Abstract
The understanding of factors affecting resource uptake is important to elucidate community dynamics in aquatic ecosystems. Here, we analyzed nutrient–phytoplankton and phytoplankton–zooplankton interactions in open water areas without macrophytes and in areas with floating and submerged macrophytes in the tropical Cajueiro reservoir, semiarid Brazil. Phytoplankton species were classified into functional groups according to life form and cell size, and zooplankton species were grouped based on functional feeding groups and trophic guilds. Macrophytes favored the effect of nutrients on phytoplankton and the availability of prey for the zooplankton. In open water, unicellular and flagellated phytoplankton were positively influenced by nitrate and inorganic phosphate, while colonial, filamentous, small, medium and large phytoplankton were positively influenced by total phosphorus and nitrite. Colonial phytoplankton, mainly filamentous cyanobacteria, was positively associated with zooplankton in areas with macrophytes, while flagellated phytoplankton was negatively related to zooplankton in open water areas. Our results showed that the presence of floating and submerged macrophytes has different influences on the effect of nutrients on phytoplankton and on the effects of phytoplankton on zooplankton in Cajueiro reservoir, and this should be considered when analyzing these communities’ dynamics in similar environments, i.e. tropical reservoirs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trophic interactions have been studied in aquatic ecology for more than five decades (Martin 1970; McQueen et al. 1986; Brett and Goldman 1997; Sommer 2008; Diniz et al. 2019; Severiano et al. 2021). The food chain structure can be strongly regulated by bottom-up effects, that is, when the energy flow in the food chain occurs as a function of available resources (McQueen et al. 1989). In this sense, nutrients are the main factors controlling phytoplankton growth (Carpenter et al. 1985), with nitrogen and phosphorus being the limiting nutrients (Müller and Mitrovic 2015; Paerl et al. 2018; Lewis et al. 2020; Liu et al. 2021).
The effects of nutrients on phytoplankton have been widely demonstrated in several ecosystems (Smith and Lancelot 2004; Jeppesen et al. 2012; Liu et al. 2018). More recently, studies have shown that bottom-up effects are more common in phytoplankton communities than top-down regulation by zooplankton (Li et al. 2020; Frau et al. 2021). For zooplankton, trophic interactions are influenced by functional feeding groups and tropic guilds, species body size, and selectivity and physiological tolerance to ingested toxins (DeMott 1986; Kiørboe et al. 2018; Gomes et al. 2019), and by defense characteristics of the prey (Ger et al. 2014; Lürling 2021). In addition, the strength of bottom-up effects on zooplankton is dependent on the degree of predation pressure by fish as top-down effects (Braun et al. 2021).
Nutrient uptake by phytoplankton is related to the size and structure of organisms, with intermediate-sized phytoplankton being able to exploit available nutrients more efficiently (Marañón 2015; Mousing et al. 2018). Along with nutrient availability, water turbulences also influence phytoplankton metabolism, since they increase nutrient uptake, especially in larger organisms (Naselli-Flores et al. 2021).
Throughout evolution, phytoplankton developed different and efficient morphological, physiological and behavioral strategies to avoid predation by zooplankton, among which the main morphological strategies are the formation of spines and colonies and the increase in size (Lürling 2021). These strategies can be described as functional traits based on their role in regulating predation by zooplankton (Colina et al. 2016). They are informative in explaining the dynamics and structure of the phytoplankton community and its relationship with environmental factors (Chen et al. 2019; Kruk et al. 2021), such as the climatic, chemical and biological factors of the environment (Reynolds et al. 2002).
In freshwater ecosystems, macrophytes have several effects on nutrient cycling, including a reduction in sediment resuspension through water column stabilization (Nurminen and Horppila 2009). Many of the nutrients taken up by macrophytes return to the environment through the decomposition of plant tissues (Banks and Frost 2017; Xiao et al. 2017), or by the release of nutrients from the reductive sediment through thermal stratification of the water column induced by macrophytes (Vilas et al. 2018), or by fluctuation in water level (Keitel et al. 2016). Although little is known about the effects of macrophytes on the availability of resources for aquatic communities in tropical reservoirs, studies have shown that nutrients released by macrophytes favor phytoplankton growth (Vilas et al. 2018; Wang et al. 2018), and also metaphyton, which consists of large sized algae (e.g. Oedogonium sp., Oscillatoria sp.) that are associated with the presence of macrophytes (Barrow et al. 2019). In this sense, macrophytes are also important in the interaction between phytoplankton and zooplankton, as they increase the availability of prey for zooplankton (Fischer and Pusch 2001; Kovalenko et al. 2012).
The main question of the present study was: do macrophytes enhance resource uptake by phytoplankton and zooplankton communities? To answer this question, we examined the effects of resource availability on phytoplankton and zooplankton in open water areas without macrophytes and in areas with floating and submerged macrophytes in a tropical reservoir, in semiarid Brazil. For this, we analyzed the potential factors controlling phytoplankton (nutrients) and zooplankton (prey availability) in a field study. We tested the following hypotheses: (i) the interaction between phytoplankton–nutrients and phytoplankton–zooplankton is favored by the presence of macrophytes, by increasing the availability of resources, and (ii) functional groups of phytoplankton and zooplankton respond differently the resource availability in areas with and without the presence of macrophytes.
Materials and methods
Study area and sampling
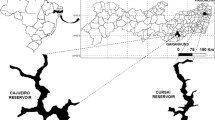
The study was carried out in Cajueiro reservoir (8°59′21.3″ S and 36°28′19.9″ W), municipality of Garanhuns, state of Pernambuco, Northeastern Brazil (Fig. 1). The regional climate is tropical “As” (Alvares et al. 2013), characterized by dry summers and rainy winters. The dry period is concentrated between September and March, and the rainy period between April and August (APAC 2019). The reservoir is shallow, with an average depth of 5.33 ± 0.88 m, and has multiple uses, such as water supply for the municipality, irrigation, fishing and recreation for the population. The Cajueiro reservoir is eutrophic, and the banks are colonized by extensive banks of floating macrophytes Nymphoides indica (L.) Kuntze, Eichhornia crassipes (Mart.) Solms, Salvinia auriculata Aubl. and Salvinia oblongifolia Martius, and banks of submerged macrophytes Chara sp., Egeria densa (Planch.) Casp. and Myriophyllum aquaticum (Vell.) Verdc.
Samples for analysis of abiotic variables, phytoplankton and zooplankton were taken in open water areas (absence of macrophytes, n = 3) and in areas with floating macrophyte banks (n = 3) and submerged macrophyte banks (n = 3), quarterly between November 2018 and August 2019, comprising an annual cycle. The sampling sites were located approximately 400 m equidistant from each other (Fig. 1). Water temperature (°C), dissolved oxygen (mg L−1), pH, electrical conductivity (µS cm−1) and total dissolved solids (mg L−1) were measured using a HANNA multiparameter probe (HI 9829). The Secchi disk was used to estimate the water transparency, in meters, and the light intensity (µmol photons m−1 s−2) was measured with a photometer (LI-250A). Reservoir water samples were collected 10 cm below the water surface using graduated buckets with a capacity of 10 L in open water areas and in areas with floating macrophytes, while in areas with submerged macrophytes, water was collected at a depth of 0.86 ± 0.13 m using a van Dorn bottle. The mean depth in the open water sites was 5.33 ± 0.88 m, and 1.06 ± 0.42 m and 0.86 ± 0.12 in the floating and submerged macrophyte banks, respectively.
Water for nutrient analysis was sampled and immediately placed in 300 mL plastic bottles and kept under refrigeration in a Styrofoam box with ice and transported to the laboratory, where samples were frozen until analysis. Samples for phytoplankton counting were taken directly from the water reservoir at the sampling sites and immediately placed in 150 mL amber flasks and fixed with 1% acetic Lugol. For taxonomic analysis of phytoplankton, water was filtered through a plankton net with 25 µm mesh, placed in 150 mL vials and fixed with 4% formaldehyde. Samples for identification and quantification of zooplankton were collected by filtering 100 L reservoir water from open water areas and areas with floating and submerged macrophytes, following the procedures for collecting water used for phytoplankton analysis, but using a plankton net with 50 µm mesh. Subsequently, samples were placed in 200 mL plastic bottles and fixed with 4% formaldehyde.
Laboratory analysis
The concentration of nutrients (μg L−1), including nitrite (NO2‾), nitrate (NO3‾) and ammoniacal nitrogen (NH4+) was determined according to Golterman et al. (1978), Mackereth et al. (1978) and Koroleff (1976), respectively. Dissolved inorganic nitrogen (DIN) concentrations were obtained by the sum of concentrations of NO2‾, NO3‾ and NH4+. Orthophosphate (PO43‾), inorganic phosphate (Pi) and total phosphorus (TP) were quantified according to the A.P.H.A. (2005).
Phytoplankton species were identified under an optical microscope using specific literature (Anagnostidis and Komárek 1988; Komárek and Anagnostidis 1999, 2005; Komárek and Cronberg 2001; Prescott et al. 1982; Krammer and Lange-Bertalot 1991; Popovský and Pfiester 1990; John et al. 2002), and then classified into morphofunctional groups according to life form (unicellular, colonial, filamentous and flagellar) and cell size (small: < 50 µm, medium: 50–100 µm and large: > 100 µm). Phytoplankton density (ind mL−1) was estimated according to Utermöhl (1958) using sedimentation chambers and an inverted microscope (Bioval XDS-1B) at 400 × magnification. Biovolume (mm3 L−1) was estimated based on the volume of species calculated using geometric models by Hillebrand et al. (1999), and multiplied by the population density for each species. Biovolume (mm3 L−1) was converted to biomass (mg L−1) according to Wetzel and Likens (2000).
Zooplankton species were identified using specific literature (Koste 1978; Reid 1985; Montú and Goeden 1986; Elmoor-Loureiro 1997; Neumann-Leitão et al. 1989). The density of zooplanktonic species (ind L−1) was calculated by counting the organisms under an optical microscope (Opton TNB 41B) with samples concentrated to 100 mL and counting three sub-samples of 2 mL in a Sedgewick-Rafter chamber. Species-specific biomass of zooplankton (μg DW m−3, where DW is dry weight) was determined through the density and the average length and body weight of the taxa according to the regression equations of Ruttner-Kolisko (1977) for rotifers, and of Dumont et al. (1975) for copepods and cladocerans. Zooplankton species were grouped based on functional feeding groups and trophic guilds: rotifers as microphagous (Obertegger et al. 2011), cladocerans as filter-feeders and filter-scrapers (Barnett et al. 2007), adult Calanoida copepods as herbivorous (Frau et al. 2019) and Copepoda nauplii as microphagous. Only groups that can feed on phytoplankton (herbivorous or omnivorous zooplankton) were considered.
Data analysis
Potential differences in phytoplankton and zooplankton biomass and nutrient concentrations between sampling sites (open waters, with floating macrophytes and with submerged macrophytes) were tested by an Analysis of Variance (one-way ANOVA) followed by a Tukey’s post hoc test. Normality of variance was tested by the Kolmogorov–Smirnov test, and homoscedasticity by the Bartlett test. For heteroscedastic variances, we applied the non-parametric Kruskal-Walllis test, followed by the pairwise Mann–Whitney U-test.
Generalized additive models (GAM) were applied to test the effect of nutrients on phytoplankton and effects of phytoplankton on zooplankton in open waters and in sites with floating and submerged macrophytes. The estimated degree of freedom (e.d.f.) was used to evaluate the smoothing of models, and the fit and significance of the model were evaluated using adjusted R2 and p values. Dissolved inorganic nitrogen and orthophosphate for phytoplankton, and total phytoplankton biomass for zooplankton, were used as the predictor variable, respectively. Data were log-transformed (x + 1) before statistical analysis.
Redundancy Analysis (RDA) was used to check the influence of nutrients on phytoplankton groups and the influence of phytoplankton groups on zooplankton groups. For this, the analysis was applied based on the length of the first axis of the Detrended Correspondence Analysis (DCA) (Legendre et al. 2011). Dependent variables were log transformed and explanatory variables were standardized. Only variables with variance inflation factor below 20 and with a significance of p < 0.05 were considered in the final RDA models through the Ordistep function.
Analyses were performed using the vegan (Oksanen et al. 2013) and mgcv (Wood 2004, 2011) package in R software (R Development Core Team 2019) with a significance of p < 0.05.
Results
Nutrients
The limnological variables found in Cajueiro reservoir in areas without macrophytes and areas with floating and submerged macrophytes throughout the study period are provided in the supplementary material (Table S1). High concentrations of nutrients were found in the floating macrophyte banks; however, no statistical variation was observed between areas without macrophytes and areas with macrophytes for nitrate (F = 0.03, p = 0.962), nitrite (F = 0.15, p = 0.857), ammoniacal nitrogen (F = 0.52, p = 0.609), dissolved inorganic nitrogen (F = 0.52, p = 0.609), orthophosphate (F = 1.38, p = 0.298), inorganic phosphate (H = 0.47, p = 0.788) and total phosphorus (F = 1.92, p = 0.201) (Fig. 2).
Variation in concentrations (µg L−1) of nitrate (a), nitrite (b), ammoniacal nitrogen (c), dissolved inorganic nitrogen (DIN, d), orthophosphate (e), inorganic phosphate (f) and total phosphorus (g) between open water areas (OW) and areas with floating (FM) and submerged (SM) macrophytes in Cajueiro reservoir, Northeastern Brazil. Lowercase letters represent statistical differences based on the post-hoc test (p < 0.05) among sampling sites
Biomass of phytoplankton and zooplankton
Eighty-four phytoplankton taxa were identified in Cajueiro reservoir, which were grouped into seven functional groups based on cell size and life form (Table S2). The colonial cyanobacteria Coelomoron tropicale P.A.C.Senna, A.C.Peres & Komárek and the filamentous cyanobacteria Raphidiopsis raciborskii (Wołoszyńska) Aguilera, Berrendero Gómez, Kastovsky, Echenique & Salerno were the dominant species in the phytoplankton community of the reservoir, representing 30.99% and 10.32% of the total biomass of phytoplankton and constituting the colonial and filamentous groups, respectively. In addition to cyanobacteria, the diatom Cyclotella meneghiniana Kützing (9.43%) and dinoflagellate Ceratium furcoides (Levander) Langhans (8.55%), belonging to the unicellular and flagellate groups, respectively, were dominant in Cajueiro reservoir. The group of algae with flagella was mainly represented by dinoflagellates and cryptophyceans. Large and medium filamentous species represented 15.20% total biomass of phytoplankton, and were composed of the diazotrophic cyanobacteria Raphidiopsis and Dolichospermum, and the non-diazotrophic cyanobacteria Anagnostidinema, Oscillatoria, Phormidium, Planktothrix and Pseudanabaena.
In areas with floating and submerged macrophytes, a significant increase was found for the biomass of unicellular (H = 9.78, p = 0.007), colonial (F = 3.37, p = 0.046), filamentous (F = 7.34, p = 0.002), small (F = 7.63, p = 0.001) and large (F = 3.76, p = 0.034) functional groups, except for medium-sized (H = 4.54, p = 0.103) and flagellated phytoplankton (F = 0.73, p = 0.489; Fig. 3a–g). The total phytoplankton biomass differed significantly between the open water areas and sites with floating and submerged macrophytes, with higher biomass found in the submerged macrophyte banks (96.52 ± 65.09 mg L−1, H = 9.86, p = 0.007; Fig. 3h).
Variation in concentrations (mg L−1) of unicellular (a), colonial (b), filamentous (c), flagellated (d), small (e), medium (f) and large (g) morphofunctional groups of phytoplankton and total phytoplankton biomass (h) between open water areas (OW) and areas with floating (FM) and submerged (SM) macrophytes in Cajueiro reservoir, Northeastern Brazil. Lowercase letters represent statistical differences based on the post-hoc test (p < 0.05) among sampling sites
Fifty zooplankton taxa were identified, in addition to the class Bdelloidea and order Calanoida and Copepoda nauplii. Zooplankton taxa were grouped into four functional feeding groups and trophic guilds (Table S3). The herbivorous-microphagous group had the highest biomass during the study; however, it did not differ significantly between sampling sites (H = 5.57, p = 0.061, Fig. 4a). Higher total zooplankton biomass was observed in floating macrophyte banks (183.80 ± 146.70 µg DW m−3) and in submerged macrophyte banks (169.12 ± 218.12 µg DW m−3), significantly differing (H = 9.46, p = 0.008) from open water areas (37.88 ± 30.71 µg DW m−3; Fig. 4e). Floating and submerged macrophytes favored the increase in biomass of the filter-scraper functional group (F = 24.18, p < 0.001), and floating macrophytes favored the increase in filter-feeder biomass (F = 3.48, p = 0.042; Fig. 4c–d). Microphagous biomass did not differ significantly between sampling sites (F = 0.58, p = 0.565; Fig. 4b).
Variation in concentrations (µg DW m−3) of herbivorous (a), microphagous (b), filter-feeders (c) and filter-scrapers (d) functional groups of zooplankton and total zooplankton biomass (e) between open water areas (OW) and areas with floating (FM) and submerged (SM) macrophytes in Cajueiro reservoir, Northeastern Brazil. Lowercase letters represent statistical differences based on the post-hoc test (p < 0.05) among sampling sites
Interaction between nutrients–phytoplankton and phytoplankton–zooplankton
Generalized additive models (GAM) showed that total phytoplankton biomass had a marginally significant positive relationship with dissolved inorganic nitrogen in open water areas (p = 0.054, Fig. 5a) and a significantly negative relationship in sites with floating macrophytes (p = 0.0003, Fig. 5b). No significant relationship was observed between dissolved inorganic nitrogen and total phytoplankton biomass in areas with submerged macrophytes (p = 0.654, Fig. 5c). A positive relationship was found between orthophosphate and total phytoplankton biomass in open water areas (p = 0.039, Fig. 5d) and with submerged macrophytes (p = 0.022, Fig. 5f), while in sites with floating macrophytes, no significant relationship was observed (p = 0.076, Fig. 5e). Total zooplankton biomass was not significantly related to total phytoplankton biomass (Fig. 5g–i).
Generalized additive models (GAM) showing the relationships between nutrients–phytoplankton (a– f) and phytoplankton–zooplankton (g, h, i) in open water areas (orange line) and areas with floating (blue line) and submerged (green line) macrophytes. The solid line represents the fitted values of the general model. DIN: dissolved inorganic nitrogen, PO4: orthophosphate, TPhytoplankton: total phytoplankton biomass, TZooplankton: total zooplankton biomass
Redundancy analysis
The RDA model revealed that nutrients explained 59.65% of variation in phytoplankton community compositions (F = 11.08, p = 0.001). Axis 1 (F = 24.64, p = 0.001) and axis 2 (F = 17.62, p = 0.001) were significant for the distribution of explanatory variables, with unicellular phytoplankton influenced by inorganic phosphate (Pi), flagellate phytoplankton by nitrate (NO3‾), and other groups (colonial, filamentous, small, medium and large) influenced by total phosphorous (TP) and nitrite (NO2‾). Two groups distinguishing the sites with and without macrophytes were formed on axis 1, with TP and NO2‾ related to functional phytoplankton groups in floating and submerged macrophyte banks and Pi and NO3‾ in sites without macrophytes (Fig. 6a).
Redundancy Analysis (RDA) of (a) phytoplankton morphofunctional groups and (b) zooplankton functional feeding groups and trophic guilds in relation to variables in open water areas and areas with floating and submerged macrophytes. TP: total phosphorus, Pi: inorganic phosphate, NO2: nitrite, NO3: nitrate
For functional feeding groups and trophic guilds of the zooplankton, the RDA indicated that phytoplankton functional groups explained 41.79% of variation in zooplankton community compositions (F = 11.48, p = 0.001), with only axis 1 (F = 22.26, p = 0.001) showing the significant distribution of groups. On the axis 1, there was a separation between open water areas and sites with macrophytes, where colonial phytoplankton biomass, positioned to the right of the axis, positively influenced herbivores, filter-feeders and filter-scrapers in floating and submerged macrophyte sites, while flagellate phytoplankton were positively related to sites without macrophytes (Fig. 6b).
Discussion
In the present study, we showed the nutrient-phytoplankton and phytoplankton–zooplankton interactions in areas with and without macrophytes. The unicellular, colonial, filamentous, small and partly the large functional phytoplankton groups presented higher values of biomass in floating and submerged macrophyte banks. For zooplankton, only cladocerans (filters and scrapers) presented high biomass in floating and submerged macrophyte banks. Phytoplankton was positively correlated with orthophosphate in sites with submerged macrophytes, and negatively correlated with dissolved inorganic nitrogen in sites with floating macrophytes, while no significant relationship was found between zooplankton and phytoplankton, partially confirming our first hypothesis.
Our results showed that floating and submerged macrophytes promoted changes in the phytoplankton community. Macrophytes are responsible for providing heterogeneous ecological niches that support diverse aquatic communities (Barrow et al. 2019; Stephan et al. 2019). For phytoplankton, macrophytes act as nutrient sources because, through the decomposition of plant tissues, nutrients become bioavailable in water (Wang et al. 2018), in addition to serving as substrate for algal colonization due to structural complexity (Nascimento-Filho et al. 2021). Algarte et al. (2017) showed that both the species richness of small-motile algae and the richness of large species were positively related to the presence of macrophytes, so the presence of macrophyte banks may have facilitated the coexistence of different species of algae.
For zooplankton, macrophytes serve as a refuge against predators (Figueiredo et al. 2018) and a place for colonization and egg laying (Battauz et al. 2017), and they also increase food availability (Rossa and Bonecker 2003; Brito et al. 2020). In this way, different macrophyte species may harbor different zooplankton species (Zeng et al. 2017), and this can have different effects on phytoplankton. In the present study, although floating macrophytes harbored higher biomass of herbivorous filter-feeders and scrapers zooplankton (cladocerans), no negative effect was found on phytoplankton biomass (Fig. 5h). Differently, the negative effect of zooplankton filter-feeders on phytoplankton was evidenced in other studies (Kozak et al. 2015; Gerasimova et al. 2018), and this can be explained by their generalist habit, predominant in several species represented by cladocerans.
Our second hypothesis was partially confirmed, as the RDA analysis showed that unicellular and flagellated phytoplankton were favored by nitrate and inorganic phosphate in open water areas, and nitrite and total phosphorus positively influenced the colonial, filamentous, small, medium and large functional phytoplankton groups in sites with macrophytes. According to Zhang et al. (2020), some macrophyte species can provide suitable substrates for filamentous algal growth. Similar results were observed by Takamura et al. (2003), who showed that phytoplankton species responded differently to the presence of macrophytes, with colonial and filamentous species of medium to large size positively related to lakes with the presence of macrophytes, and flagellated and unicellular species related to both the presence and absence of macrophytes. Our results showed that this relationship can be explained by the effect of nutrients (Fig. 6a). Studies performed in semi-arid regions of Brazil showed that submerged macrophytes play an important role in providing nutrients, especially during the dry season and rewetting, common in these regions due to the reduction in water level (Keitel et al. 2016; Barbosa et al. 2020). Thus, the nutrients available in areas covered by submerged macrophytes have a positive effect on phytoplankton, favoring filamentous, unicellular or colonial species (Monteiro et al. 2021).
During the growth period, macrophytes can accumulate nutrients from water or sediment, and when they decompose, the absorbed phosphorus is returned to the aquatic ecosystem (Wang et al. 2018). The algae attached to these macrophytes absorb 3.4–8.9% phosphorus released by macrophytes (Carignan and Kalff 1982). High phosphorus concentrations favor the development of phytoplankton, especially cyanobacteria (Simić et al. 2017), and non-diazotrophic cyanobacteria due to their inability to fix atmospheric nitrogen. Reservoirs in Northeastern Brazil are susceptible to cyanobacterial blooms (Moura et al. 2018), and eutrophication represents a factor that contributes to the success of these organisms (Amorim and Moura 2021; Macêdo et al. 2021).
In contrast, although no significant difference was observed in the biomass of flagellated species between sampling sites, a positive relationship between flagellated algae and nutrients was observed in the absence of macrophytes (Fig. 6a). This suggests that flagellated algae, predominantly represented by C. furcoides in the present study, use resources more efficiently in areas without macrophytes. According to Crossetti et al. (2019), reduction in the relative stability of the water column caused by the absence of macrophytes and the increase in water transparency are decisive for the success of C. furcoides. This supports our results, as the flagellated group was related to nitrogen in sites without macrophytes, as shown in the RDA (Fig. 6a).
Colonial phytoplankton was positively related to three functional groups of zooplankton in sites with macrophytes. This result accord with Amorim et al. (2020), which showed a positive correlation between cyanobacteria and zooplankton, such as rotifers and copepod nauplii. This relationship can be explained by ability of zooplankton to coexist with cyanobacteria in eutrophic waters (Amorim et al. 2020), in addition to a poor ability to avoid the ingestion of cyanobacteria (Lürling 2003, 2021; Colina et al. 2016), leading to the exploitation of alternative food sources, such as other algae (Ger et al. 2016). In contrast, a negative relationship was found between flagellated phytoplankton and zooplankton. Herbivorous crustaceans, such as cladocerans and calanoid copepods found in our study, have a feeding preference for medium to large-sized flagellated organisms (Colina et al. 2016; Titocci et al. 2022). Any evidence was found of zooplankton grazing C. furcoides (Colina et al. 2016).
Conclusion
This study showed that the macrophytes promoted significant changes in plankton communities, favoring the growth of small and large-sized algae and unicellular, colonial and filamentous species, in addition to increasing the biomass of filter-feeder and filter-scraper zooplankton. Floating and submerged macrophytes favored nutrient-phytoplankton interactions, while phytoplankton–zooplankton interactions occurred both in the presence of submerged macrophytes and in open water sites, partially confirming our first hypothesis. Our second hypothesis was partially confirmed too, as colonial, filamentous, small, medium and large phytoplankton were favored by the presence of macrophytes, and flagellated and colonial phytoplankton, represented by cryptophyceans, C. furcoides and colonial cyanobacteria, respectively, significantly influenced zooplankton in sites with macrophytes. Our results highlight the importance of evaluating the effects of floating and submerged macrophytes on the relationship between phytoplankton and zooplanktonic communities in tropical reservoirs.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author (ariadne_moura@hotmail.com) on reasonable request.
References
Algarte VM, Siqueira T, Landeiro VL, Rodrigues L, Bonecker CC, Rodrigues LC, Santana NF, Thomaz SM, Bini LM (2017) Main predictors of periphyton species richness depend on adherence strategy and cell size. PLoS ONE 12:e0181720. https://doi.org/10.1371/journal.pone.0181720
Alvares CA, Stape JL, Sentelhas PC, de Moraes Gonçalves JL, Sparovek G (2013) Köppen’s climate classification map for Brazil. Meteorol Zeitschrift 22:711–728. https://doi.org/10.1127/0941-2948/2013/0507
Amorim CA, Moura AN (2021) Ecological impacts of freshwater algal blooms on water quality, plankton biodiversity, structure, and ecosystem functioning. Sci Total Environ 758:143605. https://doi.org/10.1016/j.scitotenv.2020.143605
Amorim CA, Dantas ÊW, Moura AN (2020) Modeling cyanobacterial blooms in tropical reservoirs: the role of physicochemical variables and trophic interactions. Sci Total Environ 744:140659. https://doi.org/10.1016/j.scitotenv.2020.140659
Anagnostidis K, Komárek J (1988) Modern approach to the classification system of cyanophytes 3: Oscillatoriales. Arch Hydrobiol Algol Stud 80:327–472
APAC 2019 Agência Pernambucana de Águas e Clima. http://www.apac.pe.gov.br/meteorologia/monitoramento-pluvio.php Accessed 19 March 2019.
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington.
Banks LK, Frost PC (2017) Biomass loss and nutrient release from decomposing aquatic macrophytes: effects of detrital mixing. Aquat Sci 79:881–890. https://doi.org/10.1007/s00027-017-0539-y
Barbosa VV, Severiano JS, Oliveira DA, Barbosa JEL (2020) Influence of submerged macrophytes on phosphorus in a eutrophic reservoir in a semiarid region. J Limnol. https://doi.org/10.4081/jlimnol.2020.1931
Barnett AJ, Finlay K, Beisner BE (2007) Functional diversity of crustacean zooplankton communities: towards a trait-based classification. Freshw Biol 52:796–813. https://doi.org/10.1111/j.1365-2427.2007.01733.x
Barrow JL, Beisner BE, Giles R, Giani A, Domaizon I, Gregory-Eaves I (2019) Macrophytes moderate the taxonomic and functional composition of phytoplankton assemblages during a nutrient loading experiment. Freshw Biol 64:1369–1381. https://doi.org/10.1111/fwb.13311
Battauz YS, Paggi SBJ, Paggi J (2017) Macrophytes as dispersal vectors of zooplankton resting stages in a subtropical riverine floodplain. Aquat Ecol 51:191–201. https://doi.org/10.1007/s10452-016-9610-3
Braun LM, Brucet S, Mehner T (2021) Top-down and bottom-up effects on zooplankton size distribution in a deep stratified lake. Aquat Ecol 55:527–543. https://doi.org/10.1007/s10452-021-09843-8
Brett MT, Goldman CR (1997) Consumer versus resource control in freshwater pelagic food webs. Science 275:384–386. https://doi.org/10.1126/science.275.5298.384
Carignan R, Kalff J (1982) Phosphorus release by submerged macrophytes: Significance to epiphyton and phytoplankton. Limnol Oceanogr 27:419–427. https://doi.org/10.4319/lo.1982.27.3.0419
Carpenter SR, Kitchell JF, Hodgson JR (1985) Cascading trophic interactions and lake productivity. Bioscience 35:634–639. https://doi.org/10.2307/1309989
Chen Q, Li QH, Ma XY, Xiong MJ, He Y, Han MS (2019) Comparison of functional groups of phytoplankton in FG, MFG, and MBFG: taking three reservoirs as an example in Guizhou Plateau. Huan Jing Ke Xue 40:4061–4071. https://doi.org/10.13227/j.hjkx.201901192 (in Chinese)
Colina M, Calliari D, Carballo C, Kruk C (2016) A trait-based approach to summarize zooplankton–phytoplankton interactions in freshwaters. Hydrobiologia 767:221–233. https://doi.org/10.1007/s10750-015-2503-y
Crossetti LO, Bicudo DC, Bini LM, Dala-Corte RB, Ferragut C, Bicudo CEM (2019) Phytoplankton species interactions and invasion by Ceratium furcoides are influenced by extreme drought and water-hyacinth removal in a shallow tropical reservoir. Hydrobiologia 831:71–85. https://doi.org/10.1007/s10750-018-3607-y
da Brito MTS, Heino J, Pozzobom UM, Landeiro VL (2020) Ecological uniqueness and species richness of zooplankton in subtropical floodplain lake. Aquat Sci. 82(43):56. https://doi.org/10.1007/s00027-020-0715-3
DeMott WR (1986) The role of taste in food selection by freshwater zooplankton. Oecologia 69:334–340. https://doi.org/10.1007/BF00377053
Diniz AS, Severiano JS, Melo-Júnior M, Dantas ÊW, Moura AN (2019) Phytoplankton–zooplankton relationships based on phytoplankton functional groups in two tropical reservoirs. Mar Freshw Res 70:721–733. https://doi.org/10.1071/MF18049
Dumont HJ, van de Velde I, Dumont S (1975) The dry weight estimate of biomass in a selection of Cladocera, Copepoda and Rotifera from the plankton, periphyton and benthos of continental waters. Oecologia 19:75–97. https://doi.org/10.1007/BF00377592
Elmoor-Loureiro LMA (1997) Manual de identificação de cladóceros límnicos do, Brasil. Universitária, Brasília
Figueiredo BR, Fiori LF, Keppeler FW, Mormul RP, Benedito E (2018) Non-lethal effects of a native and a non-native piscivorous fish on the interaction between a mesopredator and benthic and pelagic invertebrates. Aquat Invasions 13:553–563. https://doi.org/10.3391/ai.2018.13.4.12
Fischer H, Pusch M (2001) Comparison of bacterial production in sediments, epiphyton and the pelagic zone of a lowland river. Freshw Biol 46:1335–1348
Frau D, Battauz Y, Alvarenga PF, Scarabotti PA, Mayora G, Sinistro R (2019) Assessing the relevance of top-down and bottom-up effects as phytoplankton structure drivers in a subtropical hypereutrophic shallow lake. Aquat Ecol 53:265–280. https://doi.org/10.1007/s10452-019-09687-3
Frau D, Gutierrez MF, Molina FR, de Mello FT (2021) Drivers assessment of zooplankton grazing on phytoplankton under different scenarios of fish predation and turbidity in an in situ mesocosm experiment. Hydrobiologia 848:485–498. https://doi.org/10.1007/s10750-020-04456-y
Ger KA, Hansson LA, Lürling M (2014) Understanding cyanobacteria–zooplankton interactions in a more eutrophic world. Freshw Biol 59:1783–1798. https://doi.org/10.1111/fwb.12393
Ger KA, Urrutia-Cordero P, Frost PC, Hansson LA, Sarnelle O, Wilson AE, Lürling M (2016) The interaction between cyanobacteria and zooplankton in a more eutrophic world. Harmful Algae 54:128–144. https://doi.org/10.1016/j.hal.2015.12.005
Gerasimova TN, Pogozhev PI, Sadchikov AP (2018) Suppression of alga blooming by zooplankton filter feeders in small water bodies. Water Resour 45:199–204. https://doi.org/10.1134/S0097807818020070
Golterman H, Clymo R, Ohnstad M (1978) Method for the physical and chemical analysis of fresh waters. IBP Handbook, London
Gomes LF, Pereira HR, Gomes ACAM, Vieira MC, Martins PR, Roitman I, Vieira LCG (2019) Zooplankton functional-approach studies in continental aquatic environments: a systematic review. Aquat Ecol 53:191–203. https://doi.org/10.1007/s10452-019-09682-8
Hillebrand H, Dürselen CD, Kirschtel D, Pollingher U, Zohary T (1999) Biovolume calculation for pelagic and benthic microalgae. J Phycol 35:403–424. https://doi.org/10.1046/j.1529-8817.1999.3520403.x
Jeppesen E, Søndergaard M, Lauridsen TL, Davidson TA, Liu ZW, Mazzeo N, Trochine C, Özkan K, Jensen HS, Trolle D, Starling F, Lazzaro X, Johansson LS, Bjerring R, Liboriussen L, Larsen SE, Landkildehus F, Egemose S, Meerhoff M (2012) Biomanipulation as a restoration tool to combat eutrophication: recent advances and future challenges. Adv Eco Res 47:411–488. https://doi.org/10.1016/B978-0-12-398315-2.00006-5
John DM, Whitton BA, Brook AJ (2002) The freshwater algal flora of the British Isles: an identification guide to freshwater and terrestrial algae. Cambridge University Press, Cambridge
Keitel J, Zak D, Hupfer M (2016) Water level fluctuations in a tropical reservoir: the impact of sediment drying, aquatic macrophyte dieback, and oxygen availability on phosphorus mobilization. Environ Sci Pollut Res 23:6883–6894. https://doi.org/10.1007/s11356-015-5915-3
Kiørboe T, Saiz E, Tiselius P, Andersen KH (2018) Adaptive feeding behavior and functional responses in zooplankton. Limnol Oceanogr 63:308–321. https://doi.org/10.1002/lno.10632
Komárek J, Anagnostidis K (1999) Cyanoprokaryota I. Teil Chroococcales. In: Ettl H, Gärtner G, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa. Gustav Fischer Verlag, Jena
Komárek J, Anagnostidis K (2005) Cyanoprokaryota 2. Teil Oscillatoriales. In: Büdel B, Krienitz L, Gärtner G, Schagerl M (eds) Süsswasserflora von Mitteleuropa. Gustav Fischer Verlag, Jena
Komárek J, Cronberg G (2001) Some chroococcalean and oscillatorialean Cyanoprokaryotes from southern African lakes, ponds and pools. Nova Hedwigia 73:129–160. https://doi.org/10.1127/nova.hedwigia/73/2001/129
Koroleff F (1976) Determination of nutrients. In: Grasshoff K (ed) Methods of Seawater Analysis. Verlag Chemie, Germany, pp 117–181
Koste W (1978) Rotatória: die Rädertiere Mitteleroupas Ein Bestimmungswerk begrüdet von Max Voigt-Monogonta. Gebrüder Borntraeger, Berlin
Kovalenko KE, Thomaz SM, Warfe DM (2012) Habitat complexity: approaches and future directions. Hydrobiologia 685:1–17
Kozak A, Gołdyn R, Dondajewska R (2015) Phytoplankton composition and abundance in restored Maltański Reservoir under the influence of physico-chemical variables and zooplankton grazing pressure. PLoS ONE. https://doi.org/10.1371/journal.pone.0124738
Krammer K, Lange-Bertalot H (1991) Bacillariophyceae 3. Teil: Centrales, Fragilariaceae and Eunotiaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Sübwasser flora von Mitteleuropa. Gustav Fischer Verlag, Germany
Kruk C, Devercelli M, Huszar VL (2021) Reynolds Functional Groups: a trait-based pathway from patterns to predictions. Hydrobiologia 848:113–129. https://doi.org/10.1007/s10750-020-04340-9
Legendre P, Oksanen J, ter Braak CJ (2011) Testing the significance of canonical axes in redundancy analysis. Methods Ecol Evol 2:269–277
Lewis AS, Kim BS, Edwards HL, Wander HL, Garfield CM, Murphy HE, Poulin ND, Princiotta SD, Rose KC, Taylor AE, Weathers KC, Wigdahl-Perry CR, Yokota K, Richardson DC, Bruesewitz DA (2020) Prevalence of phytoplankton limitation by both nitrogen and phosphorus related to nutrient stoichiometry, land use, and primary producer biomass across the northeastern United States. Inland Waters 10:42–50. https://doi.org/10.1080/20442041.2019.1664233
Li Y, Meng J, Zhang C, Ji S, Kong Q, Wang R, Liu J (2020) Bottom-up and top-down effects on phytoplankton communities in two freshwater lakes. PLoS ONE. https://doi.org/10.1371/journal.pone.0231357
Liu Z, Hu J, Zhong P, Zhang X, Ning J, Larsen SE, Chen D, Gao Y, He H, Jeppesen E (2018) Successful restoration of a tropical shallow eutrophic lake: strong bottom-up but weak top-down effects recorded. Water Res 146:88–97. https://doi.org/10.1016/j.watres.2018.09.007
Liu X, Chen L, Zhang G, Zhang J, Wu Y, Ju H (2021) Spatiotemporal dynamics of succession and growth limitation of phytoplankton for nutrients and light in a large shallow lake. Water Res 194:116910. https://doi.org/10.1016/j.watres.2021.116910
Lürling M (2003) Daphnia growth on microcystin-producing and microcystin-free Microcystis aeruginosa in different mixtures with the green alga Scenedesmus obliquus. Limnol Oceanogr 48:2214–2220. https://doi.org/10.4319/lo.2003.48.6.2214
Lürling M (2021) Grazing resistance in phytoplankton. Hydrobiologia 848:237–249. https://doi.org/10.1007/s10750-020-04370-3
Macêdo RL, Russo P, Correa RF, Rocha O, dos Santos LN, Branco CW (2021) The drifting dinoflagellate Ceratium furcoides (Levander) Langhans 1925: fundamental niche shift during global invasion. Hydrobiologia 848:2105–2117. https://doi.org/10.1007/s10750-020-04495-5
Mackereth F, Heron J, Talling J (1978) Water analysis: some revised methods for limnologists. Freshwater Biological Association, England
Marañón E (2015) Cell size as a key determinant of phytoplankton metabolism and community structure. Ann Rev Mar Sci 7:241–264
Martin JH (1970) phytoplankton–zooplankton relationships in Narragansett Bay. Iv. The seasonal importance of grazing. Limnol Oceanogr 15:413–418. https://doi.org/10.4319/lo.1970.15.3.0413
McQueen DJ, Post JR, Mills EL (1986) Trophic relationships in freshwater pelagic ecosystems. Can J Fish Aquat Sci 43:1571–1581. https://doi.org/10.1139/f86-195
McQueen DJ, Johannes MR, Post JR, Stewart TJ, Lean DR (1989) Bottom-up and top-down impacts on freshwater pelagic community structure. Ecol Monogr 59:289–309. https://doi.org/10.2307/1942603
Monteiro FM, Moura GCD, Severiano JDS, Mendes CF, Barbosa JEDL (2021) Submerged macrophytes support cyanobacteria and microcystin production in a drawdown tropical semi-arid reservoir. Aquat Ecol 55:875–890. https://doi.org/10.1007/s10452-021-09866-1
Montú M, Goeden IM (1986) Atlas dos cladocera e copepoda (Crustacea) do Estuário da Lagoa dos Patos (Rio Grande, Brasil). Revista Nerítica 1:1–134. https://doi.org/10.5380/rn.v1i2.41190
Moura NA, Aragão-Tavares NKC, Amorim CA (2018) Cyanobacterial blooms in freshwater bodies from a semiarid region, Northeast Brazil: a review. J Limnol 77:179–188. https://doi.org/10.4081/jlimnol.2017.1646
Mousing EA, Richardson K, Ellegaard M (2018) Global patterns in phytoplankton biomass and community size structure in relation to macronutrients in the open ocean. Limnol Oceanogr 63:1298–1312. https://doi.org/10.1002/lno.10772
Müller S, Mitrovic SM (2015) Phytoplankton co-limitation by nitrogen and phosphorus in a shallow reservoir: progressing from the phosphorus limitation paradigm. Hydrobiologia 744:255–269. https://doi.org/10.1007/s10750-014-2082-3
Nascimento-Filho SL, Gama WA, Moura AN (2021) Effect of the structural complexity of aquatic macrophytes on epiphytic algal, macroinvertebrates, and their interspecific relationships. Aquat Sci 83:1–14. https://doi.org/10.1007/s00027-021-00812-9
Naselli-Flores L, Zohary T, Padisák J (2021) Life in suspension and its impact on phytoplankton morphology: an homage to Colin S. Reynolds Hydrobiologia 848:7–30. https://doi.org/10.1007/s10750-020-04217-x
Neumann-Leitão S, Nogueira-Paranhos JD, Souza FBVA (1989) Zooplâncton do Açude de Apipucos, Recife – PE (Brasil). Arq Biol Tecnol 32:803–821
Nurminen L, Horppila J (2009) Life form dependent impacts of macrophyte vegetation on the ratio of resuspended nutrients. Water Res 43:3217–3226. https://doi.org/10.1016/j.watres.2009.04.041
Obertegger U, Smith HA, Flaim G, Wallace RL (2011) Using the guild ratio to characterize pelagic rotifer communities. Hydrobiologia 662:157–162. https://doi.org/10.1007/s10750-010-0491-5
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’hara RB, Solymos P, Stevens MHH, Szoecs E, Wagner, H. (2013) Community ecology package. R Package Version 2:321–326
Paerl HW, Otten TG, Kudela R (2018) Mitigating the expansion of harmful algal blooms across the freshwater-to-marine continuum. Environ Sci Technol 52:5519–5529. https://doi.org/10.1021/acs.est.7b05950
Popovský J, Pfiester LA (1990) Dinophyceae (Dinoflagellida). In: Ettl H, Gerloff J, Heyning H, Mollenhauer D (eds) Süßwasserflora von Mitteleuropa. Gustav Fischer Verlag, Jena, pp 1–272
Prescott GW, Bicudo CEM, Vinyard WC (1982) A synopsis of North American desmids part II section 4. The University of Nebraska Press, Lincoln
R Development Core Team (2019) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reid JW (1985) Chave de identificação e lista de referências bibliográficas para as species continentais sulamericanas de vida livre da Ordem Cyclopoida. Bol Zool (crustacea, Copepoda). 9:17–143. https://doi.org/10.11606/issn.2526-3358.bolzoo.1985.122293
Reynolds CS, Huszar V, Kruk C, Naselli-Flores L, Melo S (2002) Towards a functional classification of the freshwater phytoplankton. J Plankton Res 24:417–428. https://doi.org/10.1093/plankt/24.5.417
Rossa DC, Bonecker CC (2003) Abundance of planktonic and non-planktonic rotifers in floodplain lakes of the Upper Paraná River floodplain. Amazoniana 17:567–581
Ruttner-Kolisko A (1977) Comparison of various sampling techniques, and results of repeated sampling of planktonic rotifers. Arch Hydrobiol Beiheft Ergebnisse Der Limnologie. 8:13–18
Severiano JDS, Oliveira EDS, Lucena-Silva DD, Moura GCD, Silva EAD, Barbosa JEDL (2021) Invasion of the dinoflagellate Ceratium furcoides (Levander) Langhans 1925 in South America: record of the pattern of expansion and persistence in tropical reservoirs in Northeastern Brazil. Biol Invasions. https://doi.org/10.1007/s10530-021-02641-1
Simić SB, Ðorđević NB, Milošević D (2017) The relationship between the dominance of Cyanobacteria species and environmental variables in different seasons and after extreme precipitation. Fundam Appl Limnol 190:1–11. https://doi.org/10.1127/fal/2017/0975
Smith W, Lancelot C (2004) Bottom-up versus top-down control in phytoplankton of the Southern Ocean. Antarct Sci 16:531–539. https://doi.org/10.1017/S0954102004002305
Sommer U (2008) Trophic cascades in marine and freshwater plankton. Int Rev Hydrobiol 93:506–516. https://doi.org/10.1002/iroh.200711039
Stephan LR, Beisner BE, Oliveira SGM, Castilho-Noll MSM (2019) Influence of Eichhornia crassipes (Mart) Solms on a tropical microcrustacean community based on taxonomic and functional trait diversity. Water 11:2423. https://doi.org/10.3390/w11112423
Takamura N, Kadono Y, Fukushima M, Nakagawa M, Kim BH (2003) Effects of aquatic macrophytes on water quality and phytoplankton communities in shallow lakes. Ecol Res 18:381–395. https://doi.org/10.1046/j.1440-1703.2003.00563.x
Titocci J, Bon M, Fink P (2022) Morpho-functional traits reveal differences in size fractionated phytoplankton communities but do not significantly affect zooplankton grazing. Microorganisms 10:182. https://doi.org/10.3390/microorganisms10010182
Utermöhl H (1958) Methods of collecting plankton for various purposes are discussed. Mitt - Int Ver Theor Angew Limnol 9:1–38. https://doi.org/10.1080/05384680.1958.11904091
Vilas MP, Marti CL, Oldham CE, Hipsey MR (2018) Macrophyte-induced thermal stratification in a shallow urban lake promotes conditions suitable for nitrogen-fixing cyanobacteria. Hydrobiologia 806:411–426. https://doi.org/10.1007/s10750-017-3376-z
Wang L, Liu Q, Hu C, Liang R, Qiu J, Wang Y (2018) Phosphorus release during decomposition of the submerged macrophyte Potamogeton crispus. Limnology 19:355–366. https://doi.org/10.1007/s10201-018-0538-2
Wetzel RG, Likens GE (2000) Limnological Analyses. Springer, New York
Wood SN (2004) Stable and efficient multiple smoothing parameter estimation for generalized additive models. J Am Stat Assoc 99:673–686. https://doi.org/10.1198/016214504000000980
Wood SN (2011) Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc B 73:3–36. https://doi.org/10.1111/j.1467-9868.2010.00749.x
Xiao L, Zhu B, Kumwimba MN, Jiang S (2017) Plant soaking decomposition as well as nitrogen and phosphorous release in the water-level fluctuation zone of the Three Gorges Reservoir. Sci Total Environ 592:527–534. https://doi.org/10.1016/j.scitotenv.2017.03.104
Zeng L, Liu B, Dai Z, Zhou Q, Kong L, Zhang Y, He F, Wu Z (2017) Analyzing the effects of four submerged macrophytes with two contrasting architectures on zooplankton: a mesocosm experiment. J Limnol 76:581–590. https://doi.org/10.4081/jlimnol.2017.1520
Zhang W, Shen H, Zhang J, Yu J, Xie P, Chen J (2020) Physiological differences between free-floating and periphytic filamentous algae, and specific submerged macrophytes induce proliferation of filamentous algae: a novel implication for lake restoration. Chemosphere 239:124702. https://doi.org/10.1016/j.chemosphere.2019.124702
Acknowledgements
This work was supported by Fundação de Amparo a Ciência e Tecnologia do Estado de Pernambuco—FACEPE, Brazil (process IBPG-0549-2.03/18) and by Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq, Brazil (process PQ 305829/2019-0).
Author information
Authors and Affiliations
Contributions
ASD: conceptualization, investigation, data curation, methodology, formal analysis, visualization, writing—original draft preparation, writing—review & editing. WAG: conceptualization, formal analysis, visualization, writing—review & editing. ANM: conceptualization, writing—review & editing, supervision.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Wataru Makino.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Diniz, A.S., Gama, W.A. & do Nascimento Moura, A. The effects of presence of macrophytes on resource uptake by phytoplankton and zooplankton in a tropical reservoir. Limnology 25, 11–23 (2024). https://doi.org/10.1007/s10201-023-00726-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10201-023-00726-5