Abstract
In tropical climates, free-floating aquatic plants may dominate shallow lakes and affect trophic interactions between zooplankton and their predators have been presented as an important subject to be investigated. The present study used mesocosms within a tropical lake in rural São Paulo-Brazil to test the hypothesis that free-floating macrophytes (FFM) reduce predation pressure on prey, as these aquatic plants provide refuge and shade. Fish predation by Astyanax lacustris was observed with the presence and absence of the FFM Eicchornia crassipes over a period of 5 weeks. FFM promoted a more diverse community structure by providing new habitats, especially for small- to medium-sized littoral cladocerans. Tropocyclops prasinus meridionalis were more abundant with the presence of macrophytes. Furthermore, the size distribution of zooplankton differed between treatments at the end of the experiment, in which the larger organisms were less abundant in the treatment with FFM. The present study suggests that FFM affected the interactions between zooplankton and predators, but this influence occurred due to the macrophytes acting as refuge to smaller organisms rather than large-sized individuals. The results also indicated that the macrophytes influenced the zooplankton community by contributing to an increased species richness.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fish predation is among the main influencing factors on zooplankton communities in shallow lakes (Brooks & Dodson, 1965; Jeppesen et al., 1990, 1996; Iglesias et al., 2011; Havens et al., 2015). Zooplankton communities show behavioral shifts according to the level of presence of omni-planktivorous fish (Burks et al., 2002; Meerhoff et al., 2007) or complete changes in their size structure (Brooks & Dodson, 1965; Burks et al., 2002; Brucet et al., 2005; Jeppesen et al., 2007; Iglesias et al., 2008; Brucet et al., 2010). Moreover, by removing certain zooplankton groups with specific functional traits, fish predation has been shown to affect the structure of zooplankton communities (Iglesias et al., 2008; Lavorel et al., 2008), and consequently the functional diversity.
The direct predation of fish on zooplankton can have a cascade effect throughout the aquatic food web and have an indirect influence on the phytoplankton community (Carpenter & Kitchell, 1984; Sterner, 1989; Vanni & Findlay, 1990; Iglesias et al., 2017). Zooplankton may be consumed by macroinvertebrates as well, especially by the larvae of Chaoborus sp. (Lichtenstein, 1800, larvae). These larvae are opportunistic and feed on other planktonic organisms, and in stages III and IV they feed mainly on cladocerans and copepods (Castillho-Noll & Arcifa, 2007). Invertebrate predators feed on smaller prey when compared to the prey consumed by fish. Therefore, predaceous invertebrates influence the zooplankton community in a manner different from that of fish (Arcifa et al., 2015). Chaoborus sp. is also predated by planktivorous fish, which represents an intraguild predation relationship. Thus, depending on the strength of the links of the food web, predaceous fish may indirectly favor the proliferation of small zooplankton. In this way, Chaoborus sp. larvae have been reported to uncouple the fish–zooplankton interaction (Pujoni et al., 2016).
The direct and indirect interactions between predator and prey might be influenced by certain ecosystem conditions, which include the presence of macrophytes. These aquatic plants are known to provide a higher diversity of food items for detritivorous (Debastiani-Júnior et al., 2016), omnivorous and carnivorous (Thomaz & Cunha, 2010) vertebrate and invertebrate organisms, and support higher taxonomic and functional diversity. Submerged macrophytes have been shown to influence predator–prey interactions by providing refuge for zooplankton (Timm & Moss et al., 1984; Burks et al., 2002). This idea is central to the alternative regimes hypothesis, and it has been researched substantially for temperate systems of the northern hemisphere (Blindow et al., 1993; Scheffer et al., 1993, 2003). However, some studies suggest that in subtropical climates zooplankton avoid macrophytes (Meerhoff et al., 2006, 2007) because fish predation occurs in the middle of the plants (Teixeira de Mello et al., 2009). Interactions between planktivorous fish and zooplankton communities are poorly understood in tropical climates, of which the zooplankton communities are dominated by smaller species when compared to those of a temperate climate (Havens et al., 2015). Aquatic food webs in tropical climates are also very complex with high abundance and diversity of predaceous macroinvertebrates (Castilho-Noll & Arcifa, 2007) and free-floating macrophytes (Scheffer et al., 2003).
In the absence of predators, macrophytes have been shown to influence the functional diversity and size structure of zooplankton communities by providing habitat heterogeneity and increasing ecosystem niches (Meerhoff et al., 2007; Pantel et al., 2015; Bolduc et al., 2016; Stephan et al., 2019). Therefore, it can be assumed that the influence of predation on zooplankton communities will vary according to the presence and absence of macrophytes, since these plants act as a refuge against predation (Thomaz & Cunha, 2010) or as a habitat for fish (Teixeira de Mello et al., 2009), in which case the positive effect on prey will not occur.
Thus, the present study aimed to verify how the presence of free-floating macrophytes interferes in the role of planktivorous predators on a tropical zooplankton community. We hypothesized that, in the presence of free-floating macrophytes, prey could become less visible by using these plants as refuge or by shading caused by plants, resulting in a decrease in predation pressure on prey, especially the larger ones. Consequently, the zooplankton community that suffers predation in the presence of free-floating macrophytes would be more abundant with larger individuals than communities where there is predation but no plants. In addition, zooplankton communities suffer predation where there is predation with no plants. In addition, we tested the hypothesis that the presence of free-floating macrophytes will increase species diversity when considering the potential of these plants to increase the number of microhabitats and ecological niches.
Materials and methods
The present study was carried out using mesocosms to test how free-floating macrophytes influence predation by planktivorous fish on the zooplankton community in a tropical climate (Alvares et al., 2013), following experimental designs described in Arcifa et al. (1986) and Castilho-Noll & Arcifa (2007). The experiment lasted approximately 2 months, from April 30 to June 3 of 2015, and was carried out in a pond in rural São Paulo, Brazil (48° 55′ 41.5″ W and 21° 13′ 31.4″ S). The pond is an artificial, eutrophic reservoir with Zmax = 5 m; surface area = 32,795 m2; maximum length = 315.6 m and maximum width = 157.3 m (Câmara et al., 2012; Castilho-Noll et al., 2012). This pond has low densities of rooted and free-floating macrophytes (FFM), which are concentrated in the littoral zones. The Eichhornia crassipes Mart. (Solms) is the most abundant free-floating macrophyte.
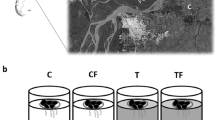
Eight cylindrical PVC enclosures (transparent; diameter = 1.3 m and length = 5 m) with a water volume of approximately 5.63 m3 (Fig. 1a) were placed in the limnetic region of the pond (Fig. 1b). The length (height) of all enclosures was sufficient to reach the bottom of the lake and encompass the entire water column. Aluminum rings were used to maintain their structure, and the enclosures were fixed and anchored with ropes penetrating ca. 0.30 m into the sediment. The mesocosms were kept attached to a plastic bottle for buoyancy in a manner that the structures were open to the atmosphere and to the bottom of the lake (Fig. 1b). A net with 1 cm mesh was attached to the bottom of the mesocosms to prevent fish from escaping. Mesocosms were filled with water from the pond to obtain a natural abundance and composition of the pond phytoplankton and zooplankton species Simocephalus latirostris Stingelin, 1906, Daphnia laevis Birge, 1879, Macrothrix spinosa King, 1853, Ilyocryptus spinifer Herrick, 1882, Thermocyclops decipiens (Kiefer, 1929), Tropocyclops prasinus meridionalis (Kiefer, 1931), Chaoborus sp. larvae (Fig. 1c). One week before, the treatments were assigned, the zooplankton composition was homogenized by pooling vertical tows from each mesocosm, and 350 ml of the resulting concentrate was redistributed into each mesocosm. The present study was carried out using two treatments with four replications (N = 8): Without free-floating macrophytes (Without FFM) and With free-floating macrophytes (With FFM). Both treatments contained the planktivorous fish.
Experimental enclosures introduced in the pelagic zone of the lake. Photographs show how the mesocosms look in various states, such as outside the water after removal. The 1.3 m diameter and 5 m in length can be seen in the picture with the men holding the plastic structure (a). Other photographs show the enclosures floating in the pelagic zone of the lake (b), With and Without free-floating macrophytes (c, d)
The present study used Astyanax lacustris (Lütken, 1875) as the predator fish species for its omnivorous–zooplanktivorous habits (Arcifa et al., 1991; Esteves & Galetti, 1995). This native fish species inhabits the entire water column in both the littoral and limnetic zones (Casatti, 2002). A. lacustris specimens were obtained from a fish farm and were stocked in the mesocosms with a length of 6.38 cm (± 0.83). The widely distributed native free-floating macrophyte Eichhornia crassipes was used due to its high abundance in the pond where the experiment was carried out. The macrophytes were obtained from the littoral zone of the pond and were gently washed before adding to the With FFM mesocosms, and were then stocked with approximately 50% coverage of the enclosure water surface (Fig. 1d). Coverage was observed during sampling periods and maintained by removing excess plants. It was necessary to remove macrophytes only on day 14 since plant growth obtained a 100% coverage of the water surface in the mesocosms.
Environmental parameter conditions were recorded at the beginning of the experiment and homogeneous conditions were shown for both treatments (Table 1). At the beginning (Day 1) of the experiment, Astyanax lacustris were stocked in the mesocosms at a density of 3 fish/m3, which is similar to their density in the environment (Rocha et al., 2009). This species occupies the entire water column since their feeding habits include consuming items in the water column (Casatti, 2002). Free-floating macrophytes were collected in the littoral zone of the pond and were gently washed before being added to the With FFM mesocosms. Plankton and environmental variables were sampled weekly (Day 1, 7, 14, 21, and 28) starting on the first day of the experiment.
At the end of the experiment (Day 28), all remaining fish individuals were removed from the mesocosms, using a coupled net in the bottom of the mescosm. The fish were anesthetized with eugenol (Lucena, et al., 2013), fixed with 10% formaldehyde, and stored in bottles with 70% alcohol for analysis of their stomach contents.
Environmental variables
Temperature, conductivity, dissolved oxygen, and pH were analyzed weekly in each mesocosm with a multiparameter (YSI Model 556) 50 cm below the water surface. Depth and water transparency were measured using a Secchi disk. Others environmental parameters measured were the concentrations of total nitrogen and total phosphorus (APHA 2012), chlorophyll a (Golterman et al., 1978), and total suspended solids (Teixeira & Kutner, 1962).
Zooplankton sampling and analysis
Zooplankton samples were collected by filtering an average of 2.5 m3 of water using vertical trawls with a conical plankton net (45 μm mesh), starting from the pond bottom to the water surface to obtain samples that represent the entire water column. In the With FFM mesocosms, the samples were taken in the middle of the plants. The captured organisms were anesthetized with gaseous water and fixed with 4% formaldehyde. Microcrustaceans (adult Copepoda and Cladocera) were identified at the species level, while copepodites were analyzed separately and rotifers were classified as Rotifera. Zooplankton were counted using three 1 mL subsamples obtained with a Stempel pipette and placed on square Petri dishes for examination under a stereoscopic microscope, or on Sedgewick-Rafter slides under an optical microscope. At least 60 individuals were counted in each subsample, and the coefficient of variation was verified to not exceed 0.20 (McCauley, 1984). Chaoborus sp. larvae and other microcrustacean species with low densities were counted from the entire sample. When possible, thirty individuals of each microcrustacean species per sample were measured to the closest µm (McCauley, 1984). Two zooplankton size classes were determined according to the feeding habits of the fish, of which were an intermediate size class of 0.300–0.600 mm and a large size class of > 0.600 mm (Bonecker et al., 2011). Abundances of each size classification was estimated for each treatment during the experiment.
Statistical analysis
Repeated measures analysis of variance (RM-ANOVA) was used to test the effect of the presence and absence of free-floating macrophytes in the treatments and the temporal variation on the zooplankton densities, diversity index, and the main environmental variables (chlorophyll a, temperature, pH, dissolved oxygen, transparency, phosphorus, and nitrogen). Differences between treatments for each day were assessed with the Tukey’s post hoc test. Student’s T test was applied to determine changes in each size class of the zooplankton between treatments at the beginning and at the end of the experimental period. Before statistical analyses, zooplankton data and environmental variables were log (x + 1) or square-root transformed to fulfill normality. Differences in the compositions of zooplankton species between treatments at the beginning and at the end of the experiment were tested using PERMANOVA and SIMPER, through Bray–Curtis distances. The food importance index (IAI) was calculated through the visual estimation of the gut volume and transformed into volumetric dominance for the calculation of the IAI (Esteves & Galleti, 1995). Fish that had 100% of digested unidentifiable material in their stomachs were excluded for this analysis.
Results
Effects of treatments on the environmental variables
Changes in environmental variables were observed with the presence of free-floating macrophytes, especially on day 14 when these plants were more abundant (Table 1). Significant differences were observed between treatments over time for chlorophyll a (F(1, 5) = 11.4, P < 0.001), suspended material (F(1, 5) = 5.3, P < 0.001), pH (F(1, 5) = 14.01, P < 0.001), dissolved oxygen (F(1, 5) = 8.87, P < 0.001), and transparency (F(1, 5) = 15.7, P < 0.001) (Table 2). The highest levels of chlorophyll a (Post hoc Tukey test, P = 0.0001) and suspended material (Post hoc Tukey test, P = 0.0009) were observed on Day 14 in the Without FFM treatment (Table 1), and the pH (Post hoc Tukey test, P = 0.01) and dissolved oxygen (Post hoc Tukey test, P = 0.002) were significantly higher in treatment the Without FFM when compared to the With FFM (Table 2). Transparency was significantly higher in the treatment With FFM on day 14 (Post hoc Tukey test, P = 0.0001) and 21 (Post hoc Tukey test, P = 0.01) (Table 1).
Effects of treatments on zooplankton community structure
Characteristics of the zooplankton community showed responses to the presence of macrophytes and temporal changes. Significant differences between treatments were observed over time for the densities of Tropocyclops prasinus meridionalis males (F(1,5) = 8.98, P = 0.001) and females (F(1,5) = 12.8, P = 0.001) (Table 2). Densities of T. prasinus meridionalis were higher With FFM until Day 21 (Post hoc Tukey test, P = 0.04) (Fig. 2d, e). Chaoborus sp. larvae exhibited a decrease in abundance for both treatments after the fish were added to the mesocosms, from day 7 until the end of the experiment, but no statistical differences were observed between treatments (Fig. 2f). Cladocera, Daphnia laevis, Copepoda, Thermocyclops decipiens males, T. decipiens females, and Rotifera also showed no significant differences between treatments over time (Table 2 and Fig. 2). However, densities of D. laevis were significantly higher in the Without FFM treatment at day 21 (Post hoc Tukey test, P = 0.01; Fig. 2).
Averages and standard errors of the densities of Daphnia laevis (a), Thermocyclops decipiens male (b), Thermocyclops decipiens female (c), Tropocyclops prasinius meridionalis male (d), Tropocyclops prasinius meridionalis female (e), Chaoborus (f) during the 5 weeks of the experiment. The chart includes averages of the replicas and the standard error bar. Tukey’s post hoc tests were performed, and the results are indicated by the letters a, b
At the beginning of the experiment, no significant differences between treatments were shown regarding thein size structure of the zooplankton, confirming the succeed of the applied homogenization among mesocosms (Table 3). On the other hand, the size structure was different between treatments and between sampling days within in each treatment, with the intermediate size being more abundant at the end of the experiment in the Without FFM (Table 3).
Species composition showed no difference between treatments at the beginning of the experiment (PERMANOVA test, F = 1.147, P = 0.43) (Table 4), whereas differences were shown at the end (PERMANOVA test, F = 3.257, P = 0.02). The SIMPER analysis showed that the main dissimilarity was due to differences in the abundances of Tropocyclops prasinus meridionalis and Thermocyclops decipiens (91.4% cumulative dissimilarity). Furthermore, the emergence of Simocephalus latirostris at the end of the experiment also contributed to the dissimilarity between the communities of both treatments (Supplementary material 1). Species richness was significantly higher in the With FFM treatment throughout the experiment (F(1, 5) = 4.54, P = 0.008; Table 5). On Days 21 and 28, increased densities were observed for several species that were not found in the first weeks such as S. latirostris, Macrothrix spinosa, Ovalona glabra (Sars, 1901), and Diaphanosoma spinulosum Herbst, 1975 (Fig. 3). Furthermore, Ilyocryptus spinifer was only found in the treatment With FFM (Fig. 3). T. decipiens and T. prasinus meridionalis were the dominant species of Copepoda, with small oscillations in the relative abundance throughout the experiment. Paracyclops chiltoni (Thomson G.M., 1883) and Ectocyclops rubescens Brady, 1904 occurred occasionally with low relative percentages (Fig. 3).
Only 24 of the initial stock of 120 fish were collected from the mesocosms on Day 28. It was not possible to recover all fish and some mortality took place, however, with the recovered fish, it was possible to compare treatments. Stomachs of nearly half of the analyzed fish were empty regardless of the treatment. The most representative items in the stomach contents of fish were digested material (56%), Chaoborus sp. larvae (20%), and Cladocera (24%) in the Without FFM treatment (Supplementary Material 2). Stomach contents of the fish in the With FFM treatment showed digested material (75%), insect remains (16%), and plant remains (9%).
Discussion
Results of the present study indicate that there were no unique responses of the zooplankton community to the presence of free-floating macrophytes and that the observed effects depended clearly on the zooplankton group considered. The presence of free-floating macrophytes appeared to have no refuge or shading effect for the relatively large, free-swimming cladoceran species (e.g., Daphnia laevis) as previously observed in a subtropical environment (Meerhoff et al., 2006, 2007), and for large Chaoborus sp. larvae. On the other hand, the macrophytes appeared to provide a protective effect for the small- and medium-sized littoral cladocerans. Furthermore, the copepods showed responses that were specific to certain species.
The responses of the zooplankton appear to be influenced by other factors beyond direct predation. Results of the present study suggest that free-floating macrophytes may serve as a habitat rather than a refuge. Previous studies have shown that Eichhornia crassipes are generally used by certain zooplankton species because the plants serve as a habitat that is ideal for their life habits or morphological adaptations (Montiel-Martínez et al., 2015). Furthermore, in subtropical region, E. crassipes was suggested to provide little or no refuge for pelagic zooplankton against predation (Meerhoff et al., 2006) since predaceous fish occur in greater abundances among macrophyte banks (Meschiatti & Arcifa, 2002; Teixeira de Mello et al., 2009). Nevertheless, the present study showed an increase in species richness with the presence of free-floating macrophytes, further supporting that these plants benefit the ecosystem by increasing the available microhabitats. The zooplankton were perhaps able to proliferate in these microhabitats by leaving eggs attached on roots or submersed parts of the macrophytes (Battauz et al., 2017), by wind dispersion (Lopes et al., 2016), or coming from sediment by hatching of resting eggs (Brendonck & De Meester, 2003).
The cladocerans Ovalona glabra, Macrothrix spinosa and Ilyocryptus spinifer are medium- to small-sized species that have morphological adaptations for fixation and creep (Fryer, 1968, 1974), allowing them to explore the microhabitat provided by the submerged parts of macrophyte roots. Simocephalus latirostris was also observed, representing another genus with adaptations for fixing to substrates while it filters the water to capture suspended solids (Fryer, 1991; Orlova-Bienkowskaja, 2001). Thus, the relative abundances of plant-attached species began to appear and increased throughout the experimental period in the treatment with macrophytes, whereas the relative abundances of larger free-swimming species, such as Daphnia laevis, were reduced.
The low density of Daphnia laevis with the presence of macrophytes was perhaps due to a decrease in phytoplankton resulted from the shading effect of macrophytes (O’Farrell et al., 2009; Fontanarrosa et al., 2010). Higher densities of free-floating macrophytes have been shown to reduce the available light for communities of phytoplankton and photosynthetic organisms, causing an overall decrease in chlorophyll a (Sinistro et al., 2006; O’Farrell et al., 2009) and consequently a lack of natural food sources for large cladocerans. In addition, results of the present study suggest that macrophytes may lead to decreases in dissolved oxygen (Villamgna & Murphy, 2010) and suspended material (De Neiff et al., 1994), and increases in pH (Bicudo et al., 2007) and transparency (Estlander et al., 2009). Increased transparency facilitates the predation efficiency of visual hunter fish, exacerbating the predation effect on the zooplankton community.
Copepods are generally considered as a main diet item of Astyanax lacustris fish in tropical aquatic environments (Maia-Barbosa & Matsumura-Tundisi, 1984; Esteves, 1996; Vilella et al., 2002; Câmara et al., 2012). The copepod Tropocyclops prasinus meridionalis is abundant in these environments and occurs in the littoral zones of shallow tropical lakes (Câmara et al., 2012; Castilho-Noll et al., 2012). Higher densities of T. prasinus meridionalis were shown with the presence of macrophytes perhaps due to the opportunity to use the macrophytes roots as refuge against predation by the A. lacustris.
Influence of macrophytes on the interactions between zooplankton and invertebrate predators may have an indirect effect on the interactions between planktivorous fish and the zooplankton community structure, of which both predators compete for the same food source and one predator feeds on the other. Furthermore, the presence or absence of the macrophytes and the refuge that they provide can influence the whole interaction complex. Astyanax lacustris was the considered as the top predator in the mesocosms of the present study, consuming both Chaoborus sp. larvae and Daphnia laevis (Arcifa et al., 1986, 2015; Câmara et al., 2012). However, the observed decrease in the densities of Chaoborus sp. larvae regardless of the presence of macrophytes indicated that predation by the fish occurred despite the potential of the macrophyte as a refuge, ultimately reducing the influence of the predaceous invertebrates on the zooplankton community.
In general, the structuring role of predaceous fish in tropical regions as observed in other studies (Esteves, 1996; Pujoni et al., 2016; Soares et al., 2016; Fernando, 1994; Pinto-Coelho et al., 2008; Bonecker et al., 2011) was experimentally confirmed in the present study. Furthermore, only the zooplankton species adapted to inhabit plant microhabitats reduced their vulnerability to fish predation by occupying the free-floating macrophytes, whereas free-swimming species remained vulnerable to fish predation regardless of the presence of a potential safety environment provided by free-floating plants. The differences in the size class structure of the zooplankton between treatments showed that the macrophytes influence predator–prey interactions. No difference in the proportion of sizes between treatments was observed but the difference between size classes was visibly lower in the treatment with the macrophytes when compared to the treatment without the plants.
In conclusion, the presence of free-floating macrophytes in tropical aquatic environments appears to influence the interactions between planktivorous predators and zooplankton. However, this influence does not occur due to the macrophytes acting as refuge to large zooplankton, but their presence still modifies the zooplankton community structure through a series of complex interactions that are still far from completely understood.
References
Alvares, C. A., J. L. Stape, P. C. Sentelhas, G. de Moraes, J. Leonardo & G. Sparovek, 2013. Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift 22: 711–728.
American Public Health Association (APHA). (2012). Standard methods for the examination of water and wastewater (22 Edn.). Washington, D.C.
Arcifa, M. S., T. G. Northcote & O. Froehlich, 1986. Fish-zooplankton interactions and their effects on water quality of a tropical Brazilian reservoir. Hydrobiologia 139: 49–58.
Arcifa, M. S., T. G. Northcote & O. Froehlich, 1991. Interactive ecology of two cohabiting characin fishes (Astyanax fasciatus and Astyanax bimaculatus) in a eutrophic Brazilian reservoir. Journal of Tropical Ecology 7: 257–268.
Arcifa, M. S., T. C. dos Santos Ferreira, C. Fileto, M. S. M. Castilho-Noll, T. C. Bunioto & W. J. Minto, 2015. A long-term study on crustacean plankton of a shallow tropical lake: the role of invertebrate predation. Journal of Limnology 74: 606–617.
Battauz, Y. S., S. B. J. de Paggi & J. C. Paggi, 2017. Macrophytes as dispersal vectors of zooplankton resting stages in a subtropical riverine floodplain. Aquatic Ecology 51: 191–201.
Bicudo, D. D. C., B. M. Fonseca, L. M. Bini, L. O. Crossetti, C. E. D. M. Bicudo & T. Araújo-Jesus, 2007. Undesirable side-effects of water hyacinth control in a shallow tropical reservoir. Freshwater Biology 52: 1120–1133.
Blindow, I., G. Andersson, A. Hargeby & S. Johansson, 1993. Long term pattern of alternative stable states in two shallow eutrophic lakes. Freshwater Biology 30: 159–167.
Bolduc, P., A. Bertolo & B. Pinel-Alloul, 2016. Does submerged aquatic vegetation shape zooplankton community structure and functional diversity? A test with a shallow fluvial lake system. Hydrobiologia 778: 151–165.
Bonecker, C. C., F. D. Azevedo & N. R. Simões, 2011. Zooplankton body-size structure and biomass in tropical floodplain lakes: relationship with planktivorous fishes. Acta Limnologica Brasiliensia 23: 217–228.
Brendonck, L. & L. De Meester, 2003. Egg banks in freshwater zooplankton: evolutionary and ecological archives in the sediment. Hydrobiologia 491: 65–84.
Brooks, J. L. & S. I. Dodson, 1965. Predation, body size and composition of plankton. Science 150: 28–35.
Brucet, S., D. Boix, R. López-Flores, A. Badosa, R. Moreno-Amich & X. D. Quintana, 2005. Zooplankton structure and dynamics in permanent and temporary Mediterranean salt marshes: taxon-based and size-based approaches. Archiv für Hydrobiologie 162: 535–555.
Brucet, S., D. Boix, X. D. Quintana, E. Jensen, L. W. Nathansen, C. Trochine, M. Meerhoff, S. Gascón & E. Jeppesen, 2010. Factors influencing zooplankton size structure at contrasting temperatures in coastal shallow lakes: implications for effects of climate change. Limnology and Oceanography 55: 1697–1711.
Burks, R. L., D. M. Lodge, E. Jeppesen & T. L. Lauridsen, 2002. Diel horizontal migration of zooplankton: costs and benefits of inhabiting the littoral. Freshwater Biology 47: 343–365.
Câmara, C. F., M. S. M. Castilho-Noll & M. S. Arcifa, 2012. Predation on microcrustaceans in evidence: the role of chaoborid larvae and fish in two shallow and small Neotropical reservoirs. Nauplius 20: 1–14.
Carpenter, S. R. & J. F. Kitchell, 1984. Plankton community structure and limnetic primary production. American Naturalist 124: 159–172.
Casatti, L., 2002. Alimentação dos peixes em um riacho do Parque Estadual Morro do Diabo, bacia do alto rio Paraná, sudeste do Brasil. Biota Neotropica 2002(2): 1–14.
Castilho-Noll, M. S. M. & M. S. Arcifa, 2007. Mesocosm experiment on the impact of invertebrate predation on zooplankton of a tropical lake. Aquatic Ecology 41: 587–598.
Castilho-Noll, M. S. M., C. F. Câmara, M. F. Chicone, É. H. Shibata & L. R. Stephan, 2012. Copepods (Crustacea, Maxillopoda) from shallow reservoirs. Acta Limnologica Brasiliensia 24: 149–159.
De Neiff, A. P., J. J. Neiff, O. Orfeo & R. Carignan, 1994. Quantitative importance of particulate matter retention by the roots of Eichhornia crassipes in the Paraná floodplain. Aquatic Botany 47: 213–223.
Debastiani-Júnior, J. R., L. M. A. Elmoor-Loureiro & M. G. Nogueira, 2016. Habitat architecture influencing microcrustaceans composition: a case study on freshwater Cladocera (Crustacea Branchiopoda). Brazilian Journal of Biology 76: 93–100.
Esteves, K. E., 1996. Feeding ecology of three Astyanax species (Characidae, Tetragonopterinae) from a floodplain lake of Mogi-Guaçú River, Paraná River basin, Brazil. Environmental Biology of Fishes 46: 83–101.
Esteves, K. E. & P. M. Galetti Jr., 1995. Food partitioning among some characids of a small Brazilian floodplain lake from the Paraná River basin. Environmental Biology of Fishes 42: 375–389.
Estlander, S., L. Nurminen, M. Olin, M. Vinni & J. Horppila, 2009. Seasonal fluctuations in macrophyte cover and water transparency of four brown-water lakes: implications for crustacean zooplankton in littoral and pelagic habitats. Hydrobiologia 620: 109–120.
Fernando, C. H., 1994. Zooplankton, fish and fisheries in tropical freshwaters. Hydrobiologia 272: 105–123.
Fontanarrosa, M. S., G. Chaparro, P. de Tezanos-Pinto, P. Rodriguez & I. O’Farrell, 2010. Zooplankton response to shading effects of free-floating plants in shallow warm temperate lakes: a field mesocosm experiment. Hydrobiologia 646: 231–242.
Fryer, G., 1968. Evolution and adaptive radiation in the Chydoridae (Crustacea: Cladocera): a study in comparative functional morphology and ecology. Philosophical Transactions of the Royal Society of London B: Biological Sciences 254: 221–384.
Fryer, G., 1974. Evolution and adaptive radiation in the Macrothricidae (Crustacea: Cladocera): a study in comparative functional morphology and ecology. Philosophical Transactions of the Royal Society of London B: Biological Sciences 269: 137–274.
Fryer, G., 1991. Functional morphology and the adaptive radiation of the Daphniidae (Branchiopoda: Anomopoda). Philosophical Transactions: Biological Sciences 331: 1–99.
Golterman, H. L., R. S. Clymo & M. Onstad, 1978. Methods for Physical and Chemical Analysis of Freshwaters. Blackwell Scientific Publication, Oxford: 214.
Havens, K. E., J. R. Beaver, E. E. Manis & T. L. East, 2015. Inter-lake comparisons indicate that fish predation, rather than high temperature, is the major driver of summer decline in Daphnia and other changes among cladoceran zooplankton in subtropical Florida lakes. Hydrobiologia 750: 57–67.
Iglesias, C., N. Mazzeo, G. Goyenola, C. Fosalba, F. Teixeira De Mello, S. García & E. Jeppesen, 2008. Field and experimental evidence of the effect of Jenynsia multidentata, a small omnivorous–planktivorous fish, on the size distribution of zooplankton in subtropical lakes. Freshwater Biology 53: 1797–1807.
Iglesias, C., N. Mazzeo, M. Meerhoff, G. Lacerot, J. Clemente, F. Scasso, C. Kruk, G. Goyenola, J. García, S. L. Amsinck, J. C. Paggi, S. José de Paggi & E. Jeppesen, 2011. High predation is the key factor for dominance of small-bodied zooplankton in warm lakes–evidence from lakes, fish enclosures and surface sediment. Hydrobiologia 667: 133–147.
Iglesias, C., E. Jeppesen, N. Mazzeo, J. P. Pacheco, F. Teixeira de Mello & F. Landkildehus, 2017. Fish but not macroinvertebrates promote trophic cascading effects in high density submersed plant experimental lake food webs in two contrasting climate regions. Water 9: 514.
Jeppesen, E., M. Søndergaard, O. Sortkjoær, E. Mortensen & P. Kristensen, 1990. Interactions between phytoplankton, zooplankton and fish in a shallow, hypertrophic lake: a study of phytoplankton collapses in Lake Søbygård, Denmark. Hydrobiologia 191: 149–164.
Jeppesen, E., M. Søndergaard, J. P. Jensen, E. Mortensen & O. Sortkjær, 1996. Fish-induced changes in zooplankton grazing on phytoplankton and bacterioplankton: a long-term study in shallow hypertrophic Lake Søbygaard. Journal of Plankton Research 18: 1605–1625.
Jeppesen, E., M. Meerhoff, B. A. Jacobsen, R. S. Hansen, M. Søndergaard, J. P. Jensen, T. L. Lauridsen, N. Mazzeo & C. W. C. Branco, 2007. Restoration of shallow lakes by nutrient control and biomanipulation – the successful strategy varies with lake size and climate. Hydrobiologia 581: 269–285.
Lavorel, S., K. Grigulis, S. McIntyre, N. S. G. Williams, D. Garden, D. Josh, S. Berman, F. Quétier, A. Trébault & A. Bonis, 2008. Assessing functional diversity in the field – methodology matters! Functional Ecology 22: 134–147.
Lopes, P. M., R. Bozelli, L. M. Bini, J. M. Santangelo & S. A. Declerck, 2016. Contributions of airborne dispersal and dormant propagule recruitment to the assembly of rotifer and crustacean zooplankton communities in temporary ponds. Freshwater Biology 61: 658–669.
Lucena, C. A. S., B. B. Calegari, E. H. L. Pereira & E. Dallegrave, 2013. O uso de óleo de cravo na eutanásia de peixes. Boletim Sociedade Brasileira de Ictiologia 105: 20–24.
Maia-Barbosa, P. M. & T. Matsumura-Tundisi, 1984. Consumption of zooplanktonic organisms by Astyanax fasciatus Cuvier, 1819 (Osteichthyes, Characidae) in Lobo (Broa) Reservoir, São Carlos, SP, Brazil. Hydrobiologia 113: 171–181.
McCauley, E., 1984. The estimation of the abundance and biomass of zooplankton in samples. In Downing, J. A. & F. H. Rigler (eds), A Manual on Methods for Assessment of Secondary Productivity in Freshwaters. Blackwell Scientific Publishing, Oxford: 228–265.
Meerhoff, M., C. Fosalba, C. Bruzzone, N. Mazzeo, W. Noordoven & E. Jeppesen, 2006. An experimental study of habitat choice by Daphnia: plants signal danger more than refuge in subtropical lakes. Freshwater Biology 51: 1320–1330.
Meerhoff, M., C. Iglesias, F. Teixeira De Mello, J. M. Clemente, E. Jensen, T. L. Lauridsen & E. Jeppesen, 2007. Effects of habitat complexity on community structure and predator avoidance behavior of littoral zooplankton in temperate versus subtropical shallow lakes. Freshwater Biology 52: 1009–1021.
Meschiatti, A. J. & M. S. Arcifa, 2002. Early life stages of fish and the relationships with zooplankton in a tropical Brazilian reservoir: Lake Monte Alegre. Brazilian Journal of Biology 62: 41–50.
Montiel-Martínez, A., J. Ciros-Pérez & G. Corkidi, 2015. Littoral zooplankton–water hyacinth interactions: habitat or refuge? Hydrobiologia 755: 173–182.
O’Farrell, I., P. T. Pinto, P. L. Rodriguez, G. Chaparro & H. N. Pizarro, 2009. Experimental evidence of the dynamic effect of free-floating plants on phytoplankton ecology. Freshwater Biology 54: 363–375.
Orlova-Bienkowskaja, M. Y., 2001. Cladocera: Anomopoda, Daphiniidae: Genus Simocephalus. In Drumont, H. J. F. (ed.), Guides to identification of the Microinvertebrates of the Continental Waters of the World. Backhuys Publishers, Leiden: 1–127.
Pantel, J. H., C. Duvivier & L. D. Meester, 2015. Rapid local adaptation mediates zooplankton community assembly in experimental mesocosms. Ecology Letters 18: 992–1000.
Pinto-Coelho, R. M., J. F. Bezerra-Neto, F. Miranda, T. G. Mota, R. Resck, A. M. Santos, P. M. Maia-Barbosa, N. A. S. T. Mello, M. M. Marques, M. O. Campos & F. A. R. Barbosa, 2008. The inverted trophic cascade in tropical plankton communities: impacts of exotic fish in the Middle Rio Doce lake district, Minas Gerais, Brazil. Brazilian Journal of Biology 68: 1025–1037.
Pujoni, D. G. F., P. M. Maia-Barbosa, F. A. R. Barbosa, C. R. Fragoso Jr. & E. H. Van Nes, 2016. Effects of food web complexity on top-down control in tropical lakes. Ecological Modeling 320: 358–365.
Rocha, F. C. D., L. Casatti, F. R. Carvalho & A. M. D. Silva, 2009. Fish assemblages in stream stretches occupied by cattail (Typhaceae, Angiospermae) stands in Southeast Brazil. Neotropical Ichthyology 7: 241–250.
Scheffer, M., S. H. Hosper, M. L. Meijer, B. Moss & E. Jeppesen, 1993. Alternative equilibria in shallow lakes. Trends in Ecology and Evolution 8: 275–279.
Scheffer, M., S. Szabo, A. Gragnani, E. H. van Nes, S. Rinaldi & N. Kautsky, 2003. Floating plant dominance as a stable state. Proceedings of the National Academy of Sciences of the United States of America 100: 4040–4045.
Sinistro, R., I. Izaguirre & V. Asikian, 2006. Experimental study on the microbial plankton community in a South American wetland (Lower Paraná River Basin) and the effect of the light deficiency due to the floating macrophytes. Journal of Plankton Research 28: 753–768.
Soares, C. M., C. Hayashi, A. C. E. Faria-Soares & E. M. Galdioli, 2016. Impact of Brazilian fish species at early developmental stages on plankton communities and water chemical parameters. Acta Scientiarum Biological Sciences 38: 263–272.
Stephan, L. R., B. E. Beisner, S. G. M. Oliveira & M. S. M. Castilho-Noll, 2019. Influence of macrophytes on a tropical microcrustacean community based on taxonomic and functional trait diversity. Water 1(11): 2423.
Sterner, R. W., 1989. The role of grazers in phytoplankton succession. In Sommer, U. (ed.), Plankton Ecology: Succession in Plankton Communities. Springer, New York: 107–169.
Teixeira de Mello, F., M. Meerhoff, Z. Pekcan-hekim & E. Jeppesen, 2009. Substantial differences in littoral fish community structure and dynamics in subtropical and temperate shallow lakes. Freshwater Biology 54: 1202–1215.
Teixeira, C. & M. B. Kutner, 1962. Plankton studies in a mangrove environment: I first assessment of standing stock and principal ecological factors. Boletim do Instituto Oceanográfico 12: 101–124.
Thomaz, S. M. & E. R. D. Cunha, 2010. The role of macrophytes in habitat structuring in aquatic ecosystems: methods of measurement, causes and consequences on animal assemblages composition and biodiversity. Acta Limnologica Brasiliensia 22: 218–236.
Timms, R. M. & B. Moss, 1984. Prevention of growth of potentially dense phytoplankton populations by zooplankton grazing, in the presence of zooplanktivorous fish, in a shallow wetland ecosystem. Limnology and Oceanography 29: 472–486.
Vanni, M. J. & D. L. Findlay, 1990. Trophic cascades and phytoplankton community structure. Ecology 71: 921–937.
Vilella, F. S., F. G. Becker & S. M. Hartz, 2002. Diet of Astyanax species (Teleostei, Characidae) in an Atlantic forest river in Southern Brazil. Brazilian Archives of Biology and Technology 45: 223–232.
Villamagna, A. M. & B. R. Murphy, 2010. Ecological and socio-economic impacts of invasive water hyacinth (Eichhornia crassipes): a review. Freshwater Biology 55: 282–298.
Acknowledgements
We would like to thank all our colleagues from the Laboratório de Ecologia de Zooplâncton (Zooplankton Ecology Laboratory) during the field work, IBILCE/UNESP for providing its facilities, those responsible for the Fazenda Experimental of Pólo Regional Centro Norte, the anonymous reviewers, especially Mariana Meerhoff, the associate editor, for their comments on the manuscript. We thank “The São Paulo State Research Foundation (FAPESP) (process 2013/19848-0) for the financial support for this study. Finally, we would like to thank Dr. Dallas L. Flickinger for the English revision.
Funding
The research that led to the presented results was mainly financed by “The São Paulo State Research Foundation (FAPESP)” (Process Number 2013/19848-0), SNI (ANII-Uruguay) and by PEDECIBA, and CAPES which provided the scholarship awarded to the author during his Master’s degree.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Mariana Meerhoff
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
dos Santos, N.G., Stephan, L.R., Otero, A. et al. How free-floating macrophytes influence interactions between planktivorous fish and zooplankton in tropical environments? An in-lake mesocosm approach. Hydrobiologia 847, 1357–1370 (2020). https://doi.org/10.1007/s10750-020-04194-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-020-04194-1