Abstract
Human activities may change beta diversity—the spatial variation in species composition—in different ways. Positive and negative trends in beta diversity are referred as biotic differentiation and homogenization, respectively. In this context, river damming is likely to be a major cause of changes in beta diversity over time. Here, we evaluated the impact of damming on zooplankton beta diversity in two Brazilian reservoirs. We predicted that damming would cause biotic differentiation due to the creation of areas with different hydrological conditions, which would allow the colonization and population growth of species belonging to different zooplankton groups. Our results for the total zooplankton community were consistent with the hypothesis of biotic differentiation, either due to the increased mean beta diversity or due to the tendency of increasing beta diversity over time after damming. An indicator species analysis also showed that a large proportion of taxa that can be categorized as euplanktonic were mainly indicators of the period after damming, whereas the opposite was true for testate amoebae. Increased beta diversity should be interpreted as an impact of damming. However, we speculate that, under a process of water quality deterioration, biotic homogenization is likely to occur, reversing the patterns we observed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The temporal increase in compositional similarity among a set of local communities is called biotic homogenization (McKinney & Lockwood, 1999). In general, during this process, native species with restricted distribution are replaced by common, widespread and, in many cases, exotic species (Olden & Poff, 2003; Olden et al., 2007, 2018; Petsch, 2016). Various anthropogenic activities that alter the structure and functioning of ecosystems can cause biotic homogenization (e.g., eutrophication, damming, and urbanization; Rahel, 2002; McKinney, 2006; Rogalski et al., 2017; Monchamp et al., 2018; Liu et al., 2020). The process of biotic homogenization can be analyzed by beta diversity metrics, as they quantify the variation in species composition among a set of local communities in a specific region and point in time (Anderson, 2006; Anderson et al. 2006, 2011). Thus, if there is a decrease in spatial beta diversity over time, it can be inferred that a process of homogenization is occurring (Olden & Rooney, 2006). On the other hand, if there is an increase in spatial beta diversity over the time, one can infer that a process of biotic differentiation is occurring (Olden & Poff, 2003).
River damming is one of the main impacts on freshwater ecosystems that can lead to changes in biodiversity (Rahel, 2002; Poff et al., 2007; Agostinho et al., 2016; Turgeon et al., 2019). The abrupt change in hydrological conditions after damming, transforming a lotic system into one with lentic characteristics (Thornton et al., 1990), triggers various changes in the physical, chemical, and biological characteristics of lotic ecosystems (Baxter, 1977; Thornton et al., 1990; Agostinho et al., 2016). At large spatial scales, the construction of reservoirs homogenizes hydrological regimes, causing profound impacts on biodiversity (Poff et al., 2007; Kirk et al., 2020). According to Poff et al. (2007), “dams arguably have a continental scale effect of homogenizing regionally distinct environmental templates, thereby creating conditions that favor the spread of cosmopolitan, nonindigenous species at the expense of locally adapted native biota.” At the reservoir scale, however, one can expect that dams create regions with different environmental characteristics (Thornton et al., 1990). Thus, the environmental conditions of the riverine region tend to be more similar to those of the lotic environment (i.e., before damming). This region is characterized by high water flow, nutrient concentration, and turbidity. The opposite conditions characterize the lacustrine region and, in general, the transition region exhibits intermediate characteristics in relation to the previous ones. At a large spatial scale, we would also expect environmental and ecological differences between the reservoir and downstream reaches due to water flow regulation.
A large body of evidence from the last decades indicates the pivotal role of hydrology on zooplankton community structure (e.g., Thorp & Mantovani, 2005; Sluss et al., 2008; Taura & Duggan, 2020). Therefore, zooplankton beta diversity is likely to be strongly influenced by the changes in hydrology caused by damming. Specifically, studies have found that zooplankton abundance was positively correlated with water residence time (Basu & Pick, 1996) or negatively correlated with discharge (Thorp et al., 1994). The underlying mechanism here is that low water residence time (or high discharge) precludes zooplankton population growth because, even if other environmental factors are favorable, advective losses of individuals surpass any gain of individuals due to local reproduction (Pace et al., 1992; Reckendorfer et al., 1999). The positive effect of water residence time on zooplankton abundance has also been evidenced by studies showing that reservoirs are major sources of individuals to downstream reaches in impounded rivers (Pourriot et al., 1997; Havel et al., 2009; Dickerson et al., 2010; Sindt & Wolf, 2021). Thus, studies comparing rivers with different hydrological regimes or studies comparing rivers with lentic systems (either lakes or reservoirs) indicate that zooplankton communities will thrive after damming (e.g., Stephan et al., 2017; Murphy et al., 2020). In addition to an increase in abundance, beta diversity is also expected to increase because of the environmental (and mainly hydrological) differentiation caused by damming. This increase in beta diversity would be expected for the different zooplankton groups (e.g., testate amoebae, rotifers and microcrustaceans). For example, from the riverine to the lacustrine regions, densities of rotifers and, mainly, of microcrustaceans are expected to be positively impacted by the reduction in water velocity. On the other hand, testate amoebae are likely to be negatively impacted along this gradient. Thus, one can expect that trends of biotic differentiation would occur in different directions along the longitudinal gradient for the different zooplankton groups. An increase in beta diversity could also be expected for a comparison between a reservoir and free-flowing reaches (upstream or downstream the reservoir).
Zooplankton species have different environmental requirements and, in general, zooplankton communities are strongly responsive to changes in environmental conditions (e.g., Jeppesen et al., 2011). In addition, after river damming, one would expect a compartmentalization/differentiation of the environment (i.e., the creation of regions with different environmental characteristics within a reservoir and the environmental differentiation between the reservoir and downstream reaches). In its turn, the environmental differentiation, after damming, would favor the occurrence of different species compositions among sampling sites within a reservoir. Thus, we tested (i) the hypothesis that spatial beta diversity (among sampling sites within the reservoir area of influence) would increase after the damming. However, the spatial (environmental) differentiation within reservoirs, which may select for different local communities, should be more frequent in storage reservoirs, where water residence time is higher than in run-of-river reservoirs (Perbiche-Neves & Nogueira, 2013; Picapedra et al., 2020). Thus, because our study was conducted in reservoirs differing in the type of operation (see details in the Study Area), we also tested (ii) the hypothesis that the effects of damming on zooplankton beta diversity would be stronger in the storage reservoir than in the run-of-river reservoir. In addition, we expected (iii) that an increase in beta diversity, after damming, would occur for the different zooplankton groups (e.g., microcrustaceans, rotifers and testate amoebae).
Material and methods
Study aea
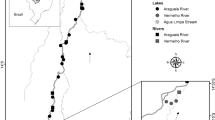
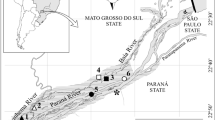
This study was conducted in two hydroelectric reservoirs located in two river basins of Brazil (Fig. 1). The Santo Antônio do Jari Reservoir (SAJ henceforth) was built in the Jari River (states of Pará and Amapá), a tributary of the Amazon River. The filling of SAJ was completed in May 2014, and in average, this reservoir has a total area of 31.7 km2, a total capacity of 133.39 × 106 m3, an average depth of 9.5 m (Vieira et al., 2017), and can be classified as run-of-river due to its short water residence time (about 1.5 days). The filling of the Serra do Facão Reservoir (SF henceforth), in the state of Goiás (São Marcos River, Paraná Basin; Fig. 1), was completed in September 2009. This reservoir can be classified as a storage reservoir, with a long water residence time (ca. in 350 days). It has a total area of 218.84 km2, a total capacity of 3,474 × 106 m3, and an average depth of 43.5 m (http://sefac.com.br/energia/ficha-tecnica/).
Sampling and laboratory analysis
In the SAJ Reservoir, sampling was conducted between February 2012 and February 2018, totaling 33 sampling events in 14 sites (with 10 and 23 months of sampling before and after damming, respectively; for the detailed sampling schedule, see Fig. S1a). In the SF Reservoir, sampling was conducted between July 2007 and June 2010, totaling 20 sampling events in 9 sites (with 14 and 6 months of sampling before and after damming, respectively; Fig. S1b). At each sampling site, 1000 L of water was filtered through a plankton net (68 µm). In both reservoirs, the samples were always collected at a depth of approximately 50 cm. The filtered samples were fixed with a buffered 4% formaldehyde solution. For quantitative and qualitative analyses, the samples were concentrated to a known and variable volume (between 75 and 300 ml), depending on the number of organisms and amount of sediment in the samples. Larger volumes were used for samples that contained higher concentrations of sediment.
After homogenizing each sample, five 1.5 ml aliquots were taken from each sample with a Hensen-Stempel pipette for counting in Sedgwick-Rafter chambers. The samples were analyzed using an optical microscope (Olympus CX31—400 ×) using a modified version of the method proposed by Bottrell et al. (1976). The identification was made to the lowest possible taxonomic level (often species level) using taxonomic keys (mainly Koste, 1978; Ogden & Hedley, 1980; Reid, 1985; Paggi, 1995; Velho & Lansac-Tôha, 1996; El Moor-Loureiro, 1997; see Table S1). The larval and juvenile forms of the copepod families Cyclopidae and Diaptomidae were considered as different taxonomic entities and included in the species list. The abundance of these groups shows a high spatio-temporal variation, and therefore, they were included in our analyses. The density of each taxon was expressed as individuals/m3.
Data analysis
Before the analysis, we log transformed [log10 (x + 1)] the species densities. We used the average distance from sampling sites to group centroids (sampling months in our study) as our measure of beta diversity (Anderson, 2006; Anderson et al., 2006). We used the Bray–Curtis index in this analysis. The higher this average, the higher the dispersion of the sampling sites around a month (group centroid) and, hence, the higher the (spatial) beta diversity. We quantified beta diversity separately for each reservoir, considering the total zooplankton community and separately for testate amoebae, rotifers, and microcrustaceans.
For each reservoir, we used an interrupted time series analysis (Manly, 1994; see also Wauchope et al., 2021) to test the effect of damming on zooplankton beta diversity (for the total community and for each group separately). Beta diversity (βt) was used as a response variable in this analysis. The following explanatory variables were included in the model: time, damming (a dummy variable, with I = zero and 1.0 before and after damming, respectively), and an interaction term between time (t) and damming (I). Thus, the following model was fitted to the data:
where βt is the beta diversity value at time t, a and b are the coefficients of a linear regression model (intercept and slope, respectively), and et indicates the error term. The immediate impact of damming is given by δ, whereas τ indicates the changes in the temporal trends before and after the impact (i.e., damming). The time series before and after the intervention can be described by the models βt = a + bt + et and βt = (a + δ) + (b + τ)t + et, respectively (see details below). We tested for residual independence, using autocorrelation functions (Zuur et al., 2009), and found that this assumption was met.
The interpretation of the models described above depends critically on the estimates of δ and τ. For example, if both coefficients did not differ significantly from zero, then one would not find evidence of an impact (Fig. S2A-C). This would be so even if b is significant (positive or negative) because the trend in beta diversity (increasing or decreasing over time) would be unrelated to the damming. On the other hand, the clearest results would be obtained when δ is significant and both b and τ are non-significant. In this case, a negative and positive δ would indicate that the damming caused an impact on beta diversity (biotic homogenization and differentiation, respectively; Fig. S2D and S2M); in other words, it would indicate a clear shift in the mean level of the time series. However, even when both δ and b are significant and τ is non-significant, one would be able to infer an impact of damming (Fig. S2E-F and Fig. S2N-O). For this combination of results, if both δ and b are negative and significant, then one could infer that the damming changed the level of the time series (biotic homogenization), but not its temporal trend (Fig. S2E). If both δ and b are positive and significant, one could infer that beta diversity increased both before and after damming and that this impact caused a positive shift in the mean level of time series between these periods (biotic differentiation; Fig. S2O).
On the other hand, if τ is significant, then one should focus on the interpretation of the interaction and, in this case, several patterns are possible (Fig. S2). Here, for the sake of brevity, we will describe the results that were more frequent in our study (see results), but the other possibilities can be visualized in Fig. S2. If τ is significant, the time series can be described by the two models described above. For example, if b is positive and also significant, one would observe an increasing in beta diversity that is unrelated to the damming (as this trend was already occurring before damming). If this result is associated with a positive and significant δ, one could infer an immediate impact of damming (biotic differentiation). If b + τ = zero, still considering a scenario where τ is significant, then one could infer that after damming the process of biotic differentiation was attenuated (which, however, do not change the fact that the mean level of the time series increased after damming; see Fig. S2Q). On the other hand, if b = zero, δ < 0 and b + τ > 0, then one could infer that beta diversity was stable before damming, decreased due to damming but showed a pattern of biotic differentiation afterwards (Fig. S2J). Our second hypothesis that the effect of damming on beta diversity would be stronger in the storage reservoir (SF) than in the run-of-river reservoir (SAJ) can be tested by comparing the estimated values of δ, assuming that τ is not significant in both reservoirs. However, if τ is significant, this hypothesis can be evaluated by comparing the slopes after damming (i.e., b + τ). Finally, our third hypothesis would be supported if, for example, the different zooplankton groups exhibited significant and positive values of δ. In case of a significant τ, this hypothesis would be confirmed by a b + τ > 0, with similar magnitudes among the groups.
We also ran an Indicator Species Analysis (INDVAL), following the procedures described by Dufrêne & Legendre (1997), to find taxa that were indicative of the sampling periods (before and after damming). We used 999 random permutations of the samples between periods to test the significance of the indicator values. Our study is focused on spatial beta diversity. However, the INDVAL would be useful to indicate, for each reservoir, the taxa that are indicative of each sampling periods (before and after damming) and that, therefore, contribute to temporal beta diversity.
We conducted all analyses in the R program (R Development Core Team, 2020) using the “stats,” “vegan,” (Oksanen et al., 2019) and “labdsv,” (Roberts, 2019) packages.
Results
We found 232 zooplankton taxa in the SAJ Reservoir (83 testate amoebae, 89 rotifers, and 60 microcrustaceans, including larval and juvenile forms of copepods). Testate amoebae species richness differed little between periods (66 species before and 65 after damming); however, for rotifers and microcrustaceans, the species richness was higher after damming (rotifers: 67 before and 75 after; microcrustaceans: 44 before and 54 after). We recorded 175 zooplankton taxa in the SF Reservoir (73 testate amoebae, 63 rotifers and 39 microcrustaceans). Testate amoebae richness declined after damming (71 before and 48 after), whereas the richness of rotifers (41 before and 55 after) and microcrustaceans (27 before and 33 after) increased after damming.
Interrupted time series models for the total zooplankton community
Zooplankton beta diversity ranged from 0.98 to 1.07 and from 0.54 to 0.67 in SAJ and SF reservoirs, respectively (Fig. 2). The models were significant in both reservoirs (SAJ: F3,29 = 11.87, P < 0.001, R2adj = 0.50; SF: F3,16 = 5.10, P = 0.012, R2adj = 0.39). In the SAJ Reservoir, beta diversity increased over time before damming and the damming significantly increased the mean level of the time series. After damming, beta diversity did not vary significantly over time (Table 1; Fig. 2a). In the SF Reservoir, beta diversity was time independent before damming. We also found a significant and negative effect of damming on the mean level of the time series; afterwards, we found a temporal increase in beta diversity (Table 1; Fig. 2b).
Temporal variation of the total zooplankton beta diversity in the Santo Antônio do Jari (a) and Serra do Facão (b) reservoirs. Open and closed circles indicate beta diversity values observed before and after damming, respectively (which are separated by dotted lines). The solid lines are the fitted values
Interrupted time series models for the different zooplankton groups
For testate amoebae beta diversity in the SAJ Reservoir, we found a positive temporal trend before damming (Table 1; Fig. 3a), whereas the model for the SF Reservoir was not significant (Table 1; Fig. 3b).
Temporal variation of the testate amoebae beta diversity in the Santo Antônio do Jari (a) and Serra do Facão (b) reservoirs. Open and closed circles indicate beta diversity values observed before and after damming, respectively (which are separated by dotted lines). The solid lines are the fitted values
Beta diversity of rotifers in the SAJ Reservoir increased significantly over time before damming, and we also found a significant (immediate) increase in beta diversity due to damming. After damming, beta diversity of rotifers did not exhibit a temporal trend (Table 1; Fig. 4a). The beta diversity of the rotifer community in the SF Reservoir varied independently of time and was not affected by damming (Table 1; Fig. 4b).
For microcrustaceans in the SAJ Reservoir, the results of the interrupted time series analysis were similar to those obtained for the total zooplankton community and for rotifers. Thus, the beta diversity of this group tended to increase significantly over time before damming, and we found an immediate and positive effect of damming on the mean level of the time series. After damming, beta diversity varied independently of time (Table 1; Fig. 5a). In the SF Reservoir, beta diversity of rotifers varied independently of time and was not affected by damming (Table 1; Fig. 5b).
Temporal variation of microcrustaceans beta diversity in the Santo Antônio do Jari (a) and Serra do Facão (b) reservoirs. Open and closed circles indicate beta diversity values observed before and after damming, respectively (which are separated by dotted lines). The solid lines are the fitted values
Indicator species analysis (INDVAL)
We found 25 testate amoebae taxa in the SAJ Reservoir with significant INDVAL indexes, and all of them were significant indicators of the period before damming (Table S2A). In the SF Reservoir, we found 13 taxa with significant INDVAL indexes, of which 10 and 3 taxa were significant indicators of the periods before and after damming, respectively (Table S2B). Centropyxis aculeata (Ehrenberg, 1838) and Centropyxis ecornis (Ehrenberg, 1841) were the taxa with the highest INDVAL indexes in both reservoirs.
Ten and eleven rotifers were significant indicators of the periods before and after damming in the SAJ Reservoir, respectively (Table S2A). The taxa with the highest INDVAL indexes, before damming, were Lepadella ovalis (Müller, 1786) and Dicranophorus sp., whereas after damming, Lecane leontina (Turner, 1892) and Plathyonus patulus macracanthus (Daday, 1905) were those with the highest indexes. In the SF Reservoir, 21 taxa were significant indicators of the period after damming (and none of the period before damming), especially Plathyas quadricornis (Ehrenberg, 1832) and Polyarthra vulgaris Carlin, 1943 (Table S2B).
For microcrustaceans, we found 5 taxa that were significant indicators of the period before damming in the SAJ Reservoir (e.g., Alona sp., Nicsmirnovius fitzpatricki (Chien, 1970) and Tropocyclops prasinus (Fischer, 1860)), whereas 11 taxa had significant INDVAL indexes for the period after damming (e.g., Cyclopoida copepodites and Moina minuta Hansen, 1899; Table S2A). In the SF Reservoirs, 21 taxa were significant indicators of the period after damming (and none of the period before damming), especially Cyclopoida and Calanoida (nauplii and copepodites; Table S2B).
Discussion
We detected different temporal trends (before and after damming) and effects of the immediate impact of damming on the beta diversity of the total zooplankton community. In SAJ Reservoir, we detected a trend of increasing beta diversity over time before the damming and an immediate impact of the damming that is consistent with the biotic differentiation hypothesis. In addition, we did not detect a trend of increasing beta diversity after damming. On the other hand, the beta diversity in the SF reservoir did not show a temporal trend before impact. In this reservoir, the immediate effect of damming was negative, indicating biotic homogenization and, after damming, we detected a significant trend of increasing beta diversity over time. Thus, in general, our results were more consistent with the biotic differentiation hypothesis, either by the increased mean level of the time series after the impact (SAJ) or by the increased trend over time also after impact (SF).
Compared to other human-induced impacts on ecosystems (e.g., urbanization; Liu et al., 2020), damming is more likely to cause biotic differentiation than homogenization of plankton communities. This may be the case because the environmental differentiation caused by damming (within the reservoir, with the creation of different regions, and between the reservoir and downstream or upstream reaches) would tend to favor different zooplankton communities. Also, the effect of damming on hydrology, with the transformation of a lotic to a lentic-like environment, is probably a key mechanism underlying biotic differentiation. High water flow is a strong environmental filter, inhibiting the development of planktonic communities (Baranyi et al., 2002). Before damming, river zooplankton communities are mainly constituted by individuals passively dispersing from areas with low water flow (e.g., backwaters and side channels; Dickerson et al., 2010). When the effects of this filter are reduced, due to damming, many species can colonize the new environment (with more lentic-like characteristics), increasing beta diversity. Indeed, studies have shown that reservoirs, as compared to rivers, are more favorable to zooplankton population growth due to the increased water residence time (Havel et al., 2009; Dickerson et al., 2010). The INDVAL results are consistent with this interpretation because euplanktonic taxa (microcrustaceans and rotifers) were mainly indicators of the after damming period. Conversely, most testate amoebae taxa, which can be regarded as pseudoplanktonic (Lansac-Tôha et al., 2008), were predominantly indicators of the before damming period, considering that these organisms are usually suspended into the water column from the substrate and associated vegetation (Alves et al., 2010).
Other studies focusing on zooplankton and fish communities have found a tendency to biotic homogenization in areas under the influence of reservoirs (Freedman et al., 2014; Sá-Oliveira et al., 2015; Braghin et al., 2018). However, clearer evidence on the effects of damming on beta diversity is more likely to be provided in studies that analyze the same sampling sites over time, as we did in our study. With this sampling design, one would control for distance effects on composition similarity (Nekola & White, 1999) and would also consider more closely the definition of biotic homogenization: “decrease in beta diversity over time” (Olden et al., 2018). To the best of our knowledge, this is one of the first studies to evaluate the effects of damming on zooplankton beta diversity using data before and after damming at the same sampling sites. Although we found evidence for biotic differentiation considering a period of a few years after damming, additional data, with the continuation of the monitoring program, may indicate a reversal of this pattern (e.g., Lopes et al., 2017). For example, eutrophication may be a cause of biotic homogenization in reservoir plankton communities (Zorzal-Almeida et al., 2017).
We expected the effects of damming on zooplankton beta diversity to vary depending on the type of reservoir operation. Storage reservoirs, such as SF Reservoir, have large volumes and longer water residence times than run-of-river reservoirs, such as the SAJ Reservoir (Poff & Hart, 2002; McManamay et al., 2016). Thus, we expected that the compositional difference between regions of a reservoir and between the reservoir and other stretches would be more pronounced in the storage reservoir. In general, our results support this expectation as we found a strong trend of increasing beta diversity, after damming, in the storage reservoir (SF). Probably, the immediate and significant decrease of beta diversity in the SF Reservoir was caused by a strong depletion of dissolved oxygen during the filling phase, an impact that is common in tropical reservoirs due to the decomposition of flooded vegetal biomass (Bianchini Jr & Santino, 2011; Agostinho et al., 2016). However, we found a positive impact of damming (i.e., a significant δ) on beta diversity even in the run-of-river reservoir (SAJ). This result may be mainly attributed to hydrological differentiation, assuming that the limnological differentiation may not be strong enough to alone increase beta diversity in this type of reservoir.
We predicted that beta diversity of the different zooplankton groups would increase after the impact. For example, the variation in community structure of testate amoebae would be increased due to a negative impact of damming on the abundance of this group in sampling sites near the dam, which would decrease, in terms of effect, towards the fluvial region (where the hydrologic conditions favor the abundance of this group; Velho et al., 2003; Alves et al., 2010). In general, due to an increase in water residence time, the opposite pattern would be expected for microcrustaceans and rotifers as the abundance of these groups would increase after damming and from the riverine to the lacustrine zone (as well as from free-flowing reaches to the reservoirs; Baranyi et al., 2002). Thus, the three zooplankton groups were expected to undergo biotic differentiation. However, our main predictions that an increase in beta diversity after damming would occur and that this increase would be greater in the storage reservoir were confirmed mainly when the total community was analyzed.
The patterns of biotic differentiation we observed should be interpreted, first, as an impact caused by the damming (i.e., more is not necessarily better). On the other hand, what should be the baseline reference for monitoring these man-made systems? Conservation efforts should be directed to keep ecosystems as pristine as possible; thus, would it be desirable to reduce beta diversity to levels similar to those found before damming (assuming that an intervention of this kind is possible)? In the long run, the trend of biotic differentiation may change to a trend of biotic homogenization (Turgeon et al., 2019). It is also important to consider that eutrophication is one of the main threats to aquatic ecosystems and recent studies have shown that this process can decrease beta diversity (Zorzal-Almeida et al., 2017; Rogalski et al., 2017; Cook et al., 2018). Thus, we believe that the high values of beta diversity observed after the damming should be the new baseline for monitoring purposes in reservoirs. Putting it in another way, we are of the opinion that high zooplankton beta diversity should be the “desirable” state because a decrease of this metric is likely to be related to water quality problems in reservoirs (especially, eutrophication).
Recently, Olden et al. (2018) carried out a systematic review and found that biotic homogenization was more frequently observed than biotic differentiation or than an absence of relationship between beta diversity and time. They also stated that the results of the studies varied considerably (between biogeographic regions and taxonomic groups) and, probably, this high variability can be partly attributed to the problems of definition and methods used. However, assuming negligible changes in water quality (e.g., eutrophication), biotic differentiation of plankton communities in reservoirs, as found in our study, may be more likely to occur because, at the reservoir scale, different regions (with different hydrological characteristics) are created (i.e., lacustrine, intermediate and riverine regions), which can favor the population growth of different zooplankton species.
Conclusions and caveats
We found evidence that zooplankton beta diversity may be increased by damming, as indicated by an increase in the mean level of the time series (SAJ) or by a positive trend after damming (SF). However, our results were complex. For example, a clear increase in mean beta diversity after damming was observed in the SAJ, a run-of-river reservoir. On the other hand, despite the positive trend after damming, the immediate impact on beta diversity was negative in SF, a storage reservoir. In terms of mechanisms, these results indicate that even a small increase in the water residence time (as in the SAJ Reservoir), which may not translate into strong limnological compartmentalization, may be enough to cause biotic differentiation of zooplankton communities within reservoirs. We cannot rule out the possibility of different patterns with the use of longer time series (both before and after the impact). In this context, pre-damming time series in tropical environments are often very short in relation to studies in temperate regions (e.g., Turgeon et al., 2019). Thus, for new hydroelectric plants, we suggest more frequent (monthly) samplings (e.g., 24 months) before and after damming to analyze their impacts on plankton communities.
The impact of damming was detected in both reservoirs only when the total zooplankton community was analyzed. For example, the effect of damming on testate amoebae beta diversity was not significant in both reservoirs, and for rotifer and microcrustaceans, the models were significant only in the SAJ Reservoir. At least indirectly, these results indicate that assessments of biotic homogenization and differentiation should consider, whenever possible, the analysis of different zooplankton groups. Finally, we emphasize that the inferences above should be restricted to plankton communities impacted by reservoirs that are not subject to eutrophication.
Availability of data and material
The original data are available upon request.
Code availability
Our R scripts are available upon request.
References
Agostinho, A. A., L. C. Gomes, N. C. L. Santos, J. C. G. Ortega & F. M. Pelicice, 2016. Fish assemblages in Neotropical reservoirs: colonization patterns, impacts and management. Fisheries Research 173: 26–36.
Alves, G. M., L. F. M. Velho, N. R. Simões & F. A. Lansac-Tôha, 2010. Biodiversity of testate amoebae (Arcellinida and Euglyphida) in different habitats of a lake in the Upper Paraná River floodplain. European Journal of Protistology 46: 310–318.
Anderson, M. J., 2006. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62: 245–253.
Anderson, M. J., K. E. Ellingsen & B. H. McArdle, 2006. Multivariate dispersion as a measure of beta diversity. Ecology Letters 9: 683–693.
Anderson, M. J., T. O. Crist, J. M. Chase, M. Vellend, B. D. Inouye, A. L. Freestone, N. J. Sanders, H. V. Cornell, L. S. Comita, K. F. Davies, S. P. Harrison, N. J. B. Kraft, J. C. Stegen & N. G. Swenson, 2011. Navigating the multiple meanings of β diversity: a roadmap for the practicing ecologist. Ecology Letters 14: 19–28.
Baranyi, C., T. Hein, C. Holarek, S. Keckeis & F. Schiemer, 2002. Zooplankton biomass and community structure in a Danube River floodplain system: effects of hydrology. Freshwater Biology 47: 473–482.
Basu, B. K. & F. R. Pick, 1996. Factors regulating phytoplankton and zooplankton biomass in temperate rivers. Limnology and Oceanography 41: 1572–1577.
Baxter, R. M., 1977. Environmental effects of dams and impoundments. Annual Review of Ecology and Systematics 8: 255–283.
Bianchini, I., Jr. & M. B. C. Santino, 2011. Model parameterization for aerobic decomposition of plant resources drowned during man-made lakes formation. Ecological Modelling 222: 1263–1271.
Bottrell, H. H., A. Ducan, Z. M. Gliwicz, E. Grygierek, A. Herzig, A. Hillbricht-Ilkowska, H. Kurasawa, P. Larsson & T. Weglenska, 1976. Review of some problems in zooplankton production studies. Norwegian Journal of Zoology 24: 419–456.
Braghin, L. S. M., B. A. Almeida, D. C. Amaral, T. F. Canella, B. C. G. Garcia & C. C. Bonecker, 2018. Effects of dams decrease zooplankton functional β-diversity in river-associated lakes. Freshwater Biology 63: 721–730.
Cook, S., L. Housley, J. A. Back & R. S. King, 2018. Freshwater eutrophication drives sharp reductions in temporal beta diversity. Ecology 99: 47–56.
Dickerson, K. D., K. A. Medley & J. E. Havel, 2010. Spatial variation in zooplankton community structure is related to hydrologic flow units in the Missouri River, USA. River Research and Applications 26: 605–618.
Dufrêne, M. & P. Legendre, 1997. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecological Monographs 67: 345–366.
El Moor-Loureiro, L. M. A., 1997. Manual de identificação de cladóceros límnicos do Brasil, Editora Universa - UCB, Brasília.
Freedman, J. A., B. D. Lorson, R. B. Taylor, R. F. Carline & J. R. Stauffer, 2014. River of the dammed: longitudinal changes in fish assemblages in response to dams. Hydrobiologia 727: 19–33.
Havel, J. E., K. A. Medley, K. D. Dickerson, T. R. Angradi, D. W. Bolgrien, P. A. Bukaveckas & T. M. Jicha, 2009. Effect of main-stem dams on zooplankton communities of the Missouri River (USA). Hydrobiologia 628: 121–135.
Jeppesen, E., P. Nõges, T. A. Davidson, T. Nõges, K. Blank, T. L. Lauridsen, M. Søndergaard, C. Sayer, R. Laugaste, L. S. Johansson, R. Bjerring & S. L. Amsinck, 2011. Zooplankton as indicators in lakes: a scientific-based plea for including zooplankton in the ecological quality assessment of lakes according to the European Water Framework Directive (WFD). Hydrobiologia 676: 279–297.
Kirk, M. A., B. M. Maitland & F. J. Rahel, 2020. Spatial scale, reservoirs and nonnative species influence the homogenization and differentiation of Great Plains—Rocky Mountain fish faunas. Hydrobiologia 847: 3743–3757.
Koste, W., 1978. Rotatoria: Die Radertiere Mitteuropas, Gebruder Borntraeger, Berlin.
Lansac-Tôha, F. A., L. M. Bini, L. F. M. Velho, C. C. Bonecker, E. M. Takahashi & L. C. Vieira, 2008. Temporal coherence of zooplankton abundance in a tropical reservoir. Hydrobiologia 614: 387–399.
Liu, P., S. Xu, J. Lin, H. Li, Q. Lin & B.-P. Han, 2020. Urbanization increases biotic homogenization of zooplankton communities in tropical reservoirs. Ecological Indicators 110: 105899.
Lopes, V. G., C. W. Castelo-Branco, B. Kozlowsky-Suzuki, I. F. Sousa-Filho, L. C. Souza & L. M. Bini, 2017. Predicting temporal variation in zooplankton beta diversity is challenging. PLoS ONE 12: e0187499.
Manly, B. F. J., 1994. The design and analysis of research studies, Cambridge University Press, Cambridge.
McKinney, M. L., 2006. Urbanization as a major cause of biotic homogenization. Biological Conservation 127: 247–260.
McKinney, M. L. & J. L. Lockwood, 1999. Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends in Ecology & Evolution 14: 450–453.
McManamay, R. A., C. O. Oigbokie, S. C. Kao & M. S. Bevelhimer, 2016. Classification of US hydropower dams by their modes of operation. River Research and Applications 32: 1450–1468.
Monchamp, M. E., P. Spaak, I. Domaizon, N. Dubois, D. Bouffard & F. Pomati, 2018. Homogenization of lake cyanobacterial communities over a century of climate change and eutrophication. Nature Ecology and Evolution 2: 317–324.
Murphy, C. A., A. Evans, B. Coffin, I. Arismendi & S. L. Johnson, 2020. Resilience of zooplankton communities in temperate reservoirs with extreme water level fluctuations. Inland Waters 10: 256–266.
Nekola, J. C. & P. S. White, 1999. The distance decay of similarity in biogeography and ecology. Journal of Biogeography 26: 867–878.
Ogden, C. G. & R. H. Hedley, 1980. An atlas of freshwater testate amoebae, Oxford University Press, London.
Oksanen, J., F. G. Blanchet, R. Kindt, P. Legendre, P. R. Minchin, R. B. O’Hara, G. L. Simpson, M. H. H. Stevens & H. Wagner, 2019. Package ‘vegan.’ Community Ecology Package, Version 2 5–6: 1–296.
Olden, J. D. & N. L. Poff, 2003. Toward a mechanistic understanding and prediction of biotic homogenization. The American Naturalist 162: 442–460.
Olden, J. D. & T. P. Rooney, 2006. On defining and quantifying biotic homogenization. Global Ecology and Biogeography 15: 113–120.
Olden, J. D., L. Comte & X. Giam, 2018. The Homogocene: a research prospectus for the study of biotic homogenisation. NeoBiota 37: 23–36.
Olden, J. D., M. J. Kennard & B. J. Pusey, 2007. Species invasions and the changing biogeography of Australian freshwater fishes. Global Ecology and Biogeography 17: 27–37.
Pace, M. L., S. E. G. Findlay & D. Lints, 1992. Zooplankton in advective environments: the Hudson River community and a comparative analysis. Canadian Journal of Fisheries and Aquatic Sciences 49: 1060–1069.
Paggi, J. C., 1995. Crustacea Cladocera. In Lopretto, E. C. & G. Tell (eds), Ecosistemas de aguas continentales: metodologias para su estudio Ediciones Sur, La Plata: 909–951.
Perbiche-Neves, G. & M. G. Nogueira, 2013. Reservoir design and operation: effects on aquatic biota-a case study of planktonic copepods. Hydrobiologia 707: 187–198.
Petsch, D. K., 2016. Causes and consequences of biotic homogenization in freshwater ecosystems. International Review of Hydrobiology 101: 113–122.
Picapedra, P. H. S., C. Fernandes, J. Taborda, G. Baumgartner & P. V. Sanches, 2020. A long-term study on zooplankton in two contrasting cascade reservoirs (Iguaçu River, Brazil): effects of inter-annual, seasonal, and environmental factors. Peer J 8: e8979.
Poff, N. L. & D. D. Hart, 2002. How dams vary and why it matters for the emerging science of dam removal. BioScience 52: 559–668.
Poff, N. L., J. D. Olden, D. M. Merritt & D. M. Pepin, 2007. Homogenization of regional river dynamics by dams and global biodiversity implications. Proceedings of the National Academy of Sciences of the United States of America 104: 5732–5737.
Pourriot, R., C. Rougier & A. Miquelis, 1997. Origin and development of river zooplankton: example of the Marne. Hydrobiologia 345: 143–148.
R Development Core Team, 2020. Computing, R: A Language and Environment for Statistical. R Foundation for Statistical Computing, Vienna, Austria, https://www.r-project.org/.
Rahel, F. J., 2002. Homogenization of freshwater faunas. Annual Review of Ecology and Systematics Annual Reviews 33: 291–315.
Reckendorfer, W., H. Keckeis, G. Winkler & F. Schiemer, 1999. Zooplankton abundance in the River Danube, Austria: the significance of inshore retention. Freshwater Biology 41: 583–591.
Reid, J. W., 1985. Chave de identificação e lista de referências bibliográficas para as espécies continentais sulamericanas de vida livre da ordem Cyclopoida (Crustacea, Copepoda). Boletim De Zoologia 9: 17–143.
Roberts, D. W., 2019. Package ‘labdsv’. Ordination and Multivariate Analysis for Ecology. R package version 2.0-1.
Rogalski, M. A., P. R. Leavitt & D. K. Skelly, 2017. Daphniid zooplankton assemblage shifts in response to eutrophication and metal contamination during the Anthropocene. Proceedings of the Royal Society B: Biological Sciences Royal Society Publishing 284: 20170865.
Sá-Oliveira, J. C., J. E. Hawes, V. J. Isaac-Nahum & C. A. Peres, 2015. Upstream and downstream responses of fish assemblages to an eastern Amazonian hydroelectric dam. Freshwater Biology 60: 2037–2050.
Sindt, A. R. & M. C. Wolf, 2021. Spatial and temporal trends of Minnesota River phytoplankton and zooplankton. River Research and Applications 37: 776–795.
Sluss, T. D., G. A. Cobbs & J. H. Thorp, 2008. Impact of turbulence on riverine zooplankton: a mesocosm experiment. Freshwater Biology 53: 1999–2010.
Stephan, L. R., M. S. M. Castilho-Noll & R. Henry, 2017. Comparison among zooplankton communities in hydrologically different lentic ecosystems. Limnetica 36: 99–112.
Taura, Y. M. & I. C. Duggan, 2020. The relative effects of willow invasion, willow control and hydrology on wetland zooplankton assemblages. Wetlands 40: 2585–2595.
Thornton, K. W., B. L. Kimmel & F. E. Payne, 1990. Reservoir limnology: ecological perspectives, Wiley, New York.
Thorp, J. H., A. R. Black & K. H. Haag, 1994. Zooplankton assemblages in the Ohio River: seasonal, tributary, and navigation dam effects. Canadian Journal of Fisheries and Aquatic Sciences 51: 1634–1643.
Thorp, J. H. & S. Mantovani, 2005. Zooplankton of turbid and hydrologically dynamic prairie rivers. Freshwater Biology 50: 1474–1491.
Turgeon, K., C. Turpin & I. Gregory-Eaves, 2019. Dams have varying impacts on fish communities across latitudes: a quantitative synthesis. Ecology Letters 22: 1501–1516.
Velho, L. F. M. & F. A. Lansac-Tôha, 1996. Testate Amoebae (Rhizopodea-Sarcodina) from Zooplankton of the High Paraná River Floodplain, State of Mato Grosso do Sul, Brazil: II. Family Difflugidae. Studies on Neotropical Fauna and Environment 31: 179–192.
Velho, L. F. M., F. A. Lansac-Tôha & L. M. Bini, 2003. Influence of environmental heterogeneity on the structure of testate amoebae (Protozoa, Rhizopoda) assemblages in the plankton of the Upper Paraná River floodplain, Brazil. International Review of Hydrobiology 88: 154–166.
Vieira, M. C., L. M. Bini, L. F. M. Velho, L. F. Gomes, J. C. Nabout & L. C. G. Vieira, 2017. Biodiversity shortcuts in biomonitoring of novel ecosystems. Ecological Indicators 82: 505–512.
Wauchope, H. S., T. Amano, J. Geldmann, A. Johnston, B. I. Simmons, W. J. Sutherland & J. P. Jones, 2021. Evaluating impact using time-series data. Trends in Ecology & Evolution 36: 196–205.
Zorzal-Almeida, S., L. M. Bini & D. C. Bicudo, 2017. Beta diversity of diatoms is driven by environmental heterogeneity, spatial extent and productivity. Hydrobiologia 800: 7–16.
Zuur, A. F., E. N. Ieno, N. J. Walker, A. A. Saveliev & G. M. Smith, 2009. Mixed effects models and extensions in ecology with R, Springer, New York.
Acknowledgements
This research was funded by the Coordination for the Improvement of Higher Level Personnel (CAPES; scholarships to MCV and JCGO) and the Brazilian Council of Research (CNPq; grants to LMB, LCGV and LFMV). This work was also developed in the context of the National Institutes for Science and Technology (INCT) in Ecology, Evolution and Biodiversity Conservation, supported by MCTIC/CNPq (proc. 465610/2014-5) and FAPEG. Two anonymous reviewers provided helpful comments on early version of this paper.
Funding
This research was funded by the Coordination for the Improvement of Higher Level Personnel (CAPES; scholarships to MCV and JCGO) and the Brazilian Council of Research (CNPq; grants to LMB, LCGV and LFMV). This work was also developed in the context of the National Institutes for Science and Technology (INCT) in Ecology, Evolution and Biodiversity Conservation, supported by MCTIC/CNPq (proc. 465610/2014-5) and FAPEG.
Author information
Authors and Affiliations
Contributions
MCV and LMB planned the study and wrote the first draft of the manuscript. MCV and JCGO conducted the statistical analysis. LCGV and LFMV obtained the data. All authors contributed to the refinement of the study and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Handling editor: Andrew Dzialowski
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vieira, M.C., Ortega, J.C.G., Vieira, L.C.G. et al. Evidence that dams promote biotic differentiation of zooplankton communities in two Brazilian reservoirs. Hydrobiologia 849, 697–709 (2022). https://doi.org/10.1007/s10750-021-04740-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-021-04740-5