Abstract
The extinction of fish species can direct and indirectly affect many groups of associated species, among which parasite communities can be the most susceptible. However, the intensity of this effect depends on the structure interaction networks. This study evaluated whether networks constituted of fish ectoparasites or endoparasites differed in their robustness to the loss of host species and to what extent these potential differences are explained by the network structures. We used path models to evaluate the direct and indirect effects of host and parasite richness, connectance, and nestedness on the robustness of ecto- and endoparasite-based networks. In most cases, nestedness was the descriptor that best explained the robustness of the fish-parasite networks, and co-extinctions are less likely when the fish species act mainly as hosts of the generalist parasites. Both the richness of the host species and connectance in the networks have an essential indirect influence on robustness. Regardless of the extinction sequence, the ectoparasite-based networks showed higher vulnerability to host species loss when compared to endoparasite-based networks. These findings highlight the importance of considering both ecto- from endoparasites to better understand the structure and vulnerability of host–parasite networks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parasites comprise a high proportion of global species diversity (Windsor, 1998; Poulin, 2014). They provide essential functions and services to ecosystems, many of which through indirect effects on host abundance (Hatcher et al., 2012; Frainer et al., 2018). The local extinction or even the reduction in the population of host species tends to negatively affect parasite communities (Dallas & Cornelius, 2015). Highly specialized parasites use few host species and are generally more vulnerable to the loss of their host species than generalist parasites that use various host species, although the latter are also negatively impacted by the extinction of their hosts (Farrell et al., 2015). Given that many species will be extinct in the next few decades due to anthropogenic activities, even species that are not considered as vulnerable to extinction may become endangered because of interspecific dependencies (Kolbert, 2014).
Marine and freshwater fishes are among the most threatened animal groups worldwide (Costello et al., 2016; Darwall & Freyhof, 2016). The local extinction of fishes is mainly driven by habitat loss, invasive species, water pollution, and overfishing (Franco, 2013; Costello et al., 2016; Darwall & Freyhof, 2016). Fish parasites, in turn, are ubiquitous in aquatic environments, being characterized by high diversity of species, life history strategies and habitat requirements (see Bush et al., 2001). Fish parasites range from species whose life cycles only include aquatic hosts (e.g., parasites with simple cycles as monogeneans, which do not have intermediate hosts, or parasites with complex cycles and all aquatic hosts) to species that use terrestrial hosts during their life cycles (e.g., birds are definitive hosts of various species of trematodes to which fish are intermediate hosts). Additionally, parasite species can be classified into two categories depending if they live on or in their hosts: ectoparasites that live on the host’s body and have direct contact with the external environment, and endoparasites that live in their host’s body and have almost no direct contact with the external environment (Bush et al., 2001).
Resource sharing within an ecosystem provides that different species interact with each other, directly or indirectly (Rynkiewicz et al., 2015). The extinction of host fish species can lead to changes not only for host–parasite interaction, but also for other species with which interact directly (e.g., species competing for resources, species that preyed or were preyed on by extinct species) or indirectly (e.g., environment use and/ or intermediate interaction by shared parasite species). The extent to which species in parasite assemblages persist after the loss of one or more host species constitutes their robustness against co-extinctions, which is an important predictor of how species extinctions cascade through ecological networks (Pascual & Dunne, 2006). In this context, analyzing the fish-parasite networks allows us to understand how parasites will respond to the extinction of their host species (Dallas & Cornelius, 2015).
The topological properties of ecological networks, such as connectance and nestedness, are emergent properties arising from species-specific differences in their specialization, adaptations and interaction constraints (Dallas & Cornelius, 2015; Vanbergen et al., 2017). These topological properties are important because mediate how the set of species responds to extinctions in ecological networks (Tylianakis et al., 2010; Vieira & Almeida-Neto, 2015). In ecological networks, the connectance measures the proportion of realized interactions in relation to the total possible (Pimm, 1982). Nestedness measures the extent to which the interactions of species with fewer connections represent a subset of the interactions performed by species with more connections (Almeida-Neto et al., 2008; Almeida-Neto & Ulrich, 2011). Both measures reflect the way parasite species use the host species available on the network, for example, lower connectance and nestedness values indicate more specialist interactions on the network. However, high connectivity values do not correspond a perfectly nested structure.
There is a wide variation in the ability of parasite species to use their hosts as resources. Nevertheless, they can be classified by specialists for their ability to infect only one host species (or a limited number of host species), while other parasite species are generalist in being able to infect many host species (Ventim et al., 2012; Walker et al., 2017). In a previous study (Bellay et al., 2015), we showed that endoparasite-based networks are characterized by higher connectance and nestedness values than ectoparasite-based networks. Therefore, the effects of host extinction on the structure of fish-parasite networks is expected to be distinct for ecto- and endoparasites considering the network structure, being fish ectoparasites generally more specialists and endoparasites less specialists in the network (Bellay et al., 2015).
When host species are removed from networks with higher connectance values, such as the fish-endoparasite networks, in general, the parasites are less prone to going extinct because many of them have alternative host species. Furthermore, the different life strategies of the parasites (e.g., ecto- or endoparasites; or simple life cycle species that no require intermediate host or complex life cycle species that need one or more intermediate hosts) may lead to variations in their ecological functions within the host fish-parasite network (Lima-Jr et al., 2012; Bellay et al., 2015, 2018; Lima et al., 2016).
The primary aim of this study was to evaluate whether the primary extinction of host fish species results in different patterns of the co-extinction of endoparasites and ectoparasites. In a previous study, Dallas & Cornelius (2015) demonstrated that the random extinction host species lead to a faster loss of parasite species when compared to the extinction sequence starting with the host species with the smallest to the greatest parasitic communities. These authors evaluated different models of host removal considering the number of interactions between species in a binary host fish-parasite network but did not evaluate the effects of this removal on robustness considering ecto- and endoparasites separately. These two groups of fish parasites have a great diversity of species (see Bush et al., 2001; Eiras et al. 2008) and, although each group may be composed of members of different taxa (for example: ectoparasites—monogeneans, copepods, branchiura and others; endoparasites—nematodes, trematodes, cestodes, acanthocephalans and others), members of each group share morphophysiological and ecological characteristics that allow them to occupy microhabitats outside the host body (ectoparasites—exposed, for example, to environmental variations) or inside (endoparasites—exposed, for example, to peristaltic movements, gastric juices of the host).
Nevertheless, as ectoparasites are exposed to more extreme environmental conditions (variation of water currents), they change their morphology and biology (such as mobility and reproductive strategies; Bush et al., 2001). These changes tend to lead, for example, to the need to have robust organs for attachment and copulation, which can be the basis for more effective speciation on the one host species (Rohde & Heat, 1998). These authors reinforce that such robust attachments and copulatory organs are absent in digenean and other helminth endoparasites, and their speciation may depend on the speciation of their hosts. Here, we expected that ectoparasites are more susceptible to host fish extinction due to their higher host specialization (i.e., lower connectance) and lower host overlap (i.e., lower nestedness) when compared to endoparasites (see Bellay et al., 2015). Thus, fish-ectoparasite networks submitted to the simulated loss of hosts were expected to present a greater decrease in the richness of the ectoparasites when compared to the reduction of endoparasites.
Materials and methods
Study system
We gathered from the literature 22 binary host–parasite networks composed of fish species and their metazoan parasites species from worldwide marine and freshwater ecosystems (Table S1). The number of host and parasite species in the networks range from 6 to 91 and from 20 to 420, respectively. Bellay et al. (2015) brought these networks together by searching The Interaction Web Database (IWDB, available in https://iwdb.nceas.ucsb.edu/resources.html; access date: April 7th, 2020), articles available on Google academic searches using “fish parasite”, “fish host–parasite interaction” as search terms; after the searches, only studies with data that characterized networks were selected (S. Bellay, personal communication; see Bellay et al., 2015 for data availability). Because they are binary networks, we only deal with aspects related to species richness of parasites by fish species and the number of host species that each parasite species occurs, and data on the abundance/intensity of interactions are not used.
The criterion for selecting the networks that were analyzed in this study was that these were composed of both ecto- and endoparasites. Our analyzes do not cover data from small aquatic environments (i.e. streams, temporary lakes) due to the lack of enough data on fish-parasite interactions in the network approach. Each network was decoupled into two subnetworks: (i) those with only ectoparasites (or ectoparasite-based networks), (ii) those with only endoparasites (or endoparasite-based networks). Because both ecto- and endoparasites were presented in all networks we were able to perform within-network comparisons between these two parasite groups.
The taxonomic groups of ectoparasites were Acari, Branchiura, Copepoda, Hirudinea, Isopoda, Mollusca, Monogenea, and Myxosporea. In turn, the endoparasites were represented by Acanthocephala, Aspidobothrea, Cestoda, Digenea, Nematoda, and Pentastomida. Following the studies conducted by Vázquez et al. (2005) and Bellay et al. (2013, 2015), we considered the different stages of the parasites as different “functional species” in the networks.
Network structure and robustness
The selected descriptors of the networks were connectance and nestedness. Connectance is the sum of interactions in the network divided by number of possible interactions (i.e. number of parasite species times number of host species) in binary bipartite network (see Dunne et al., 2002). Connectance was calculate in R Programming Environment (R Core Team, 2019), with bipartite package, networklevel fuction and connectance index (Dormann et al., 2008). Nestedness values were obtained from Bellay et al. (2015) and can be calculated using the NODF index (Almeida-Neto et al., 2008) in bipartite package. NODF index measures, for each species pair, the proportion of interactions of the more specialized species that are predicted by the less specialized ones. The NODF value for the entire network is then calculated as the mean nestedness value for all species pairs. Connectance and nestedness values are available as supplementary material (Data S1).
We evaluated the effect of host extinction on the robustness of ecto- and endoparasite-based networks according to two criteria: (1) the random removal of host fish species; and (2) the sequential removal of host fish species from the least to the most parasitized. The second criterion follows the assumption that less parasitized hosts are less abundant or less susceptible to the parasites, requiring more specific conditions that make them more susceptible to extinction. For each criterion, whenever a host species was removed, all parasite species that had no remaining host species were also removed from the network.
We calculated the robustness of the parasite species against coextinctions using the robustness index (R), which ranges from 0 to 1 and measures the area under the coextinction curve (Burgos et al., 2007). The robustness approaches its maximum value when most of the parasite species remain in the network after most of the host species have been removed and tends to 0 when the networks collapse with the removal of a few host species (Burgos et al., 2007). We generated the extinction curve using the second.extinct function (with 100 randomizations) of the bipartite package. We obtained the co-extinction curves through two primary extinction sequences mentioned earlier.
Statistical analysis
A paired t test (Zar, 2010) was used to verify significant differences between the robustness values of ecto- and endoparasite-based networks, considering the proposed scenarios for the removal of host species. We used the path analysis to investigate the effects of connectance and nestedness on network robustness, controlling the potential for direct and indirect effects of host richness and parasite richness. The application of the R index for both proposed removal procedures resulted in four path models when considering the networks with ecto- and endoparasites separately. We considered host richness (log) and parasite richness (log) as exogenous explanatory variables that can affect robustness both directly and indirectly, mediated by their effects on connectance and nestedness (NODF). The effect of connectance on robustness was also decoupled in a direct effect and an indirect effect, mediated by nestedness. Therefore, we assumed that the variations in nestedness depend on the degree of overall specialization (i.e., the connectance) of the host–parasite networks. Only the direct effect of nestedness on robustness were considered in the path models.
We assessed the model fit through the chi-squared test and by examining the Tucker–Lewis Fit Index (TLI), the Comparative Fit Index (CFI), and the Root Mean Square Error of Approximation (RMSEA). We used the approach introduced by MacCallum et al. (1996) to determine the statistical power of the path models. The statistical power was calculated using RMSEA through the R code developed by Preacher & Coffman (2006). Normality was verified using Mardia’s test, and outliers were inspected using the Mahalanobis distance. We conducted the path analyses using the AMOS 5.0 software (Arbuckle, 2003).
Results
The random extinctions of host fishes resulted in higher coextinctions of the ectoparasites than of the endoparasites in the same networks, which indicates that endoparasite networks were more robust against random primary extinctions (Fig. 1a; t = 4.37; DF = 21; P < 0.0001). Similarly, endoparasite-based networks were more robust than ectoparasite-based networks against the removal of host fish species from those least to most connected to different parasite species (Fig. 1b; t = 2.62; DF = 21; P = 0.0173).
Robustness from 22 host–parasite networks with ecto- and endoparasites analyzed separately. a Random primary extinction; b Primary extinction from least to most connected host species. Details on the networks are presented in the supplementary material. Lines link endoparasite- and ectoparasite-based subnetworks from the fish-parasite network. In red the mean line of robustness values (mean ± SE)
The selected path models used to explain the robustness of parasite assemblages against the loss of host species showed an appropriate fit (Table 1). The explained variation in robustness in the path models for endoparasites was of 79% and 59% for random and sequential extinction of the hosts from the least to the most connected fish species, respectively (Fig. 2). For ectoparasites, the path models accounted for 57% and 27% of the variation in robustness for random and sequential host extinction from the least to the most connected fish species, respectively.
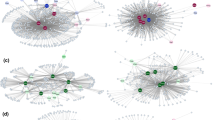
Path models for robustness with two methods of primary extinction (random and from host least to most connected) according to the structures of host–parasite networks: a, b fish-endoparasite networks; c, d fish-ectoparasite networks. Blue and red arrows represent significant positive and negative effects, respectively. Dashed arrows mean non-significant effects
The influence of the network structure on the robustness against secondary extinctions showed marked differences between ecto- and endoparasite-based networks (Fig. 2). For host-endoparasite networks, nestedness was the network structure that most influenced robustness, showing direct positive effects on robustness for both random and sequential removal of host fish species (Fig. 2a, b). On the other hand, nestedness presented no significant effect on parasite coextinctions considering the random removal of hosts for host-ectoparasite networks but showed a positive effect on robustness considering the sequential removal of host species (Fig. 2c, d). In addition, connectance had no significant direct effect on robustness for endoparasite-based networks but showed positive and negative significant effects for ectoparasite-based networks considering both random and sequential removal of host species.
The direct and indirect effects of host richness and parasite richness on robustness against extinction also varied between ecto- and endoparasite-based networks. Regarding the random extinction of the fish species, the richness of host species showed a strong positive effect on robustness for ectoparasites but no direct effect for endoparasites. However, because the indirect effect of host richness on robustness was even more significant than its direct effect for ectoparasite networks, the total net effect did not differ between ecto- and endoparasite-based networks (Table 2). In addition, parasite species richness had significant, but opposite, effects on robustness in endoparasite and ectoparasite networks under the random extinction of the host species. We verified a significant direct effect in the richness of the host species for endoparasite-based networks regarding the sequential extinction from the least to the most connected host fish species (Fig. 2b).
Discussion
We found that simulated primary extinction of fish species lead to a faster lost of ectoparasites when compared to the lost of co-occuring endoparasites. This finding was consistent regardless of the fish extinction sequence (i.e., random extinction or from least to most connected host species) used in the coextinction simulations. In addition, results using path analyses show that the influence of network structure on the vulnerability of fish parasite faunas to the extinction of their host differ between ectoparasite- and endoparasite-based networks.
Positive effects of nestedness on the robustness of ecological networks have been shown in both empirical and simulated networks (Memmott et al., 2004; Santamaria et al., 2014), especially when primary extinction occurs from the least to most connected species (Burgos et al., 2007). For the endoparasite assemblages studied here, nestedness was the network structure that best explained their vulnerability to host species loss in both sequences of primary extinctions, showing a positive effect on the robustness of the endoparasite faunas. In the case of ectoparasite faunas, nestedness also had a major importance in reducing coextinctions, but only when host fishes were extinct from the least to the most connected. The explanation for the positive effect of nestedness on the robustness of fish-parasite networks is that coextinctions are less likely when those fish species harboring fewer parasite species usually act as hosts of the generalist parasites. In a nested network when occur events host species loss, it is expected that parasite species will remain longer in time because generalist species have several host species and specialist species tend to occur in locally less endangered host species. Regarding the difference in the importance of nestedness to network robustness between endoparasite- and ectoparasite-based networks, the simplest explanation is that the former show higher levels of nestedness than the latter (see Bellay et al., 2015).
The positive effect of connectance on the robustness of parasites faunas against coextinctions seems to be mostly a consequence of the strong positive correlation between connectance and nestedness. For endoparasite-based networks, connectance had no direct effect on network robustness in both sequences of host loss. On the other hand, for ectoparasite networks, the direct effect of connectance on robustness against coextinctions was positive under random removal of host fishes, but negative when the fish species are removed in the inverse order of their parasite richness. These findings are in partial disagreement with the prediction that higher connectance (i.e., less specialization) lead to more robust networks (e.g., Dunne et al., 2002; Gilbert, 2009; but see Vieira & Almeida-Neto, 2015).
If generalist species contributes positively to connectance and consequently to nestedness, then these species can lead to fast-occurring disturbances when compared to the removal of a specialist species that have more restricted interactions (see Olesen et al., 2007). The probability of a parasite finding its host is one of the factors governing host–parasite interactions (Strona et al., 2013). This probability theoretically tends to increase, for example, when a host has a wide distribution or high local abundance. Thus, while hosts with higher distribution and abundance are theoretically less vulnerable to extinction (Strona, 2015), they are also exposed to a greater richness and abundance of parasites (see Strona et al., 2013). Currently, even not usually considered as threatened species, many specialized fish parasites share the extinction risk of their host species (see Farrell et al., 2015).
We found positive effects of host richness and negative effects of parasite richness on nestedness for both ecto- and endoparasite-based networks. The negative effect of parasite richness on nestedness may have been due to a decrease in shared hosts and to an increase in the number of highly specialized parasite species. Every parasite has a degree of specialization either by site of infection or host species (see Bush et al., 2001). Thus, the higher the number of species in the network, the occurrence of species with specific requirements is expected. The effects of host and parasite richness on connectance and nestedness modulates the net effects of host and parasite richness on robustness in both ectoparasite- and endoparasite-based networks. Under random primary extinctions of the host species, both host richness and parasite richness had negative net effects on robustness. On the other hand, when primary extinctions occur from host species with lower parasite richness to host species with higher parasite richness, then host richness show a positive effect on network robustness. Because we controlled for the indirect effects of host richness and parasite richness through connectance and nestedness, their net effects must be interpreted as the consequence of both ecological and sampling-related effects. The ecological effects are related to true variation in the relative richness of specialist parasites. The sampling-related effects are related to the negative relationship between sampling effort and connectance (Bersier et al., 1999; Martinez et al., 1999), especially for species-rich networks with many low abundant species.
Different factors may be involved in the organization of fish ecto- and endoparasite-based networks, among which the relative richness of specialist ectoparasites seems to be of major importance (Bellay et al., 2015). The presence of monogeneans, that are highly specialized ectoparasites, in the networks studied seem to be a key factor associated to the higher vulnerability of ectoparasites to host species loss when compared to endoparasites, however in general all taxonomic groups of ectoparasites have species with restricted host numbers, relatively few species occurring with a high number of host species in network (see supplementary material from Bellay et al. 2015). Already in endoparasite networks there was a greater variation among endoparasite groups with a greater number of host species due to environmental characteristics of each network, considering that in general endoparasites need intermediate hosts and environmental variations (abiotic and biotic factors) can benefit one group over another (see supplementary material from Bellay et al., 2015 to check the diversity of the groups). Even if the ecto- and endoparasites of a network are considered together and even if their robustness against coextinctions is high, the subnetwork composed by ectoparasites may be relatively more fragile to local extinctions. This difference suggests that the establishment of different life history traits among parasitic groups may have strong ecological and conservation implications for host–parasite networks.
Conclusion
The present study contributes to knowledge about host fish-parasite interactions, demonstrating that the ectoparasite-based networks tend to be more susceptible to the extinction of host species in large aquatic waterbodies. The study also shows that nestedness was the best predictor of network robustness. The findings highlight the importance of decoupled the components of host–parasite networks to better understand their structure and vulnerability to the primary extinction of host species. Therefore, in the face of the environmental change scenarios, robust networks can present groups of species that are more vulnerable and therefore, more susceptible to extinction (Strona & Lafferty, 2016). Thus, future studies of host–parasite networks should consider whether the networks present groups of species with different patterns of interaction and evaluate them separately.
References
Almeida-Neto, M. & W. Ulrich, 2011. A straightforward computational approach for measuring nestedness using quantitative matrices. Environmental Modelling & Software 26(2): 173–178.
Almeida-Neto, M., P. Guimarães, P. R. Guimarães, R. D. Loyola & W. Ulrich, 2008. A consistent metric for nestedness analysis in ecological systems: reconciling concept and measurement. Oikos 117(8): 1227–1239.
Arbuckle, J. L., 2003. AMOS (Version 5.0). Small Waters Corporation, Chicago.
Bellay, S., E. F. de Oliveira, M. Almeida-Neto, D. P. Lima-Jr, R. M. Takemoto & J. L. Luque, 2013. Developmental stage of parasites influences the structure of fish-parasite networks. PLoS ONE 8(10): e75710.
Bellay, S., E. F. de Oliveira, M. Almeida-Neto, M. A. R. Mello, R. M. Takemoto & J. L. Luque, 2015. Ectoparasites and endoparasites of fish form networks with different structures. Parasitology 142(7): 901–909.
Bellay, S., F. H. Oda, K. M. Campião, F. H. Yamada, R. M. Takemoto & E. F. de Oliveira, 2018. Host-parasite networks: an integrative overview with tropical examples. In Dáttilo, W. & V. Rico-Gray (eds), Ecological Networks in the Tropics. Springer, Cham: 127–140. https://doi.org/10.1007/978-3-319-68228-0_9.
Bersier, L. F., P. Dixon & G. Sugihara, 1999. Scale-invariant or scale-dependent behavior of the link density property in food webs: a matter of sampling effort? American Naturalist 153(6):676–682.
Burgos, E., H. Ceva, R. P. J. Perazzo, M. Devoto, D. Medan, M. Zimmermann & A. María Delbue, 2007. Why nestedness in mutualistic networks? Journal of Theoretical Biology 249(2): 307–313.
Bush, A. O., J. C. Fernández, G. W. Esch & J. R. Seed (eds), 2001. Parasitism: The Diversity and Ecology of Animal Parasites. Cambridge University Press, Cambridge: 566 pp.
Costello, C., D. Ovando, T. Clavelle, C. K. Strauss, R. Hilborn, M. C. Melnychuk, T. A. Branchc, S. D. Gainesa, C. S. Szuwalskia, R. B. Cabrala, D. N. Raderb & A. Leland, 2016. Global fishery prospects under contrasting management regimes. Proceedings of the National Academy of Sciences of the United States of America 113(18): 5125–5129.
Dallas, T. & E. Cornelius, 2015. Co-extinction in a host–parasite network: identifying key hosts for network stability. Scientific Reports 5: 13185.
Darwall, W. R. T. & J. Freyhof, 2016. Lost fishes, who is counting? The extent of the threat to freshwater fish biodiversity. In G. P. Closs, M. Krkosek & J. D. Olden (eds), Conservation of Freshwater Fishes. Cambridge University Press, Cambridge.
Dormann, C. F., B. Gruber & J. Fründ, 2008. Introducing the bipartite Package: analysing ecological networks. R News 8: 8–11.
Dunne, J. A., R. J. Williams & N. D. Martinez, 2002. Network structure and biodiversity loss in food webs: robustness increases with connectance. Ecology Letters 5(4): 558–567.
Eiras, J., H. Segner, T. Wahli & B. G. Kapoor (eds), 2008. Fish Diseases, 2 vols. CRC Press, Boca Raton: 1340 pp.
Farrell, M. J., P. R. Stephens, L. Berrang-Ford, J. L. Gittleman & T. J. Davies, 2015. The path to host extinction can lead to loss of generalist parasites. Journal of Animal Ecology 84: 978–984.
Frainer, A., B. G. McKie, P. Amundsen, R. Knudsen & K. D. Lafferty, 2018. Parasitism and the biodiversity–functioning relationship. Trends in Ecology and Evolution 33(4):260-268.
Franco, J. L. de A., 2013. The concept of biodiversity and the history of conservation biology: from wilderness preservation to biodiversity conservation. História (São Paulo) 32(2): 21–48.
Gilbert, A. J., 2009. Connectance indicates the robustness of food webs when subjected to species loss. Ecological Indicators 9(1): 72–80.
Hatcher, M. J., J. T. Dick & A. M. Dunn, 2012. Diverse effects of parasites in ecosystems: linking interdependent processes. Frontiers in Ecology and the Environment 10(4): 186–194.
Kolbert, E., 2014. The Sixth Extinction: An Unnatural History. Henry Holt and Company, New York: 319 pp.
Lima, L. B., S. Bellay, H. C. Giacomini, A. Isaac & D. P. Lima-Jr, 2016. Influence of host diet and phylogeny on parasite sharing by fish in a diverse tropical floodplain. Parasitology 143(3): 343–349.
Lima-Jr, D. P., H. C. Giacomini, R. M. Takemoto, A. A. Agostinho & L. M. Bini, 2012. Patterns of interactions of a large fish–parasite network in a tropical floodplain. Journal of Animal Ecology 81(4): 905–913.
MacCallum, R. C., M. W. Browne & H. M. Sugawara, 1996. Power analysis and determination of sample size for covariance structure modeling. Psychological Methods 1(2): 130–149.
Martinez, N. D., B. A. Hawkins, H. A. Dawah & B. P. Feifarek, 1999. Effects of sampling effort on characterization of food-web structure. Ecology 80(3): 1044–1055.
Memmott, J., N. W. Waser & M. V. G. Price, 2004. Tolerance of pollinator networks to species extinctions. Proceedings of the Royal Society B 271(1557): 2605–2611.
Olesen, J. M., J. Bascompte, Y. L. Dupont & P. Jordano, 2007. The modularity of pollination networks. Proceedings of the National Academy of Sciences of the United States of America 104: 19891–19896.
Pascual, M. & J. A. Dunne (eds), 2006. Ecological Networks: Linking Structure to Dynamics in Food Webs. Oxford University Press, New York: 386 pp.
Pimm, S. L., 1982. Food Webs. Chapman & Hall, London: 219 pp.
Poulin, R., 2014. Parasite biodiversity revisited: frontiers and constraints. International Journal for Parasitology 44(9): 581–589.
Preacher, K. J. & D. L. Coffman, 2006. Computing power and minimum sample size for RMSEA. https://quantpsy.org/.
R Development Core Team, 2019. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna.
Rohde, K. & M. Keap, 1998. Latitudinal differences in species and community richness and in community structure of metazoan endo- and ectoparasites of marine teleost fish. International Journal of Parasitology 28: 461–474.
Rynkiewicz, E. C., A. B. Pedersen & A. Fenton, 2015. An ecosystem approach to understanding and managing within-host parasite community dynamics. Trends in Parasitology 31: 212–221.
Santamaria, S., J. Galeano, J. M. Pastor & M. Méndez, 2014. Robustness of alpine pollination networks: effects of network structure and consequences for endemic plants. Arctic, Antarctic, and Alpine Research 46(3): 568–580.
Strona, G., 2015. Past, present and future of host–parasite co-extinctions. International Journal for Parasitology: Parasites and Wildlife 4(3): 431–441.
Strona, G. & K. D. Lafferty, 2016. Environmental change makes robust ecological networks fragile. Nature Communications 7(1): 12462.
Strona, G., P. Galli & S. Fattorini, 2013. Fish parasites resolve the paradox of missing coextinctions. Nature Communications 4: 1718.
Tylianakis, J. M., E. Laliberté, A. Nielsen & J. Bascompte, 2010. Conservation of species interaction networks. Biological Conservation 143(10): 2270–2279.
Vanbergen, A. J., B. A. Woodcock, M. S. Heard & D. S. Chapman, 2017. Network size, structure and mutualism dependence affect the propensity for plant-pollinator extinction cascades. Functional Ecology 31(6): 1285–1293.
Vázquez, D. P., R. Poulin, B. R. Krasnov & G. I. Shenbrot, 2005. Species abundance and the distribution of specialization in host–parasite interaction networks. Journal of Animal Ecology 74: 946–955.
Ventim, R., J. Morais, S. Pardal, L. Mendes, J. A. Ramos & J. Pérez-Tris, 2012. Host–parasite associations and host-specificity in haemoparasites of reed bed passerines. Parasitology 139: 310–316.
Vieira, M. C. & M. Almeida-Neto, 2015. A simple stochastic model for complex coextinctions in mutualistic networks: robustness decreases with connectance. Ecology Letters 18(2): 144–152.
Walker, J. G., A. Hurford, J. Cable, A. R. Ellison, S. J. Price & C. E. Cressler, 2017. Host allometry influences the evolution of parasite host-generalism: theory and meta-analysis. Philosophical Transactions of the Royal Society B: Biological Sciences 372: 20160089.
Windsor, D. A., 1998. Controversies in parasitology. Most of the species on Earth are parasites. International Journal for Parasitology 28(12): 1939–1941.
Zar, J. R., 2010. Biostatistical Analysis. Pearson Prentice-Hall, Upper Saddle River: 944 pp.
Acknowledgements
We thank the anonymous reviewers for their helpful comments and suggestions on this manuscript. Manuscript funded by Programa de Pós-Graduação em Ecologia de Ambientes Aquáticos Continentais (PEA/UEM), Coordenação de Aperfeiçoamento Pessoal de Nível Superior/Programa de Excelência Acadêmica, Pró-Reitoria de Pesquisa e Pós-Graduação da Universidade Tecnológica Federal do Paraná (PROPPG/UTFPR), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). S.B. was sponsored by Programa Nacional de Pós-doutorado da Coordenação de Aperfeiçoamento Pessoal de Nível Superior (Grant number 88882.315824/2019-00). M.A.N received funding (Grants 310461/2015-4, 408567/2016-3) and R.M.T (Grant number 308197/2018-6) from CNPq.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Eric R. Larson
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bellay, S., de Oliveira, E.F., Almeida-Neto, M. et al. Ectoparasites are more vulnerable to host extinction than co-occurring endoparasites: evidence from metazoan parasites of freshwater and marine fishes. Hydrobiologia 847, 2873–2882 (2020). https://doi.org/10.1007/s10750-020-04279-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-020-04279-x