Abstract
Population densities in different sites are frequently observed to fluctuate synchronously, a phenomenon termed temporal coherence. The two main causes of temporal coherence are dispersal and the effects of a regional factor (Moran effect) that influence each of the populations similarly. If synchronous patterns are observed, it is possible to infer that there is a regional process (e.g., climate) exerting a uniform influence over the entire region. Here, we evaluate patterns of temporal coherence in total densities of zooplankton groups. Data were gathered at 11 sites in the Corumbá Reservoir (Central Brazil) between 1996 and 2000 (n = 27 months). These sites were distributed in the main channel, arms, and tributaries of the reservoir. Reservoir-wide correlations (as estimated by the average Spearman rank correlation between temporal trajectories of abundances) were low (−0.01, 0.06, 0.23, and 0.14 for cladocerans, copepods, rotifers, and testate amoebae, respectively). In general, high temporal coherence was detected only between geographically adjacent sites and/or between sites with similar limnological characteristics. Contrary to many recent studies, these results illustrate that, in a small geographic area (i.e., a single reservoir of approximately 65 km2), local processes may override the effects of regional processes or dispersal. Moreover, they demonstrate that the lack of regional trajectories (i.e., time series of population densities with asynchronous patterns of fluctuation) should be considered in interpreting results obtained in long-term studies or monitoring programs based on a single site per ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The degree to which the temporal series of limnological variables (biotic and abiotic), obtained in a subset of aquatic environments within a predefined realm, are positively correlated (i.e., vary synchronously) is defined as temporal coherence (George et al., 2000). Following Magnuson et al. (1990), Rusak et al. (1999) defined temporal coherence “as the phenomenon of synchronous fluctuations in one or more parameters among locations within a geographic region.” Detecting temporal coherence has different implications. Firstly, a significant pattern of temporal coherence or synchrony might indicate the prevalence of regional, extrinsic factors (e.g., climate) on the dynamics of the variables of interest (e.g., plankton population density; Kratz et al., 1987). Conversely, if a low level of temporal coherence is found, one may infer the predominance of local-scale regulators (Rusak et al., 1999). In this way, some of the main local mechanisms in maintaining population synchrony are environmental drivers, predation, food source, and/or local spatial complexity (Kent et al., 2007 and references therein). Secondly, from an applied perspective, a high level of temporal coherence is a paramount condition to a reliable extrapolation of the results obtained in a set of sites to a larger region (Stoddard et al., 1998).
Previous studies addressing temporal coherence were made across a series of distinct lakes at different temporal and spatial scales (see Kratz & Frost, 2000). For instance, George et al. (2000) demonstrated the effects of climatic driving variables (e.g., North Atlantic Oscillation (NAO), wind-mixing) affecting the levels of coherence of winter temperature and summer abundance of zooplankton in five lakes in the English Lake District. In contrast, low levels of temporal coherence were estimated by Baron & Caine (2000), despite the physiographic similarity of the lakes in two alpine lake basins of the Colorado Front Range (USA). In the system studied by Kling et al. (2000) encompassing a lake district in arctic Alaska (USA), the best predictor of temporal coherence was the geographical proximity between the lakes. Webster et al. (2000) demonstrated how the position of the lakes in the landscape (lowland versus highland lakes) and the level of hydrogeologic complexity within a lake district may affect the levels of temporal coherence. Benson et al. (2000) examined the degree of temporal coherence of lake thermal variables of four lake districts in the Upper Great Lakes region of North America and found a high general level of temporal coherence (within and between lake districts). Baines et al. (2000) demonstrated a high level of temporal coherence for temperature, calcium, and chlorophyll in lakes of Northern Wisconsin and suggested the usefulness of carefully selected sentinel lakes to predict the regional effects of climatic variability on aquatic ecosystems. In a last example, Weyhenmeyer (2004) has shown that synchronous relationships between NAO and water chemistry were restricted to variables strongly driven by surface-water temperature.
All aforementioned studies were developed in discrete systems (e.g., distinct lakes). Independently of the area of these systems, only one time series was gathered for each system and the levels of temporal coherence (correlation between time series) were estimated. However, the level of temporal coherence may vary even within a single system. Thus, the degree of temporal coherence must also be investigated within ecosystems by correlating the temporal trajectories of limnological variables measured in different sampling sites within a single ecosystem in order to examine at what spatial extent temporal coherence may be detected. This is especially true for monitoring purposes as a way to verify whether the time series obtained in a single site can be extrapolated to other regions (e.g., arms of a reservoir) of the ecosystem monitored. Scale is an important issue in this discussion. Without the verification of temporal coherence, most conclusions derived from monitoring a single sampling site within an ecosystem cannot be easily extrapolated for the ecosystem of interest as a whole. Because the definition of an ecosystem is controversial (O’Neill, 2001) in this article we used this term in a broad context to refer to a realm or unity of management or study.
In this study, we analyzed temporal fluctuations in the total density of zooplankton groups in a tropical reservoir (Corumbá Reservoir, Brazil) to determine the relative strength of the endogenous (local) and exogenous factors in determining population dynamics and to verify our capability to estimate reservoir-wide density trajectories, which are relevant for the purpose of ecosystem management.
Methods
Study area
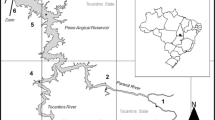
Corumbá Reservoir (Central Brazil) is situated at 17°59′ S and 48°31′ W, in southern Goiás State near the border with Minas Gerais State. It is formed by the Corumbá River and other small tributaries (Fig. 1). The drainage basin of the Corumbá Reservoir covers an area of 27,800 km2. The Corumbá River was dammed in September 1996, flooding an area of 65 km2. It is approximately 60 km long with an average depth of 23 m. The theoretical water residence time (estimated as a ratio of the annual mean reservoir volume to the annual mean outflow) is ca. 30 days.
Sampling
Sampling was performed monthly between November 1996 and November 1997, and every 2 months between January 1998 and March 2000 (n = 27 months), at 11 sites distributed among the reservoir main channel (RES1, RES2, RES3, RES4, and RES5), arms (ARM1 and ARM2), Corumbá River upstream and downstream of the reservoir (RIV1 and RIV2), and tributaries (TRI1 and TRI2) (Fig. 1). All sites were sampled at all time points except for one, RES4, where n = 25. For each sample, 1,000 l of water was collected just below the surface with a motorized pump and filtered through a 68-μm mesh plankton net. The samples were fixed in 4% buffered formalin and concentrated in a final volume of 150 ml. Total abundances for each taxonomic group (cladocerans, copepods, rotifers, and testate amoebae) were determined by counting subsamples (2.5 ml) taken with a Hensen-Stempell pipette. At least 200 individuals of each group were counted per sample using a Sedgwick-Rafter counting cell and an optical microscope (about of 10% of whole). If samples had few individuals, the whole sample was counted. Density was expressed as individuals m−3.
The following limnological variables were determined twice: once in the rainy season (March 1999) and once during the dry season (September 1999), at all stations: water temperature (°C, by digital thermometer), pH (portable pH meter), dissolved oxygen (mg l−1, by the Winkler method, modified by Golterman et al., 1978), electrical conductivity (μS cm−1), using a glass electrode, alkalinity (μmol l−1, according to Mackereth et al., 1978), and turbidity (NTU, LaMotte portable turbidimeter). Moreover, 5 l of water was collected for laboratory determination of chlorophyll-a concentration (μg l−1, Golterman et al., 1978), dissolved organic carbon (mg l−1, Carbon Analyzer Schimadzu TOC 5000), total phosphorus (μg l−1, according to Golterman et al., 1978), and total Kjeldahl nitrogen (μg l−1, according to Mackereth et al., 1978).
Data analysis
Spearman rank correlation coefficients between the time series of two sites (no time lags were considered) were calculated as a measure of temporal coherence between these sites. Accordingly, for each of the considered zooplankton groups, we constructed a triangular site × site matrix of these correlation coefficients (S). Each off-diagonal value in this matrix (one matrix for each group) represents the strength of the relationship between the temporal trajectories of the abundances measured in a pair of sites. The reservoir-wide level of temporal coherence was estimated as the average Spearman rank correlation from all sites. Following Bjørnstad et al. (1999a), a bootstrap confidence interval for each average (one per taxonomic group) was estimated by sampling with replacement among sites.
Variation in the level of temporal coherence between sites (S) was modeled considering geographical distance (G) and limnological dissimilarity (L) (data obtained in March and September 1999) as explanatory matrices. Matrix L was based on the following variables: dissolved oxygen, conductivity, pH, total phosphorous, total nitrogen, dissolved organic carbon, turbidity, and chlorophyll-a. Geographical distance between sites was expressed in kilometers, whereas the standardized Euclidean distance was used to quantify the limnological dissimilarities between sites. The correlations between S and G or between S and L were quantified by the standardized Mantel statistic (Legendre & Fortin, 1989). The Mantel’s (1967) statistic is designed to measure the relationship between two (usually) symmetrical matrices and, in its standardized form, the test criterion is
where s ij (i.e., temporal coherence between sites i and j) and g ij (i.e., geographical distance between sites i and j) are the previously standardized (off-diagonal) elements (by subtracting the mean of all the elements in the matrix from each observation and then dividing by the standard deviation) of the matrices S and G, respectively. A high negative (and significant) value of the statistic r indicates that temporal coherence decreases as geographical distance increases.
By using a series of partial Mantel tests (Smouse et al., 1986), we also evaluated the relationships between S and L after taking into account the geographical patterns (G) and between S and G after taking into account the limnological distances among sampling sites (L). The partial Mantel statistic is designed to evaluate the relationship between two matrices (say S and L) while controlling for the effects of a third matrix (i.e., G). A detailed description of the testing procedure is given by Legendre (2000). All tests were performed using the library VEGAN version 1.6–7 (Dixon, 2003; Oksanen, 2005) in R (R Development Core Team, 2004).
Results
Data of some limnological variables are presented in Table 1. According to data obtained in March and September 1999, there is clear spatial and temporal variation in the water chemistry variables across the Corumbá Reservoir. The surface oxygen concentration varied from 5.7 to 9.4 mg l−1. Conductivity ranged between 15 and 55.7 μS cm−1 and pH between 5.9 and 9.6. Concentrations of total phosphorous and total nitrogen varied from 14 to 162 μg l−1 and from 157 to 2,200 μg l−1, respectively. Dissolved organic carbon ranged from 1.1 to 7.6 mg l−1. In general, due to high turbidity (1.9–561 NTU) and low residence time, phytoplankton biomass in the reservoir (as indicated by chlorophyll-a concentration) is low, varying from values below the limit of detection to 52 μg l−1. In general, higher values of DOC, turbidity, total phosphorous, and total nitrogen were recorded in rainy season (March), while the higher values of chlorophyll-a, alkalinity, and electrical conductivity were observed in dry period (September).
Total zooplankton abundance varied from 520 to 447,657 ind. m−3. The highest densities occurred in lentic habitats (reservoir and arms), whereas the lowest densities were found in the lotic ones (riverine region and tributaries). Microcrustaceans (mainly copepods) and rotifers dominated in lentic habitats, while testate amoebae and rotifers were predominant in lotic habitats.
The most abundant species among testate amoebae were Centropyxis aculeata (Ehrenberg), C. ecornis (Ehrenberg), Cyclopyxis kahli (Deflandre), Arcella vulgaris Ehrenberg, A. discoides Ehrenberg, and Difflugia gramen Pènard. Rotifers were mainly represented by Synchaeta pectinata Ehrenberg, Polyarthra vulgaris Carlin, P. dolychoptera Idelson, Ptygura sp., Trichocerca cylindrica (Imhof), Keratella americana Carlin, and Brachionus calyciflorus Pallas. Among microcrustaceans, Ceriodaphnia cornuta Sars, Diaphanosoma spinulosum Herbst, Bosminopsis deitersi Richard, Bosmina hagmanni Stingelin, and Moina minuta Hansen were the most abundant cladoceran species, and Thermocyclops minutus (Lowndes), T. decipiens (Kiefer), and Notodiaptomus iheringi (Wright) dominated among copepods.
The degree of temporal coherence, as estimated by the Spearman rank correlation between the series, ranged from −0.4 to 0.63 for testate amoebae (mean = 0.14; estimated by summing all off-diagonal elements of the matrix S and dividing by the total number of elements), from −0.14 to 0.78 for rotifers (mean = 0.23), from −0.56 to 0.67 for cladocerans (mean = −0.01), and from −0.45 to 0.57 for copepods (mean = 0.06). The confidence intervals for the mean correlation were positive for testate amoebae (CI95% = 0.07, 0.21; see Table 2) and rotifers (CI95% = 0.18, 0.39; Table 2), indicating that the dynamics of these groups were significantly correlated. On the other hand, the confidence intervals for the mean correlation of cladocerans (CI95% = −0.08, 0.062) and copepods (CI95% = −0.012, 0.13) included negative values, indicating that the overall levels of temporal coherence (reservoir-wide) for these groups were not significant (Table 2).
For testate amoebae, temporal coherence between pairs of sites located in the upper reach of the reservoir (including sites RIV1, RES1, RES2, TRI1, and TRI2, Fig. 2A) was always higher than 0.5. The highest value of temporal coherence was measured between sites RIV1 and RES1 (Spearman’s correlation = 0.63). However, even sites not directly connected by water flow exhibited a high level of temporal coherence in this part of the reservoir, for example, between pairs TRI1–TRI2 (0.62) and TRI1–RIV1 (0.60).
Spearman rank correlations between sites for each taxonomic group analyzed: (A) testate amoebae, (B) rotifers, (C) cladocerans, (D) copepods. Only values ≥0.49 are shown. Solid arrows were used when the sites in comparison are directly “connected” by water flow. Otherwise, dashed arrows were used. RES, reservoir main channel; ARM, arms; RIV, Corumbá River; and TRI, tributaries
The highest level of temporal coherence for rotifers was also found between sites RIV1 and RES1 (0.78). However, temporal coherence was also detected in the southern part of the reservoir (Fig. 2B), where the lentic conditions were predominant. Similar results were obtained for cladocerans (Fig. 2C) and copepods (Fig. 2D). For this last group, however, high levels of temporal coherence were detected only among the sites ARM1 and RES4 (0.52) and between sites RES3 and RES4 (0.57). Surprisingly, considering the level of temporal coherence between sites ARM1–RES4 and RES3 and RES4 (Fig. 2C and D) as well as the spatial adjacency, low levels of temporal coherence between sites ARM1 and RES3 were detected for both copepods (0.22) and cladocerans (0.32).
The highest levels of synchrony in density fluctuations of testate amoebae were found among sites located in the upper reach of the reservoir, where lotic conditions predominated and where this group was more abundant (Fig. 3A). On the other hand, microcrustaceans (cladocerans and copepods) were more abundant and presented evident synchronous patterns in the southern part of the reservoir, where the lentic conditions predominated (Fig. 4A–B). In relation to rotifers, although differences in density were not so remarkable, they were also more abundant in the lower reach but synchronous patterns could be found, in general, in both stretches of the reservoir (Fig. 3B).
Temporal variation in density (log X) of testate amoebae (A) and rotifers (B). ○, RIV1; □, RES1; ◊, RES2; ∆, TRI1; ●, ARM1; ■, RES3; ♦, RES4; ▲, ARM2; +, TRI2; ✴, RES5; −, RIV2. RES, reservoir main channel; ARM, arms; RIV, Corumbá River; and TRI, tributaries. The first vertical dotted line indicates when the reservoir reached the maximum water level; the second vertical dotted line means started operation
Temporal variation in density (log X) of cladocerans (A) and copepods (B). ○, RIV1; □, RES1; ◊, RES2; ∆, TRI1; ●, ARM1; ■, RES3; ♦, RES4; ▲, ARM2; +, TRI2; ✴, RES5; −, RIV2. RES, reservoir main channel; ARM, arms; RIV, Corumbá River; and TRI, tributaries. The first vertical dotted line indicates when the reservoir reached the maximum water level; the second vertical dotted line means started operation
A decline in temporal coherence with increasing geographical distance between site pairs (matrix G) was found for cladocerans, copepods, and rotifers, as indicated by the Mantel test (Table 3). The strongest predictor of temporal coherence was the limnological distance between sites calculated for March 1999 (matrix L; Table 3). However, in September 1999, S was significantly correlated with L only for cladocerans (Table 3).
In March 1999, except for testate amoebae, results of partial Mantel tests indicated a significant effect of limnological characteristics on patterns of temporal coherence, after controlling for spatial effects. The more different the limnological conditions among sites the lower the concordance and, therefore, the signs of the correlations are negative. On the other hand, no significant spatial autocorrelation was detected when limnological variation was removed (Table 3).
Results obtained in September 1999 (dry season) were rather different from those registered in March 1999 (rainy season). Specifically, only matrix G was a significant predictor of S (for microcrustaceans and rotifers), when limnological variation was statistically controlled for (Table 3).
Discussion
Before discussing our results, some of their potential caveats must be mentioned. First, most studies on temporal coherence were based on time series longer than the one analyzed by us (e.g., Baines et al., 2000). For instance, short-term time series may preclude the detection of synchronous dynamics and trends due to low statistical power (Urquhart et al., 1998). Also, the beginning of our study coincided with the creation of the reservoir (November 1996), and therefore, the temporal dynamics we recorded may be atypical. Thus, our results should be interpreted cautiously. We also estimated the temporal coherence only within the major groups of zooplankton, whereas analyses based on species or guilds are more common (Jenkins & Buikema, 1998; but see Kent et al., 2007). However, aggregated variables (e.g., total density of a given taxonomic group) have lower background variability than species density (Cottingham & Carpenter 1998), and this property may be useful when detecting temporal coherence.
Considering that our data were gathered for a single system, we would expect a much higher level of temporal coherence, similar to those previously detected in aquatic ecosystems (George et al., 2000 and references therein; Kent et al., 2007). However, the low synchrony indeed suggests that local community varies rather independently within the reservoir and that dispersal of organisms among locations cannot overwhelm out these local dynamics. The fact that there is an association between the pattern of covariance and the limnological characteristics of sites suggests that local factors may be responsible for local dynamics (Rusak et al., 1999) and the overall pattern of low synchrony. Also, one important reason for the difference with other studies, in terms of the level of synchrony, may be that our system was very heterogeneous in habitat type, i.e., a combination of lotic and lentic environments.
Variations in the levels of temporal coherence in the dynamics of microcrustaceans and rotifers, during the rainy season (March), were significantly predicted by limnological differences (see partial Mantel tests results). Thus, highest levels of temporal coherence were detected when sites presented similar limnological conditions, independently of spatial proximity. Interestingly, limnological differences estimated in the rainy season (March 1999) better predicted the variability in the levels of temporal coherence than did the limnological differences estimated in the dry season, where the opposite results were detected and geographic distance was a significant predictor of the temporal coherence variation. During the dry season, we detected a lower level of spatial variability in limnological characteristics (see Table 1).
The variation in the levels of temporal coherence detected in this study is probably accounted for by the two main mechanisms that may synchronize abundance dynamics (dispersal and Moran’s effect; see Moran, 1953; Royama, 1992; Hanski & Woiwod, 1993; Heino et al., 1997; Bjørnstad et al., 1999b; Hudson & Cattadori, 1999; Paradis et al., 1999). As indicated below, the relative importance of these mechanisms, however, was dependent on the taxonomic group analyzed and on the geographic position of the sampling sites.
Particularly, the incidence of dispersal, which includes the transport of individuals by water flow, for testate amoebae and rotifers may be inferred considering the high temporal coherence between sites with a hydrological connection (e.g., sites RIV1 and RES1). Indeed, the influence of hydrological factors on testate amoebae and rotifer abundances, transporting individuals from substrates to the water column, is observed frequently in intertropical ecosystems (Lansac-Tôha et al., 2004). On the other hand, the temporal fluctuations in the total abundance of testate amoebae at sites TRI1 and TRI2 were also synchronized, despite the absence of a direct spatial and hydrological connection between these sites. Thus, dispersal is not supported as a mechanism or at least is not the only explanation, and the influence of a reservoir-wide factor is the most parsimonious explanation for this specific result. Variations in water discharge, which transport individuals from upstream to downstream sites, may also be synchronous due to the regional effects of precipitation on the entire reservoir’s watershed. Recently, a number of studies have indicated the importance of spatial configuration of sampling sites in a hydrological network for predictive and interpretative purposes (Ganio et al., 2005; Cressie et al., 2006; Peterson & Urquhart, 2006; Peterson et al., 2006; Ver Hoef et al., 2006). Studies of temporal coherence in lotic systems would benefit highly from this approach, mainly after taking into account the idea of “hydrologic distance weighted by discharge volume” (Peterson et al., 2007).
In September 1999, the levels of temporal coherence for microcrustaceans and rotifers were significantly correlated with geographical distances, after statistically controlling for limnological variation. Dispersal and the Moran effect (environmental synchrony that declines with distance) may also explain the patterns of decrease in temporal coherence between sites as the distance between them increases (Swanson & Johnson, 1999). Unfortunately, there are no abiotic time-series data allowing a direct estimate of environmental synchrony. This could allow us to verify if abiotic variables show a decline with distance equivalent to that observed in animal populations, which could give support to the Moran effect as an explanation for the pattern (Ranta et al., 1999; Koenig 2002).
It is important to emphasize the low levels of temporal coherence found for microcrustaceans, indicating the preponderance of intrinsic or site-specific factors (Rusak et al., 1999; Hessen et al., 2006). This result may be explained by the low potential of dispersing individuals to alter population density in the downstream sites (Michels et al., 2001). As predicted by Beisner et al. (2006 and references therein), one should expect, under this scenario, the preponderance of local factors over (i.e., spatial distribution of sampling sites and their connections on the reservoir) to explain patterns of temporal coherence. Jenkins (1995) and Jenkins & Underwood (1998) also highlighted the infrequency of rotifer dispersal.
Independently of the mechanisms that could explain these patterns, our results have at least one implication for the design of monitoring programs in reservoirs. The extent to which local data (i.e., obtained at each site) can be used to infer population trajectories across larger spatial scales depends on the level of synchrony (or coherent variation) among different sites. If temporal coherence is high, data gathering in few sites can be effective to estimate regional trajectories (for the entire reservoir) because the temporal patterns of variation are similar independently of the sampling site. On the other hand, where spatial synchrony is low, data obtained in a single site cannot be extrapolated for the entire environment of interest (see Urquhart et al., 1998 for a similar discussion). In these more complicated cases, the statistical power to detect regional trends in the abundance of a particular taxonomic group may be improved by the measurement of relevant covariates that explain inter-site variation or by the identification of sites with similar patterns of variability (Urquhart et al., 1998).
In short, our study indicates that the highest levels of temporal coherence (population synchrony) were detected for rotifers and testate amoebae in the upper part of the reservoir. Many species belonging to these groups are considered pseudoplanktonic (Lansac-Tôha et al., 2004). Thus, densities may be correlated with the stochastic fluctuations in water flow that wash out (“disperse”) individuals from sediments and other substrates to the water column. This process may occur between adjacent sites or between sites located in different basins which are, nevertheless, affected by the same regional factor (e.g., precipitation) that causes synchrony in water flow. Alternatively, water flow fluctuations may also modify local growth conditions. On the other hand, reservoir-wide synchrony estimated for microcrustaceans was not significant, indicating the preponderance of local factors. Identifying the environmental factors that may explain the spatial variability in the temporal trajectories of this group is the main way to improve our power to detect reservoir-wide trends in the total abundance of these organisms.
References
Baines, S. B., K. E. Webster, T. K. Kratz, S. R. Carpenter & J. J. Magnuson, 2000. Synchronous behavior of temperature, calcium, and chlorophyll in lakes of northern Wisconsin. Ecology 81: 815–825.
Baron, J. & N. E. L. Caine, 2000. Temporal coherence of two alpine lake basins of the Colorado Front Range, U.S.A. Freshwater Biology 43: 463–476.
Beisner, B. E., P. R. Peres-Neto, E. S. Lindström, A. Barnett & M. L. Longhi, 2006. The role of environmental and spatial processes in structuring lake communities from bacteria to fish. Ecology 87: 2985–2991.
Benson, B. J., D. John, J. J. Magnuson, M. Stubbs, K. K. Timothy, J. Peter, R. E. Hecky & R. C. Lathrop, 2000. Regional coherence of climatic and lake thermal variables of four lake districts in the Upper Great Lakes Region of North America. Freshwater Biology 43: 517–527.
Bjørnstad, O. N., N. C. Stenseth & T. Saitoh, 1999a. Synchrony and scaling in dynamics of voles and mice in northern Japan. Ecology 80: 622–637.
Bjørnstad, O. N., R. A. Ims & X. Lambin, 1999b. Spatial population dynamics: analyzing patterns and processes of population synchrony. Trends in Ecology & Evolution 14: 427–432.
Cottingham, K. L. & S. R. Carpenter, 1998. Population, community, and ecosystem variates as ecological indicators: Phytoplankton responses to whole-lake enrichment. Ecological Applications 8: 508–530.
Cressie, N., J. Frey, B. Harch & M. Smith, 2006. Spatial prediction on a river network. Journal of Agricultural Biological and Environmental Statistics 11: 127–150.
Dixon, P., 2003. VEGAN, a package of R functions for community ecology. Journal of Vegetation Science 14: 927–930.
Ganio, L. M., C. E. Torgersen & R. E. Gresswell, 2005. A geostatistical approach for describing spatial pattern in stream networks. Frontiers in Ecology and the Environment 3: 138–144.
George, D. G., J. F. Talling & E. Rigg, 2000. Factors influencing the temporal coherence of five lakes in the English Lake District. Freshwater Biology 43: 449–461.
Golterman, H. L., R. S. Clymo & M. A. M. Ohmstad, 1978. Methods for Physical and Chemical Analysis of Fresh Waters. Blackwell Scientific, Oxford: 214 pp.
Hanski, I. & I. P. Woiwod, 1993. Spatial synchrony in the dynamics of moth and aphid populations. Journal of Animal Ecology 62: 656–668.
Heino, M., V. Kaitala, E. Ranta & J. Lindstrom, 1997. Synchronous dynamics and rates of extinction in spatially structured populations. Proceedings of the Royal Society of London Series B-Biological Sciences 264: 481–486.
Hessen, D. O., B. A. Faafeng, V. H. Smith, V. B. Bakkestuen & B. Walsen, 2006. Extrinsic and intrinsic controls of zooplankton diversity in lakes. Ecology 87: 433–443.
Hudson, P. J. & I. M. Cattadori, 1999. The Moran effect: A cause of population synchrony. Trends in Ecology & Evolution 14: 1–2.
Jenkins, D. G., 1995. Dispersal limited zooplankton distribution and community composition in new ponds. Hydrobiologia 313(314): 15–20.
Jenkins, D. G. & A. L. Buikema, 1998. Do similar communities develop in similar sites? A test with zooplankton structure and function. Ecological Monographs 68: 421–443.
Jenkins, D. G. & M. O. Underwood, 1998. Zooplankton may not disperse readily in wind, rain, or waterfowl. Hydrobiologia 387(388): 15–21.
Kent, A. D., A. C. Yannarell, J. A. Rusak, E. W. Triplett & K. D. McMahon, 2007. Synchrony in aquatic microbial community dynamics. The ISME Journal 1: 38–47.
Kling, G. W., G. W. Kipphut, M. M. Miller & W. J. O’Brien, 2000. Integration of lakes and streams in a landscape perspective: The importance of material processing on spatial patterns and temporal coherence. Freshwater Biology 43: 477–497.
Koenig, W. D., 2002. Global patterns of environmental synchrony and the Moran effect. Ecography 25: 283–288.
Kratz, T. K. & T. M. Frost, 2000. The ecological organization of lake districts: general introduction. Freshwater Biology 43: 297–299.
Kratz, T. K., T. M. Frost & J. J. Magnuson, 1987. Inferences from spatial and temporal variability in ecosystems—Long-term zooplankton data from lakes. American Naturalist 129: 830–846.
Lansac-Tôha, F. A., C. C. Bonecker & L. F. M. Velho, 2004. Composition, species richness and abundance of the zooplankton community. In Thomaz, S. M., A. A. Agostinho & N. S. Hahn (eds), The Upper Paraná River and its floodplain: Physical aspects, ecology and conservation. Backhuys Publishers, Leiden: 145–190.
Legendre, P., 2000. Comparison of permutation methods for the partial correlation and partial Mantel tests. Journal of Statistical Computation and Simulation 67: 37–73.
Legendre, P. & M. J. Fortin, 1989. Spatial pattern and ecological analysis. Vegetatio 80: 107–138.
Mackereth, F. Y. H., J. Heron & J. J. Talling, 1978. Water analysis: Some revised methods for limnologists. Freshwater Biological Association 36: 1–120.
Magnuson, J. J., B. J. Benson & T. K. Kratz, 1990. Temporal coherence in the limnology of a suite of lakes in Wisconsin, USA. Freshwater Biology 23: 145–159.
Mantel, N., 1967. The detection of disease clustering and a generalized regression approach. Cancer Research 27: 209–220.
Michels, E., K. Cottenie, L. Neys & L. De Meester, 2001. Zooplankton on the move: First results on the quantification of dispersal of zooplankton in a set of interconnected ponds. Hydrobiologia 442: 117–126.
Moran, P. A. P., 1953. The statistical analysis of the Canadian Lynx Cycle. 2. Synchronization and meteorology. Australian Journal of Zoology 1: 291–298.
Oksanen, J., 2005. VEGAN: Community ecology package. R package version 1.6–7 [URL: http://cc.oulu.fi/~jarioksa/].
O’Neill, R. V., 2001. Is it time to bury the ecosystem concept? (with full military honors, of course!). Ecology 82: 3275–3284.
Paradis, E., S. R. Baillie, W. J. Sutherland & R. D. Gregory, 1999. Dispersal and spatial scale affect synchrony in spatial population dynamics. Ecology Letters 2: 114–120.
Peterson, E. E. & N. S. Urquhart, 2006. Predicting water quality impaired stream segments using landscape-scale data and a regional geostatistical model: A case study in Maryland. Environmental Monitoring and Assessment 121: 615–638.
Peterson, E. E., A. A. Merton, D. M. Theobald & N. S. Urquhart, 2006. Patterns of spatial autocorrelation in stream water chemistry. Environmental Monitoring and Assessment 121: 571–596.
Peterson, E. E., D. M. Theobald & J. M. V. Hoef, 2007. Geostatistical modelling on stream networks: Developing valid covariance matrices based on hydrologic distance and stream flow. Freshwater Biology 52: 267–279.
R Development Core Team, 2004. R: A language and environment for statistical computing [URL: http://www.R-project.org].
Ranta, E., V. Kaitala & J. Lindström, 1999. Spatially autocorrelated disturbances and patterns in population synchrony. Proceedings of the Royal Society of London—Series B 266: 1851–1856.
Royama, T., 1992. Analytical Population Dynamics. Chapman and Hall, London: 392 pp.
Rusak, J. A., N. D. Yan, K. M. Somers & D. J. McQueen, 1999. The temporal coherence of zooplankton population abundances in neighboring north-temperate lakes. American Naturalist 153: 46–58.
Smouse, P. E., J. C. Long & R. R. Sokal, 1986. Multiple regression and correlation extensions of the Mantel test of matrix correspondence. Systematic Zoology 35: 627–632.
Stoddard, J. L., C. T. Driscoll, J. S. Kahl & J. P. Kellogg, 1998. Can site-specific trends be extrapolated to a region? An acidification example for the northeast. Ecological Applications 8: 288–299.
Swanson, B. J. & D. R. Johnson, 1999. Distinguishing causes of intraspecific synchrony in population dynamics. Oikos 86: 265–274.
Urquhart, N. S., S. G. Paulsen & D. P. Larsen, 1998. Monitoring for policy-relevant regional trends over time. Ecological Applications 8: 246–257.
Ver Hoef, J. M., E. Peterson & D. Theobald, 2006. Spatial statistical models that use flow and stream distance. Environmental and Ecological Statistics 13: 449–464.
Webster, K. E., P. A. Soranno, S. B. Baines, T. K. Kratz, C. J. Bowser, P. J. Dillon, P. Campbell, J. Everett & R. E. Hecky, 2000. Structuring features of lake districts: Landscape controls on lake chemical responses to drought. Freshwater Biology 43: 499–515.
Weyhenmeyer, G. A., 2004. Synchrony in relationships between the North Atlantic Oscillation and water chemistry among Sweden’s largest lakes. Limnology and Oceanography 49: 1191–1201.
Acknowledgments
We thank to Furnas Centrais Elétricas and Nupélia/UEM for financial and logistic support. Work by FALT, LMB, LFMV, and CCB have been continuously supported by CNPq productivity fellowships. Grants from the Fundação de Amparo à Pesquisa da Universidade Federal de Goiás (FUNAPE/UFG) to LMB are also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: S. Declerck
Rights and permissions
About this article
Cite this article
Lansac-Tôha, F.A., Bini, L.M., Velho, L.F.M. et al. Temporal coherence of zooplankton abundance in a tropical reservoir. Hydrobiologia 614, 387–399 (2008). https://doi.org/10.1007/s10750-008-9526-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-008-9526-6