes often promote the homogenization of habitats, reduction the diversity species and life forms (submerged, free floating, emergent) of aquatic macrophytes (Amorim et al., 2015; Thomaz et al., 2015). These invaders may also negatively affect other aquatic communities, reducing the richness of fish species (Carniatto et al., 2013), modifying the composition of the associated fauna (Houston & Duivenvoorden, 2002; Midgley et al., 2006; Mormul et al., 2010; Coetzee, 2014) and decreasing the abundance of phytoplankton (Villamagna & Murphy, 2010).
In estuaries, the introduction of exotic species is of major concern, since these ecosystems have high biodiversity and are important nursery grounds for several species (Odum, 1988; Levin et al., 2001). The great diversity and richness of species in these ecosystems derive from the longitudinal gradients of salinity, flooding and nutrient concentration due to the influence of seawater (Ribeiro et al., 2011; Nunes et al., 2019). These longitudinal gradients are decisive in the distribution of aquatic macrophyte species. In tropical estuaries, there is a marked longitudinal salinity gradient, with low salinity in the upper estuary (predominance of fresh water), intermediate salinity in the middle estuary, and high salinity in the lower estuary (predominance of salt water) (Nunes & Camargo, 2018). Thus, salinity is an important variable to determine the distribution of aquatic macrophytes and the colonization by invasive macrophytes (Nunes & Camargo, 2018). For instance, conditions of high salinity may prevent the expansion of freshwater macrophytes (Thouvenot & Thiébaut, 2018), while selecting individuals and increasing the possibility of invasion by species adapted to saline environments (Xue et al., 2018). Furthermore, the increase in salinity in response to sea level rise due to climatic changes can affect coastal plant communities to a greater or lesser extent, depending on location, conditions and plant tolerances (Short et al., 2016), which increases the importance of studies on estuarine regions. Salinity is the main abiotic factor that limits the development and primary production of plants (Houle et al., 2001; Esteves & Suzuki, 2009), since it may cause ionic disequilibrium and oxidative stress (Esteves & Suzuki, 2009). However, some species have the ability to adapt and/or tolerate salinity, as a result of different types of adjustments, such as osmotic (Flowers & Colmer, 2008), morphological (Esteves & Suzuki, 2009), physiological (Larcher, 2000) and biochemical (Gratão et al., 2005).
Oxidative stress is an important indicator of salt stress, since the plant under the effect of salt may increase the production of reactive oxygen species (ROS) and suffer cell damage (Koyro et al., 2013; Gil et al., 2020). Among the ROS, hydrogen peroxide (H2O2) is one of the main cellular metabolites that, when at low concentrations, acts as an important signaling factor in the cell defense metabolism (Gill & Tuteja, 2010; Hussain et al., 2015; Foyer et al., 2017). On the other hand, H2O2 at high concentrations and in the presence of transition metals may generate the hydroxyl radical (OH⋅) that is capable of transposing and disintegrating cell membranes (Barreiros et al., 2006), forming small fragments of hydrocarbons, such as ketones, malondialdehydes (MDA), among other products related to lipid peroxidation (Garg & Manchanda, 2009; Halliwel & Gutteridge, 2015). In order to deal with the effects of ROS, an enzymatic complex (Foyer & Noctor, 2005; Gratão et al., 2012) and a non-enzymatic antioxidant mechanism (Carvalho et al., 2010; Foyer & Noctor, 2013) act in cell detoxification, preventing severe cell damage (Gratão et al., 2005; Hafsi et al., 2010), allowing the plant to grow in places with greater salinity.
Urochloa arrecta (Hack. ex Durand & Schinz) Morrone & Zuloaga is an invasive plant species originally from Africa, which occurs in all regions of Brazil (Flora of Brazil, 2020) and has been observed in different aquatic ecosystems, such as in the wetlands of the State of Mato Grosso (Pott et al., 2011), reservoirs (Michelan et al., 2010b; Rodrigues et al., 2017), coastal rivers and lakes (Amorim et al., 2015; Ferreira et al., 2017). Although this species has a preference for freshwater environments, U. arrecta forms dense and extensive stands in estuaries in the south and southeast of Brazil (Reinert et al., 2007). U. arrecta, like other Poaceae species, has salt glands (Bora et al, 2020), which explains its occurrence in estuarine areas.
Despite its importance, only two studies have evaluated the distribution of U. arrecta in estuaries and obtained salinity data at their place of occurrence. Nunes et al. (2019) observed this species in the upper estuary, in places of freshwater, while Bora et al. (2020) observed stands of this species in a middle estuary with salinity of approximately 3 ppt. These latter authors observed that this species grows at intermediate levels of salinity and develops roots and shoots also at 4.0 ppt of salinity, in which mangrove vegetation occurs. This demonstrates that populations of U. arrecta exposed to salinity are more resistant to high salinity. In the southeastern and southern estuaries of Brazil, the native aquatic macrophytes Spartina alterniflora Loisel (Poaceae) and Crinum americanum L. (Amaryllidaceae) are the main species that occur along a longitudinal salinity gradient (lower to upper estuary). In general, S. alterniflora occurs in the lower estuary (salinity from 20 to 30 ppt), C. americanum occurs in the upper estuary (salinity from 0 to 5 ppt) and both species form mixed stands in the middle estuary (salinity from 5 to 20 ppt). Our question is: Can U. arrecta expand to the middle and lower estuary areas, occupying the space of the native species and affecting areas critical for conservation?
Understanding the distribution and potential expansion of an invasive species depends on the adaptation and response mechanisms to a stressful condition. Since the increase in oxidative stress and the reduction in growth are effects that the plant may present in the presence of salt stress, we made an experiment with the aim of evaluating the effect of the salinity in low estuary areas (20 and 30 ppt) on biomass gain and on the H2O2 and MDA contents of U. arrecta. Our hypotheses regarding the responses of U. arrecta at the three levels of salinity tested are that: (i) at the salinity of 0 ppt U. arrecta will present greater biomass gain and less production of H2O2 and MDA; (ii) at 20 ppt U. arrecta will present reduced biomass gain and H2O2 production, and intermediate MDA; (iii) at 30 ppt U. arrecta will not grow and will have a large production of H2O2 and MDA.
Methods
Species and sampling area
The species U. arrecta is an emergent aquatic macrophyte belonging to the Poaceae family. This species is originally from Africa and has been previously described as U. subquadripara (Thomaz et al., 2009; Michelan et al., 2010b; Alves et al., 2017). It is invasive and common in several areas of humid ecosystems (Pott et al., 2011; Amorim et al., 2015). U. arrecta has roots fixed on the margins and floating stems (nodes + internodes + leaves = stems) that extend to limnetic regions (Michelan et al., 2017). It is a perennial and stoloniferous aquatic macrophyte, with insignificant seminiferous propagation, and fast growing from fragments (Michelan et al., 2010a). On the Itanhaém River, which is located on the South coast of the State of São Paulo (Brazil) (23° 50′ and 24° 15′ S; 46° 35′ and 47° 00′ W), the stands of U. arrecta are distributed in the upper and middle area of the estuary (Umetsu et al., 2018).
Experiment
From September to December 2019 we conducted an experiment at the Faculty of Agricultural and Veterinary Sciences, UNESP—São Paulo State University, Jaboticabal, SP, Brazil (21° 14′ 05″ S, 48° 17′ 09″ W, and 615.01 m of altitude). The climate classification is Aw (tropical) according to Köppen and Geiger. The minimum and maximum temperatures in the period of the experiment were respectively 19.5°C and 32.6°C. The intensity of light (hours) and global solar radiation (Wh m−2) was respectively 220.6 and 20.6 (FCAV/UNESP Agroclimatological Station—Jaboticabal Campus, Brazil).
We performed a completely randomized single-factor experiment (1 species × 3 salinities × 5 repetitions = 15 experimental units) to assess U. arrecta growth and oxidative stress at different levels of salinity (0, 20 and 30 ppt). The experiment lasted 121 days and was conducted in a greenhouse (Fig. 1). The initial density was 8 macrophytes per experimental unit, based on Nunes and Camargo (2018, 2020). We considered each U. arrecta stem as an individual, that is, each clonal emergence above the substrate.
The U. arrecta individuals used in this experiment were composed of stem fragments with three nodes (approximately 15 cm) that were taken from a population of a monospecific stand of U. arrecta in the upper estuary of the Itanhaém River. The individuals were washed to remove materials adhered to the fragments and planted (approximately 3 cm deep inside the substrate) in the experimental units. As the substrate for the development of the aquatic macrophyte, we used a volume of 13 l of fine-grained washed sand (grains from 0.05 to 0.42 mm) in plastic boxes with a volume of 26 l and an area of 0.13 m2. After planting, the aquatic macrophytes were kept in acclimatization for 31 days with addition of 700 ml of modified Hoagland nutrient solution (Hoagland & Arnon, 1950; Mendelssohn et al., 2001) in each experimental unit every 15 days. From the 31st day, 2 l of saline solution and 700 ml of the same nutrient solution were added to the experimental units. The saline solutions consisted in 20 g l−1 and 30 g l−1, respectively, in the treatments with salinity of 20 and 30 ppt. The salt concentrations we used were based on the salinity of the sediments of the middle and lower estuary of the Itanhaém River, places where the native species S. alterniflora and C. americanum occur. For the saline solutions we used formulated sea salt (Aquaforest Sea Salt) which, in addition to sodium chloride, contains sulfates, calcium, potassium, magnesium and other minerals found in seawater (Nunes & Camargo, 2020). In the treatment of 0 ppt, in substitution to the saline solution, 2 l of tap water were added. In addition, we added tap water whenever necessary to maintain the water level of the boxes. We controlled the salinity level in the sediment every 15 days by placing 15 g of fresh substrate in 500 ml of distilled water, and measuring the salinity (ppt) of the solution using the Horiba equipment, model U-50. The salinity mean values and standard deviation (SD) in each treatment throughout the experiment were: 0 ppt (without variation), 19.6 ppt (SD = 2.81) and 29.8 ppt (SD = 3.78). The salinity remained significantly different (P < 0.05) among all treatments throughout the experiment.
Biomass and nutrient concentration
At the beginning of the experiment and at intervals of 15 days, we performed measurements of the length (meter) of the emergent fraction of each individual to estimate the aboveground biomass (AB) during the experiment and obtain the growth curves of the emergent fraction of U. arrecta. To obtain AB values, we used the non-destructive method proposed by Nunes and Camargo (2017). We estimated the aboveground fraction dry weight by a simple linear regression equation between height and dry mass (Eq. 1). The models we found were
where AB is the aboveground biomass and \(h\) is the height of each individual in meters. The numbers in the equation correspond to the slope of the line and the Y-intercept. We calculated the gain of aboveground biomass—GAB (biomass of day 121 minus biomass of day 1) in each experimental unit and applied an analysis of variance (ANOVA) to verify the occurrence of significant differences among the treatments. We considered the initial biomass of the belowground fraction equal to zero. Thus, the gain of belowground biomass (GBB) was calculated from the dry mass obtained at the end of the experiment. At the end of the experiment, we separated the aboveground fraction (stems) and the belowground fraction (roots) of U. arrecta individuals from each experimental unit and washed them to remove any adhered material. After washing, we collected approximately 10 g of leaves (green and healthy) and roots (healthy) that were then wrapped in aluminum foil and immediately inserted in liquid nitrogen at the collection site. Subsequently, these samples were stored in a freezer at − 80°C for subsequent analysis of the content of MDA and H2O2 in the laboratory. The remaining aboveground and belowground fractions were dried in an oven at 60°C and ground in a mill. From the powdered plant material, we determined the total nitrogen (TN) content by the Kjeldahl method and total phosphorus (TP) (Allen et al., 1974) in aboveground and belowground fractions in the laboratory.
Oxidative stress indicators
The content of hydrogen peroxide (H2O2) was measured according to the method of Alexieva et al. (2001). We used the leaves (0.8 g) and roots (1 g) stored at − 80°C for the extraction. The absorbance was read at 560 nm and the results were expressed in µmol g−1 of fresh weight.
Lipid peroxidation was determined by the production of metabolites that are reactive to 2-thiobarbituric acid (TBARS), mainly MDA, according to the method of Heath and Packer (1968). The extraction followed the same protocol for H2O2. The concentration of MDA equivalents was measured by spectrophotometry between 535 and 600 nm; the results were obtained using an extinction coefficient of 1.55 × 10−5 mol−1 cm−1 (Gratão et al., 2012).
Statistical analysis
The salinity levels (0, 20 and 30 ppt) were used as predictor variables and the variables of GAB, GBB, TN, TP levels and stress indicators (H2O2 and MDA) as response variables. First, we submitted the data (GAB, GBB, TN, TP, H2O2 and MDA) to the Cramer–Von–Misses and Bartllet test, to verify the normality of the residual and the homoscedasticity of data variance, respectively. After meeting these premises, the analysis of variance (ANOVA) was used to verify the occurrence of significant differences among the treatments. When this difference was statistically significant (P < 0.05), the Tukey’s test was applied for the multiple comparison of the means with a 95% confidence interval. Statistical analyses were performed using the software R, version 3.4 (2017), and the graph and figures were prepared using Microsoft Excel® (2010).
Results
The GAB of U. arrecta was significantly higher at 0 ppt, intermediate at 20 ppt and lower at 30 ppt, while the GBB was significantly higher at 0 ppt and lower at 20 and 30 ppt (Fig. 2; Table S1). The growth curve of the aboveground fraction of U. arrecta was similar for the different treatments up to day 46, and on day 121 of the experiment, the growth was higher at 0 ppt, intermediate at 20 ppt and lower at 30 ppt (Fig. 3). The content of TN (%) in the aboveground fraction of U. arrecta showed no significant difference among the treatments. Nonetheless, in the belowground fraction, the TN content was greater at 20 and 30 ppt than at 0 ppt. The TP content (%) of the aboveground fraction was significantly higher at 0 ppt and lower at 30 ppt. However, in the belowground fraction the TP content was significantly higher at 20 and 30 ppt and lower at 0 ppt (Table 1).
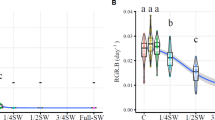
The H2O2 content (µmol g−1 of fresh mass) was higher in the aboveground fraction of U. arrecta in the treatments with salinity of 20 and 30 ppt than at 0 ppt. Nevertheless, the MDA content (nmol g−1 of fresh mass) was higher in the treatment with salinity of 30 ppt than at 0 and 20 ppt, indicating that salinity induces a greater production of reactive oxygen species (ROS) at both concentrations, leading to lipid peroxidation in aquatic macrophytes submitted to higher salinity. However, in the belowground fraction of U. arrecta, there was no significant difference in the contents of H2O2 and MDA among the salinity levels (Fig. 4; Table S2), indicating that salinity did not induce oxidative stress in the belowground fraction.
Mean and standard deviation values of A H2O2 (µmol g−1 of fresh weight) and B MDA (nmol g−1 fresh weight) contents in aboveground (light gray) and belowground (dark gray) of U. arrecta in salinities of 0, 20 and 30 ppt. Bars followed by different letters are statistically different P ≤ 0.05 (Tukey’s test)
Discussion
The results of our experiment showed that salinity limits the growth and biomass gain of U. arrecta, which corroborates our initial hypothesis. This result is similar to those obtained by other authors. Bora et al. (2020) observed that salinity was stressful for U. arrecta, reducing the growth and biomass gain of individuals from both freshwater and brackish water populations. Other emerging species tolerant to salinity, such as Distichlis spicata (L.) Greene (Poaceae) and Scirpus robustus Pursh (Cyperaceae), also had a reduction in aerial biomass in response to salinity (Crain et al., 2004).
A possible explanation for this response could be related to the high loads of energy expended by the plant to adjust to salt stress (Esteves & Suzuki, 2009). In fact, mangrove species need to maintain continuous water absorption, in addition to regulating ionic absorption and compartmentalization against a strong external salt gradient (Ball, 1996; Krauss et al., 2008). To maintain water absorption during salt stress, plants do not only need to reduce water loss by morphological and physiological adaptations, but they also need to keep water potentials sufficiently low (Krauss et al., 2008). In Poaceae species, the excretion of salt through the salt glands plays an important role in the regulation of ionic balance, contributing to tolerance to salinity (Manousaki & Kalogerakis, 2011; Céccoli et al., 2015; Atia et al., 2019). Nevertheless, salt excretion is an energy-dependent process that moves ions against large electrochemical potentials within the leaves (Lüttge, 2007). The removal of excessive salt also occurs by the translocation of ions to older leaves, which consequently promotes leaf senescence (Cram et al., 2002; Munns & Tester, 2008).
Therefore, a shorter life expectancy may accelerate leaf renewal, increasing the proportion of young leaves with a high photosynthetic rate and reducing the cost associated with maintaining older leaves. In addition, under natural conditions, variations in sediment salinity over time contribute to reactivate growth after prolonged periods of hypersaline conditions, preventing plant death (Suárez & Medina, 2005). In this sense, it is possible to assume that the lower growth and GAB of U. arrecta in treatments with 20 and 30 ppt derives from factors such as higher energy demand for mechanisms of salt tolerance, high rate of leaf senescence and absence of variation in salinity throughout the experiment.
Plant species tolerant to salinity also need a well-adjusted mechanism to combat the effects of oxidative stress promoted by salinity (Esteves & Suzuki, 2009; Koyro et al., 2013; Gil et al., 2020). In our experiment, salinity promoted increased ROS production, observed by the higher H2O2 contents, which corroborates our hypothesis. Nonetheless, regarding lipid peroxidation in the treatments with salinity of 20 and 30 ppt, our hypothesis was corroborated for individuals at salinity of 30 ppt, but not for individuals at salinity of 20 ppt. This answer shows us that, at high salinity, stress was greater, and the antioxidant mechanism was less efficient.
At intermediate salinity, the antioxidant mechanism of the aquatic macrophyte was more efficient in combating the effects of H2O2 in the cell. There is a close relationship between ROS production and reduced plant growth during salt stress. Koyro et al. (2013) identified, in the species Panicum turgidum Forssk. (Poaceae) exposed to salinity, an increase in H2O2, reducing the activity of the Calvin cycle, as well as carbon fixation and the aerial biomass of the aquatic macrophyte. Salinity affects photosynthesis because, during saline stress, the plant’s ability to absorb water from the sediment is reduced and stomatal closure is triggered to reduce water losses in the form of evaporated water (Ahanger et al., 2017). Stomatal closure promotes a series of reactions that facilitate the formation of ROS, a reduction in the photosynthetic rate that compromises CO2 availability for carboxylation reactions (Munns & Tester, 2008; Esteves & Suzuki, 2009; Parihar et al., 2015) and plant growth (Li et al., 2019). Thus, contrarily to U. arrecta individuals under 30 ppt of salinity, individuals present at 20 ppt are probably exposed to lower selective pressure and less stress, which could be attributed to the greater efficiency of the mechanism responsible for the elimination of ROS, allowing the growth and lower biomass gain of this species under this condition.
In the belowground fraction, the absence of difference in the contents of MDA and H2O2 among the treatments indicates that the oxidative stress in this fraction was lower than in the aboveground fraction and, probably, this was not one of the factors responsible for the reduction in GBB. Although salinity promotes hypoxia in the soil, reducing transpiration, nutrient absorption and, consequently, plant growth (Morard & Silvestre, 1996), in the present study, the N and P contents of the individuals were not affected by salinity. A possible explanation for the reduction in biomass in the belowground fraction may be related to the strategy that some species adopt to combat salinity, and it may be due to root elongation. Root elongation at a higher salinity may assist in sediment aeration and minimize the negative effects of salt stress (Li et al., 2019), and consequently facilitate nutrient uptake by the roots. Although root length was not assessed in our study, Bora et al. (2020) observed that in treatments with higher salinity, U. arrecta showed greater root elongation at the expense of biomass allocation, probably as a salt tolerance strategy.
The adoption of strategies that also help the plants in nutrient absorption are important, because when a species is under saline stress, the physiological requirement for N is usually higher (Crain, 2007; Nunes & Camargo, 2020). Crain (2007) observed that Spartina patens Muhl. (Poaceae) present in swamps with high salinity levels showed a higher percentage of N in their tissues, which may be related to the increasing demand for N to help with salinity tolerance and not due to the availability of N in the sediment, since these environments tend to have less availability of this nutrient. Nunes & Camargo (2020) observed that C. americanum, when cultivated in sediments with a salinity of 20 ppt, has a higher N content in the emerging fraction. One explanation for this higher N content is the result of an osmotic adjustment to maintain a positive turgor pressure (Flowers et al., 2015). Nitrogen compounds are an important and efficient solute to adjust ion balance in vacuoles and promote the osmotic adjustment of the plant (Esteves & Suzuki, 2009). In this sense, it is possible that the higher absorption and concentration of N in U. arrecta, in the treatments with salinity of 20 and 30 ppt, is also a strategy of the aquatic macrophyte to adjust osmotically and tolerate the salt.
Although in our experiment U. arrecta tolerated the stress and grew in 20 ppt of salinity, in the Itanhaém river estuary, this does not occur in the area with this salinity in the sediment. Probably, other biotic factors, e.g. competition, are responsible for the absence of this invasive species in the middle estuary area. Some studies attribute the absence of freshwater species in salt swamp regions to salt stress (Crain et al., 2004; Engels & Jensen, 2010). However, Nunes & Camargo (2018) demonstrated that competition in saline environments may also be a limiting factor for a freshwater species. In addition, the lower areas of the estuaries are more subject to variations in the water level because of their proximity to the ocean, which can also be a stress factor for some emerging species (Deegan et al., 2007; Ribeiro et al., 2011).
Rising water levels in the oceans due to climate change will affect the distribution of aquatic macrophytes in estuaries. In fact, the sixth report by the IPCC Working Group I shows that there will be a temperature increase of at least 1.5°C over the next two decades. Between the years 1901 and 2018, the global mean sea level increased by 0.20 m and by 2100 it is estimated that these levels may exceed 1 m if carbon emissions are not reduced (IPCC, 2021). According to Callaway et al. (2007), the increase, however small, in salinity due to sea level rise can lead to changes in the distribution of estuarine species, with freshwater swamps being replaced by brackish swamps and brackish swamps converted to salt marsh communities. This will imply the reduction of freshwater areas (high estuary) and may increase the competition of U. arrecta with native species. The saline intrusion in the freshwater areas of the upper estuary may select salinity-tolerant populations of U. arrecta that will occupy areas currently colonized by native species tolerant to brackish waters, such as C. americanum and S. alterniflora.
Based on the results of our experiment, we conclude that U. arrecta develops physiological adjustments that allow its survival in a salinity of 20 ppt (medium estuarine sediment salinity), as the cell stress is controlled and the GAB was greater than 60% with the increase in salinity. It is important to note that only biomass gain and oxidative stress responses of U. arrecta in two salinities are not sufficient to predict the invasiveness of the species. Other abiotic and biotic factors are also important with regard to the successful invasion of a particular species. Thus, this study is only an indication that U. arrecta is probably able to adjust to new environmental conditions, complementing what was observed by Bora et al. (2020). Although the invasion status of this species in the middle and lower estuary area is low, our results show the possibility of expansion of colonization in areas colonized by native species. These areas are very critical for conservation and, therefore, more complex studies are needed to better estimate the invasiveness of U. arrecta in saline ecosystems and to promote control or management policies for this species in these areas.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ahanger, M. A., N. S. Tomar, M. Tittal, S. Argal & R. M. Agarwal, 2017. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiology and Molecular Biology of Plants 23: 731–744.
Alexieva, V., I. Sergiev, S. Mapelli & E. Karanov, 2001. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant, Cell and Environment 24: 1337–1344.
Allen, S. E., H. M. Grimshaw, J. A. Parkinson & C. Quarmby, 1974. Chemical Analysis of Ecological Materials, Blackwell, Oxford:
Alves, R. M. A., M. B. Albuquerque & L. G. Barbosa, 2017. Status of the invasion of a Poaceae species in tropical semiarid reservoirs. Planta Daninha 35: 1–8.
Amorim, S. R., C. A. Umetsu, D. Toledo & A. F. M. Camargo, 2015. Effects of a non native species of Poaceae on aquatic macrophyte community composition: a comparison with a native species. Journal of Aquatic Plant Management 53: 191–196.
Atia, A., A. Debez, M. Rabhi, Z. Barhoumi, C. C. Haouari, H. Gouia, C. Abdelly & A. Smaoui, 2019. Salt Tolerance and Potential Uses for Saline Agriculture of Halophytes from the Poaceae. 223–237.
Ball, M. C., 1996. Comparative ecophysiology of mangrove forest and tropical lowland moist rainforest. Tropical Forest Plant Ecophysiology 461–462.
Barreiros, A. L. B. S., J. M. David & J. P. David, 2006. Estresse oxidativo: Relação entre geração de espécies reativas e defesa do organismo. Quimica Nova 29: 113–123.
Bora, L. S., S. M. Thomaz & A. A. Padial, 2020. Evidence of rapid evolution of an invasive poaceae in response to salinity. Aquatic Sciences 82: 1–16.
Callaway, J. C., V. T. Parker, M. C. Vasey & L. M. Schile, 2007. Emerging issues for the restoration of tidal marsh ecosystems in the context of predicted climate change. BioOne 54: 234–248.
Carniatto, N., S. M. Thomaz, E. R. Cunha, R. Fugi & R. R. Ota, 2013. Effects of an invasive alien Poaceae on aquatic macrophytes and fish communities in a neotropical reservoir. Biotropica 45: 747–754.
Carvalho, R. F., V. Quecini & L. E. P. Peres, 2010. Hormonal modulation of photomorphogenesis-controlled anthocyanin accumulation in tomato (Solanum lycopersicum L. cv Micro-Tom) hypocotyls: physiological and genetic studies. Plant Science 178: 258–264.
Céccoli, G., J. Ramos, V. Pilatti, I. Dellaferrera & J. C. Tivano, 2015. Salt glands in the Poaceae family and their relationship to salinity tolerance. The Botanical Review 81: 162–178.
Coetzee, J. A., 2014. Water hyacinth, Eichhornia crassipes (Pontederiaceae), reduces benthic macroinvertebrate diversity in a protected subtropical lake in South Africa. Biodiversity and Conservation 23: 1319–1330.
Crain, C. M., 2007. Shifting nutrient limitation and eutrophication effects in marsh vegetation across estuarine salinity gradients. Estuaries and Coasts 30: 26–34.
Crain, C. M., B. R. Silliman, S. L. Bertness & M. D. Bertness, 2004. Physical and biotic drivers of plant distribution across estuarine salinity gradients. Ecology 85: 2539–2549.
Cram, J. W., P. G. Torr & D. A. Rose, 2002. Salt allocation during leaf development and leaf fall in mangroves. Trees - Structure and Function 16: 112–119.
Deegan, B. M., S. D. White & G. G. Ganf, 2007. The influence of water level fluctuations on the growth of four emergent macrophyte species. Aquatic Botany 86: 309–315.
Engels, J. G. & K. Jensen, 2010. Role of biotic interactions and physical factors in determining the distribution of marsh species along an estuarine salinity gradient. Oikos 119: 679–685.
Esteves, B. S. & M. S. Suzuki, 2009. Efeito da salinidade sobre as plantas. Oecologia Australis 12: 662–679.
Ferreira, J. P. R., G. Hassemer & R. Trevisan, 2017. Aquatic macrophyte flora of coastal lakes in Santa Catarina, southern Brazil. Iheringia - Serie Botanica 72: 409–419.
Flora do Brasil, 2020. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/
Flowers, T. J. & T. D. Colmer, 2008. Salinity tolerance in halophytes. New Phytologist 179: 945–963.
Flowers, T. J., R. Munns & T. D. Colmer, 2015. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Annals of Botany 115: 419–431.
Foyer, C. H. & G. Noctor, 2005. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17: 1866–1875.
Foyer, C. H. & G. Noctor, 2013. Redox signaling in plants. Antioxidants & Redox Signaling 18: 2087–2090.
Foyer, C. H., A. V. Ruban & G. Noctor, 2017. Viewing oxidative stress through the lens of oxidative signalling rather than damage. Biochemical Journal 474: 877–883.
Garg, N. & G. Manchanda, 2009. ROS generation in plants: boon or bane? Plant Biosystems 143: 81–96.
Gil, L., X. Capó, S. Tejada, G. Mateu-Vicens, P. Ferriol, S. Pinya & A. Sureda, 2020. Salt variation induces oxidative stress response in aquatic macrophytes: the case of the Eurasian water-milfoil Myriophyllum spicatum L. (Saxifragales: Haloragaceae). Estuarine, Coastal and Shelf Science 239: 1–6.
Gill, S. S. & N. Tuteja, 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry 48: 909–930.
Gratão, P. L., A. Polle, P. J. Lea & R. A. Azevedo, 2005. Making the life of heavy metal-stressed plants a little easier. Functional Plant Biology 32: 481.
Gratão, P. L., C. C. Monteiro, R. F. Carvalho, T. Tezotto, F. A. Piotto, L. E. P. Peres & R. A. Azevedo, 2012. Biochemical dissection of diageotropica and Never ripe tomato mutants to Cd-stressful conditions. Plant Physiology and Biochemistry 56: 79–96.
Hafsi, C., M. C. Romero-Puertas, D. K. Gupta, L. A. del Río, L. M. Sandalio & C. Abdelly, 2010. Moderate salinity enhances the antioxidative response in the halophyte Hordeum maritimum L. under potassium deficiency. Environmental and Experimental Botany 69: 129–136.
Halliwel, B. & J. M. Gutteridge, 2015. No Free Radicals in Biology and Medicine, Oxford University Press, Oxford:
Heath, R. L. & L. Packe, 1968. Photoperoxidation in isolated chloro- plasts. I. Kinetics and stochiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics 125: 189–198.
Hoagland, D. C. & D. I. Arnon, 1950. The water culture method for growing plant without soil. California Agricultural Experiment. Circular 337. The College of Agriculture University of California, Berkeley.
Houle, G., L. Morel, C. E. Reynolds & J. Siegel, 2001. The effect of salinity on different developmental stages of an endemic annual plant, Aster laurentianus (Asteraceae). Botanical Society of America 88: 62–67.
Houston, W. A. & L. J. Duivenvoorden, 2002. Replacement of littoral native vegetation with the ponded pasture grass Hymenachne amplexicaulis: effects on plant, macroinvertebrate and fish biodiversity of backwaters in the Fitzroy River, Central Queensland, Australia. Marine and Freshwater Research 53: 1235–1244.
Hussain, T., H. W. Koyro, B. Huchzermeyer & M. A. Khan, 2015. Eco-physiological adaptations of Panicum antidotale to hyperosmotic salinity: water and ion relations and anti-oxidant feedback. Flora: Morphology, Distribution, Functional Ecology of Plants 212: 30–37.
IPCC, 2021. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S. L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J. B. R. Matthews, T. K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds)]. Cambridge University Press. In Press.
Koyro, H. W., T. Hussain, B. Huchzermeyer & M. A. Khan, 2013. Photosynthetic and growth responses of a perennial halophytic grass Panicum turgidum to increasing NaCl concentrations. Environmental and Experimental Botany 91: 22–29.
Krauss, K. W., C. E. Lovelock, K. L. McKee, L. López-Hoffman, S. M. L. Ewe & W. P. Sousa, 2008. Environmental drivers in mangrove establishment and early development: a review. Aquatic Botany 89: 105–127.
Larcher., 2000. Ecofisiologia vegetal. São Carlos. Rima.
Levin, L. A., D. F. Boesch, A. Covich, C. Dahm, C. Erséus, K. C. Ewel, R. T. Kneib, A. Moldenke, M. A. Palmer, P. Snelgrove, D. Strayer & J. M. Weslawski, 2001. The function of marine critical transition zones and the importance of sediment biodiversity. Ecosystems 4: 430–451.
Li, Y., W. Niu, X. Cao, J. Wang, M. Zhang, X. Duan & Z. Zhang, 2019. Effect of soil aeration on root morphology and photosynthetic characteristics of potted tomato plants (Solanum lycopersicum) at different NaCl salinity levels. BMC Plant Biology 19: 1–15.
Lüttge, U., 2007. Physiological Ecology of Tropical Plants, Springer, Germany:
Manousaki, E. & N. Kalogerakis, 2011. Halophytes-an emerging trend in phytoremediation. International Journal of Phytoremediation 13: 959–969.
Mendelssohn, I. A., K. L. McKee & T. Kong, 2001. A comparison of physiological indicators of sublethal cadmium stress in wetland plants. Environmental and Experimental Botany 46: 263–275.
Michelan, T. S., S. M. Thomaz, P. Carvalho, R. B. Rodrigues & M. J. Silveira, 2010a. Regeneration and colonization of an invasive macrophyte grass in response to desiccation. Natureza & Conservação 8: 133–139.
Michelan, T. S., S. M. Thomaz, R. P. Mormul & P. Carvalho, 2010b. Effects of an exotic invasive macrophyte (tropical signalgrass) on native plant community composition, species richness and functional diversity. Freshwater Biology 55: 1315–1326.
Michelan, T., M. Dainez Filho & S. Thomaz, 2017. Aquatic macrophyte mats as dispersers of one invasive plant species. Brazilian Journal of Biology 78: 5–8.
Midgley, J. M., M. P. Hill & M. H. Villet, 2006. The effect of water hyacinth, Eichhornia crassipes (Martius) Solms-Laubach (Pontederiaceae), on benthic biodiversity in two impoundments on the New Year’s River, South Africa. African Journal of Aquatic Science 31: 25–30.
Morard, P. & J. Silvestre, 1996. Plant injury due to oxygen deficiency in the root environment of soilless culture: a review. Plant and Soil 184: 243–254.
Mormul, R. P., S. M. Thomaz, J. Higuti & K. Martens, 2010. Ostracod (Crustacea) colonization of a native and a non-native macrophyte species of Hydrocharitaceae in the Upper Paraná floodplain (Brazil): an experimental evaluation. Hydrobiologia 644: 185–193.
Munns, R. & M. Tester, 2008. Mechanisms of salinity tolerance. Annual Review of Plant Biology 59: 651–681.
Nunes, L. S. C. & A. F. M. Camargo, 2017. A simple non-destructive method for estimating aboveground biomass of emergent aquatic macrophytes. Acta Limnologica Brasiliensia 49: 1–9.
Nunes, L. S. C. & A. F. M. Camargo, 2018. Do interspecific competition and salinity explain plant zonation in a tropical estuary? Hydrobiologia 812: 67–77.
Nunes, L. S. C. & A. F. M. Camargo, 2020. Effects of salinity on growth, competitive interaction and total nitrogen content of two estuarine macrophyte species cultivated on artificial substrate. Aquatic Ecology 54: 973–983.
Nunes, L. S. C., C. A. Umetsu, M. E. F. Rodrigues, V. J. Pott & A. F. M. Camargo, 2019. Inventory of aquatic macrophyte species in coastal rivers of the São Paulo state, Brazil. Oecologia Australis 23: 829–845.
Odum, W. E., 1988. Comparative ecology of tidal freshwater and salt marshes. Annual Review of Ecology and Systematics 19: 147–176.
Parihar, P., S. Singh, R. Singh, V. P. Singh & S. M. Prasad, 2015. Effect of salinity stress on plants and its tolerance strategies: a review. Environmental Science and Pollution Research 22: 4056–4075.
Pott, V. J., A. Pott, L. C. P. Lima, S. N. Moreira & A. K. M. Oliveira, 2011. Aquatic macrophyte diversity of the Pantanal wetland and upper basin. Brazilian Journal of Biology 71: 255–263.
Reinert, B. L., M. R. Bornschein & C. Firkowski, 2007. Distribuição, tamanho populacional, hábitat e conservação do bicudinho-do-brejo Stymphalornis acutirostris Bornschein, Reinert e Teixeira, 1995 (Thamnophilidae). Revista Brasileira De Ornitologia 15: 493–519.
Ribeiro, J. P. N., R. S. Matsumoto, L. K. Takao, A. C. Peret & M. I. S. Lima, 2011. Spatial distribution of Crinum americanum L. in tropical blind estuary: hydrologic, edaphic and biotic drivers. Environmental and Experimental Botany 71: 287–291.
Rodrigues, M. E. F., V. C. Souza & M. L. M. Pompêo, 2017. Levantamento florístico de plantas aquáticas e palustres na Represa Guarapiranga, São Paulo, Brasil. Boletim De Botânica 35: 1–64.
Short, F. T., S. Kosten, P. A. Morgan, S. Malone & G. E. Moore, 2016. Impacts of climate change on submerged and emergent wetland plants. Aquatic Botany 135: 3–17.
Suárez, N. & E. Medina, 2005. Salinity effect on plant growth and leaf demography of the mangrove, Avicennia germinans L. Trees 19: 721–727.
Thomaz, S. M., P. Carvalho, A. A. Padial & J. T. Kobayashi, 2009. Temporal and spatial patterns of aquatic macrophyte diversity in the Upper Paraná River floodplain. Brazilian Journal of Biology 69: 617–625.
Thomaz, S. M., R. P. Mormul & T. S. Michelan, 2015. Propagule pressure, invasibility of freshwater ecosystems by macrophytes and their ecological impacts: a review of tropical freshwater ecosystems. Hydrobiologia 746: 39–59.
Thouvenot, L. & G. Thiébaut, 2018. Regeneration and colonization abilities of the invasive species Elodea canadensis and Elodea nuttallii under a salt gradient: implications for freshwater invasibility. Hydrobiologia 817: 193–203.
Umetsu, C. A., F. C. Aguiar, M. T. Ferreira, L. F. Cancian & A. F. M. Camargo, 2018. Addressing bioassessment of tropical rivers using macrophytes: the case of Itanhaém Basin, São Paulo, Brazil. Aquatic Botany 150: 53–63.
Villamagna, A. M. & B. R. Murphy, 2010. Ecological and socio-economic impacts of invasive water hyacinth (Eichhornia crassipes): a review. Freshwater Biology 55: 282–298.
Xue, L., X. Li, Z. Yan, Q. Zhang, W. Ding, X. Huang, B. Tian, Z. Ge & Q. Yin, 2018. Native and non-native halophytes resiliency against sea-level rise and saltwater intrusion. Hydrobiologia 806: 47–65.
Acknowledgments
We thank Carlos Fernando Sanches, Sonia Maria R. Carregari and Baltasar Fernandes Garcia Neto for all assistance with the experiment and analysis.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Handling editor: Andre Andrian Padial
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Santini, R., de Lima, J.P., Gratão, P.L. et al. Evaluation of growth and oxidative stress as indicative of salinity tolerance by the invasive tropical aquatic macrophyte tanner grass. Hydrobiologia 849, 1261–1271 (2022). https://doi.org/10.1007/s10750-021-04787-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-021-04787-4