Abstract
The distribution of freshwater cladocerans and copepods and the drivers of beta diversity along La Plata basin were studied. We propose that local environmental conditions, dispersal limitation, and climate affect cladocerans and copepods differently owing to their variances in terms of life strategies. We calculated beta diversity using four dissimilarity metrics, and also the relative importance of spatial, environmental, and climatic variables by partitioning variance and forward selection procedure coupled with a partial redundancy analysis. Beta diversity patterns were characterized by a high turnover in the subbasins and a small contribution of nestedness. Forward selection evidenced the influence of total nitrogen and total suspended matter for both copepods and cladocerans, suggesting a strong role of eutrophication in controlling their turnover, but spatial distance, precipitation, and mean temperature of winter were related only to copepods. The last one suggests a likely role of geographic isolation driving speciation and endemism in Copepoda and reinforces the strong effect of climatic variation resulting in the high endemism patterns one finds in the Neotropical region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Copepoda and Cladocera are the major components of the zooplankton community in lakes and reservoirs, so understanding the drivers of both groups provides important information about the functioning of entire aquatic systems. These microcrustaceans play a fundamental role on the functioning of these systems linking producers (algae) and intermediate predators (other invertebrates and fish) (Ferdous & Muktadir, 2009; Perbiche-Neves et al., 2014). Although both groups are abundant in lakes and reservoirs, Cladocera species exhibit a wider distribution over large river basins; while Copepoda species are more limited in their geographic distribution (Henriques-Silva et al., 2016). Copepods, especially Calanoida-order species occur in zoographic regions with high levels of endemism (Suárez-Morales et al., 2005; Boxshall & Defaye, 2008; Perbiche-Neves et al., 2014). In contrast, Cladocera species are generally widely distributed, although they are somewhat cryptic, concealing groups of species or species complexes (Forró et al., 2008).
There is an extensive literature on diversity and biogeographic patterns of microcrustaceans (Suárez-Morales, 2003; Suárez-Morales et al., 2005; Leibold et al., 2010; Perbiche-Neves et al., 2014). Such literature highlights the relationship with local environmental conditions (e.g., Cottenie et al., 2001; Leibold et al., 2010; Debastiani-Júnior & Nogueira, 2015) and the effects of anthropic activities (e.g., Dodson et al., 2005; Nogueira et al., 2008; Simões et al., 2015). However, a few researches have studied microcrustaceans’ spatial distribution at larger scales, especially those encompassing different climatic regions (Leibold et al., 2010; Henriques-Silva et al., 2016; Xiong et al., 2016; Zhao et al., 2017). The knowledge of large-scale patterns for Tropical region is even more incipient.
Due to the speed of degradation of aquatic environments by human activities and climate changes (Moss et al., 2001), studies exceeding biogeographic borders would be pivotal if one means to forecast future broad-scale impacts (Albert & Reis, 2011; Perbiche-Neves et al., 2014; Xiong et al., 2016). A multi climatic perspective would allow the prognostics of how local and regional effects modify beta diversity; this is because one could obtain environmental gradients and biogeographic patterns, then compare them on a continental scale (Viana et al., 2015). The La Plata River Basin encompass many of these features, and, therefore, can be a suitable system to understand how the distribution of aquatic species is organized under distinct environmental, climatic, and spatial gradients, considering also the biological interactions. This is especially interesting in this basin, since the temperature along the latitudes oscillates from 40°C to less than 0°C across 3,000 km length, in a climatic transition zone characterized by the change of 1°C each 100 km (Perbiche-Neves et al., 2015).
In contrast to the growing literature and reports on the distribution patterns of Cladocera and Copepoda over wide-ranging areas, there are far fewer studies that have analyzed the underlying processes behind these patterns (Leibold et al., 2010; Viana et al., 2015; Henriques-Silva et al., 2016; Marrone et al., 2017; Zhao et al., 2017). In this sense, the metacommunity approach tries to understand community organization through encompassing processes that act over different spatial scales. This has generated excellent opportunities for understanding the drivers of large-scale distribution patterns in Cladocera and Copepoda, for example, pointing to effects of environmental factors on the microcrustaceans’ distributions in rivers (Zhao et al., 2017). Conceptually, a metacommunity is a set of communities distributed in space but linked by the potential dispersal of species among them (Leibold et al., 2004), for example, the zooplankton varying according to several drivers such as the body size (Zhao et al., 2017) or anthropic impacts (Debastiani-Júnior et al., 2015; Xiong et al., 2016) in rivers. In this framework, it is possible to get new insights regarding the relative influence of environmental filters at local scale (toleration and resilience to limnological variables), but also considering more regional processes as dispersal between sites. It is important to stress that one can use the metacommunity framework to understand the relative importance of niche-based and stochastic processes that shape communities and beta diversity, such as in case of the variation in community compositions along time and space (Whittaker, 1967; Chase & Myers, 2011; Henriques-Silva et al., 2016). For example, local environmental factors tend to explain communities largely governed by local niche processes and with efficient dispersal capacity (Leibold et al., 2010; Pinel-Alloul et al., 2013; Viana et al., 2015; Zhao et al., 2017). Conversely, local factors are less likely to explain communities governed by stochasticity (birth, death, and colonizing chance—Vellend, 2010); rather, spatial effects as evidenced by vicariance might be a signature of dispersal limitation.
Here we investigated the possible processes underlying the patterns of beta diversity in a large geographic scale in relation to Cladocera and Copepoda, which are both groups with expected differences in their underlying drivers. Our general hypothesis is that Cladocera’s beta diversity is largely affected by local niche-based processes, with a strong deterministic component in species distribution given their strong active dispersal capacity and high relationship with limnological variation. In contrast, we expect Copepoda to be less influenced by local niche processes given their dispersal limitation preventing them from reaching all optimal local sites over a large spatial extent.
To test our general hypothesis, we simultaneously sampled both Cladocera and Copepoda over the entire La Plata basin, the tenth largest basin in the world in terms of area, and the second largest in South America. La Plata’s sampling sites were over 1,397,905 km2. We tested the following predictions:
-
(P1)
Local environmental (limnological) factors should explain better the Cladocera distribution along the basin more so than they do Copepoda, given the Cladocera species’ high potential to disperse to all optimal sites and also be replenished from habitat sources as marginal lakes and other. Macroscale spatial factors are likely to better explain Copepod distribution considering their limited dispersal capacity.
-
(P2)
Climatic factors plus spatial distance should relate strongly with Copepod distribution, suggesting evolutionary/biogeography constraints determining higher endemism in this group in comparison to Cladocera.
-
(P3)
Cladocera beta diversity would be driven mainly by niche-based processes, while copepods should have a larger part of their beta diversity explained by random variation owing to their chance of colonization.
-
(P4)
The beta diversity of Copepoda should be more related to the nested component (i.e., variation from species loss, Baselga, 2010) in comparison to Cladocera because endemic copepods would not occur in most sites. Cladocera instead should have a larger turnover in comparison to Copepoda (e.g., variation due to species replacement, Baselga, 2010) because cladocerans would be more able to colonize sites according to specifically appropriate conditions for population establishment and development.
-
(P5)
Beta diversity of both Cladocera and Copepoda should differ between the seasons (winter/dry and summer/rainy periods), given that stronger determinism typically exists under harsher conditions (Chase, 2007; Henriques-Silva et al., 2013; Zhao et al., 2017). Environmental harshness generally impose greater mortality rates and metabolic costs for all organisms (Chesson & Huntly, 1997; Siqueira et al., 2015; Zhao et al., 2017) and for zooplankton this might be associated to stressful temperatures regime or lower productivity that preclude or limit the growing of organisms (Henriques-Silva et al., 2013; Perbiche-Neves et al., 2014, 2015). In dry periods, some local variables as total nitrogen, temperature, and pH explain generally a significant portion of zooplankton community variability, different from wet season when regional spatial factors are more important (Zhao et al., 2017). In this sense, during winter, both Cladocera and Copepoda should have a stronger signature of niche-based processes (e.g., beta diversity patterns cannot be explained through a null model), different from patterns found in summer.
Materials and methods
Study area and sampling design
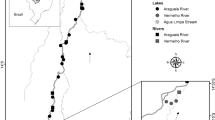
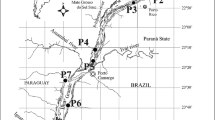
The main rivers of La Plata basin are the Paraná, Paraguay, and Uruguay, which pose a drainage area of 4.3 million km2; in our study the polygonal perimeter area considering all sampling sites was 1,397,905 km2. In each river, we located our sampling sites to ensure we were covering at least the three mains stretches: high, middle and low, including the main tributary rivers. Accordingly, we took triplicate samples in summer (January–March 2010) and in winter (June and July 2010). Specifically, zooplankton samples were obtained at 43 sites, including rivers and reservoirs (Fig. 1) distributed in Argentina, Brazil, Paraguay, Uruguay, and on the Brazil–Bolivia border.
We also took samples from 15 selected reservoirs at sites close to the dam and also in the riverine/intermediate regions (n = 30). Local governments have intensely constructed reservoirs since 1970, particularly in the upper reaches of the Paraná and Uruguay Rivers, in order to increase the hydropower generation for some of the largest cities on the continent. All sampled reservoirs have water retention times (WRT) greater than 15 days.
For rivers, we sampled 13 lotic stretches free of dams in the Paraguay River, and middle and low Paraná and Uruguay Rivers. The complete and detailed description of the sampling area and methodology for water and zooplankton samples is presented by Perbiche-Neves et al. (2015).
Zooplankton sampling
We took our samples in the main river channel and, in case of reservoirs, above dam (lacustrine zone) and in reservoir tail (riverine zone) using a conical plankton net with a 0.30 m mouth diameter, 0.90 m length, and a mesh size of 68 μm (see Perbiche-Neves et al., 2015). Samples were obtained by vertical hauls along the water column (from near bottom to surface) at each station. In deeper stations, the vertical hauls extended to a maximum depth of 40 m. In rivers, we took vertical hauls from a drifting boat to ensure that the hauls were not oblique. The volume of water filtered varied between 706 and 2,826 l per sample, averaging 1,766 l, and the volume was estimated using the formula for volume of a cylinder (= πr2h, where r is the radius of the plankton net mouth and h is the height of the hauls).
Laboratory analysis
We fixed all samples with formalin with a final concentration of 4%. For the qualitative analyses (species composition), we examined the entire sample and identified all species. The microcrustaceans were examined under stereo- and optic microscopes, and identified using specialized taxonomic references (see Debastiani-Júnior et al., 2015; Perbiche-Neves et al., 2015).
Environmental, climatological, and spatial variables
Limnological variables were measured through vertical profiles along the water column at each site, and excluded values for the deeper layers when thermal or chemical stratification was present. The following variables were measured: depth (Speedtech probe, USA), transparency (Transp) (Secchi disk), water temperature (WT), pH, dissolved oxygen (DO), conductivity (Cond) and turbidity (Turb) (Eureka Manta 2 probe, USA), total suspended matter (TSM) (following Cole, 1979), total phosphorus (TP) (following Strickland & Parsons, 1960), total nitrogen (TN) (following Marckereth et al., 1978), dissolved silica (Sil) (following Golterman et al., 1978), total chlorophyll (Chlor) (following Talling & Driver, 1963), and air temperature (AirT) (mercury thermometer).
The climatic variables used in this study for the La Plata basin appear on the WorldClim website (https://www.worldclim.org, accessed on 12 September 2013), which has records going back 50 years. The six combinations of temperature were as follows: mean air temperature (TMe), mean summer temperature (TMeS), mean winter temperature (TMeW), maximum temperature of the warmest month (TMaxWM), minimum temperature of the coldest month (TMinCM), and total amplitude of temperature (TAmp), plus precipitation (Prec) data.
In order to set up spatial distance matrix, we measure the distance among sampling sites via water course (i.e., linking sites via hydrography using a GIS-based approach) and do not in straight line using geographic coordinates (Landeiro et al., 2012). Principal coordinates of neighbor matrices (PCNM; Borcard & Legendre, 2002) eigenvectors were computed across water course distance matrix among sampling sites. PCNM quantifies spatial trends across a range of scales and is based on eigenvalue decomposition of a truncated matrix of distances among sampling sites (by water). PCNM eigenvectors can be considered as spatial variables in a canonical analysis.
Data analysis
The dataset was separately analyzed for copepods and cladocerans, as well as summer and winter to prevent seasonal interference in beta diversity metrics. A presence-and-absence matrix was used. We calculated beta diversity as the distance of group members to the group centroid in multivariate space according to the model proposed by Anderson (2006), based on the use of different dissimilarity metrics to calculate distance and the centroid. The first dissimilarity metric used to calculate group centroid was the Lande metric, which is an arithmetic means of species not shared between two locations: (b + c)/2 where b is the number of species that occurs exclusively at site A, and c is the number of species that occurs exclusively at site B (Lande, 1996). Despite being influenced by alpha diversity, this measure is an intuitive estimate that represents the magnitude of turnover between two sites. We used the Raup-Crick metric as a second method to calculate the group centroid, which applies a probabilistic null model to control for differences in alpha diversity. This gave us the means to control the positive relationship expected between alpha and beta diversity (Chase & Myers, 2011). Posteriorly, we applied a null model where the probability of selecting species is proportional to the species’ frequencies. By removing the influence of alpha diversity, the Raup-Crick metric let us distinguish deterministic from stochastic patterns of beta diversity and allowed for comparison among groups of organisms with distinct alpha diversity patterns (Chase & Myers, 2011). We chose a form to compute it that ranged between − 1 and 1 (Chase & Myers, 2011), and interpreted values approaching 0 as variation in community composition governed by stochastic processes. Negative values can be interpreted as a tendency to lower beta diversity than expected, such as in the case of a measure of homogenization. One can interpret positive values as a tendency to higher beta diversity than expected compared to the null model of beta diversity (Chase & Myers, 2011). Moreover, one might interpret pure niche selection when values are higher than 0.95, and strong homogenizing dispersal; intense dispersal maintains two sites with similar community compositions when values are lower than − 0.95 (Stegen et al., 2013). The third and the fourth dissimilarity metrics we used to calculate the group centroid were the components of nestedness and turnover from Sorensen dissimilarity, respectively (Baselga, 2010, 2012). Then, we compared differences in beta diversity (here represented as the average distance to group centroids) among seasons and among Cladocera and Copepoda, using the permutation-based test of multivariate homogeneity of group dispersion (PERMDISP, Anderson, 2006).
We also investigated the relative importance of environmental filters (limnological variables were log-transformed, except pH), spatial gradients and climatic variables on beta diversity using a procedure of partition variance (Borcard et al., 1992) and followed up with a partial redundancy analysis (pRDA; Legendre & Legendre, 1998; Legendre et al., 2005). Variance partitioning allows one to separate the fractions of variability (environmental filters, spatial gradients, and climatic variables) that result from canonical analysis (Borcard et al., 1992). We used the pRDA to verify specific associations between species composition and variables of one group (environmental filters, spatial gradients, and climatic variables), thereby controlling associations with variables of other groups (Legendre, 2008). Results were based on adjusted R2 values, since these are independent of the sample size and a number of predictor variables, allowing comparisons between the results (Peres-Neto et al., 2006). The filter’s significance was tested on pRDA (P < 0.05) with 999 randomizations (Legendre et al., 2011), using Blanchet method. Species composition was transformed through application of the Hellinger method (Legendre & Gallagher, 2001) prior to analysis to preserve the distance among sites and make community composition data suitable for analysis by linear methods (Legendre et al., 2005; Legendre, 2008). We explored multicollinearity by computing the variance inflation factors of the variables (VIF), which measure the proportion by which the variance of a regression coefficient inflates in the presence of other explanatory variables. We removed limnological variables with a VIF higher than 5 from our analyses. We chose the final models using forward selection of explanatory variables based on Akaike’s information criterion (AIC) and used adjusted coefficient of determination, thereby identifying those which best accounted for variation in species composition (Blanchet, 2008).
To observe the relative importance of each site to the total beta diversity across our study area, we calculated the Local Contribution to Beta Diversity as proposed by Legendre & De Cáceres (2013). The LCBD values for each site represent the site uniqueness in terms of community composition. When the value of LCBD is higher, its contribution to beta diversity strengthens. To calculate beta diversity in this case we used the Jaccard dissimilarity, but most dissimilarity metrics are suitable and provide similar results regarding LCBD (Legendre & De Cáceres, 2013). We did not calculate the Species Contribution to Beta Diversity (SCBD), since we are not aware of any method developed to partition the species contribution for incidence data.
In this study, we ran all statistical analyses using the software R 3.2.2 (R Core Team, 2011) with the packages vegan (Oksanen, 2018), PNCM (Oksanen, 2018), Biodiversity R (Kindt & Coe, 2005), and Packfor (Dray, 2013).
Results
Our study yielded identification of a total of 104 microcrustacean species (58 of Cladocera and 46 of Copepoda). During winter, the richness of Cladocera species oscillated between 1 and 48 in the distinct sampling sites, and the mean was 13.7 (± 11.6) species. In the same season, Copepods species’ richness varied between 1 and 16 with a mean of 7.9 (± 3.6). In the summer season, Cladocera richness varied from 2 to 58, wherein the mean was 16.5 (± 13.2), while Copepods richness oscillated between 1 and 18 species with mean of 8.2 (± 3.2).
Lande’s metric of beta diversity (measured through mean of species not shared between places) was approximately 5.4 species for Copepods and 9.3 species for Cladocerans in winter, and five species of Copepoda and ten of Cladocera in summer. Lande beta diversity of copepods was higher than five in more than 50% of pairwise observations, and for cladocerans this value exceeded 7.5 species, even reaching 30 species. We found differences in Lande beta diversity between Cladocera and Copepoda (Fig. 3), but not between seasons (Fig. 4).
The Raup-Crick metric resulted in values between 1 and − 1 for both Cladocera and Copepoda and in the two studied seasons. We found that 30–44% of community pairs had negative values, and 56–70% had positive ones for both groups, suggesting that both deterministic and stochastic processes are important in the structuration of these communities. This directly contrasts with P3. We found that 7–9% of community pairs had Raup-Crick values inferior to than − 0.95 (Fig. 2), indicating a much lower beta diversity than expected under random assembly, while, 17–25% of community pairs have values higher than 0.95, suggesting a much higher beta diversity than compared to a random model.
Dotplots showing results of Raup-Crick metric of beta diversity of Cladocera and Copepoda communities in winter and summer along the La Plata basin. The values are ordered from lower-to-higher value of Raup-Crick (from left to right).Values approaching 0 can be interpreted as variation in community composition governed by stochastic processes. Values below 0 can be interpreted as a tendency to lower beta diversity than expected; i.e., a measure of homogenization, while positive values can be interpreted as a tendency to higher beta diversity than expected compared to the null model of beta diversity (Chase & Myers, 2011). Moreover, pure niche selection can be interpreted when values are > 0.95, and strong mass effect when values are < − 0.95 (Stegen et al., 2013). The top right vertical values represent the percentage of pairwise communities lower than − 0.95. The values on the middle represent the percentage of pairwise communities between − 0.95 and 0 and between 0 and 0.95 (from top to bottom). The bottom right vertical values represent the percentage of pairwise communities higher than 0.95
The exception was seen for the summer Copepoda community, with only 6% of the values were higher than 0.95. This suggests weaker determinism in this season. Contrasting with P3, PERMDISP showed no differences between Cladocera and Copepoda and between seasons (contrasting P5) when using Raup-Crick metric (Figs. 3, 4), which implies seasonality only weakly influences the prevalence of determinism over stochasticity. It is worth noting that there was a decrease in pure niche selection during summer, which is a result partially supporting P3 (Fig. 4).
Boxplots showing the distance to the centroid in a multivariate space using four different distance metrics to calculate the distance to centroids. The comparison is among Cladocera and Copepoda patterns. The mean distance to centroid was used as measure of beta diversity. P values concern a permutational test of homogeneity using multivariate dispersion as proposed by Anderson (2006)
Boxplots showing the distance to the centroid in a multivariate space using four different distance measures to calculate the distances and centroids. The comparisons are among seasons for all species and also for Cladocera and Copepoda. The mean distance to centroid was used as measure of beta diversity. P values concern a permutational test of homogeneity using multivariate dispersion as proposed by Anderson (2006)
Among the components of Sorensen dissimilarity (Fig. 3) we found that turnover (51% of Sorensen dissimilarity on average) was predominant over nestedness (12% of Sorensen dissimilarity metric on average). Contrasting our P4, copepod nestedness was lower than cladocerans nestedness (Fig. 3), but they did not differ in turnover values. Cladocera had higher nestedness in winter in comparison with summer, thereby supporting our P5 (Fig. 4); this suggests harsher periods increase local species loss. As we conducted several PERMDISP without any method of multiple comparison correction, there is a possibility of inflated type I error in our results. Beside this possibility, we acknowledge that our multiple tests are independent of each other regarding the hypothesis tested.
Variables selected by forward selection in the pRDA showed the main factors correlated to the variation in microcrustacean composition were total nitrogen, total suspended matter, PCNM1, and PCNM2 (0.001). While PCNM12 represents spatial filters in smaller scales (Table 1), PCNM1 and PCNM2 exhibit the largest gradients of spatial distance pertaining to sampling sites, considering the Mantel test between Euclidian distance of PCNM1 and spatial distance matrix between sampling sites: r = 0.30, P < 0.001. Moreover, in addition to the variables above, Copepods responded also to the electrical conductivity of the water, spatial variables in smaller scales (PCNM 12), and the minimum temperature of coldest month.
We observed similar general trends on local contribution to beta diversity (LCBD) (Fig. 5A–D) for Cladocera and Copepoda in both seasons. The highest values occurred in sites located in rivers and reservoirs in the upper and middle Paraguay River, low Paraná River, all Uruguay River (all are lotic sites); the reservoirs of Paranapanema River, low Tietê River, and in Iguaçu River are mesotrophic–eutrophic reservoirs, with high values of nutrients and chlorophyll. Sampling sites located in oligotrophic reservoirs in upper Paraná River basin, and also in the end of La Plata River, generally exhibit low values of nutrients and chlorophyll. Even so, high values of local contribution occurred along all the sampled rivers.
Local contribution to the beta diversity (LCBD) in sampling sites for Cladocera in winter (A), Cladocera in summer (B), Copepoda in winter (C), and Copepoda in summer (D). Venn diagram (E–H), mean values of limnological variables (I–M), and climatic variables (N–Q) of our study. Venn diagram shows the percentage explanation of environmental, spatial, and climatic filters using a portioning variance on the beta diversity of cladocerans in winter (E) and summer (F), and copepods in winter (G) and summer (H) in the La Plata River Basin (* significant R2 adjusted). Limnological variables: I water transparency, J electrical conductivity, K total nitrogen, L total phosphorus, M total chlorophyll. Climatic variables are means over the last 50 years in this basin: N temperature amplitude, O mean temperature, P maximum temperature in warmest month, Q mean temperature in winter
Supporting our P1, partitioning variance showed that Cladocera responded only to environmental filters, while copepods species responded to spatial distance, climatic and environmental filters (Fig. 5E–H). Environmental, spatial, and climatic filters explained together 15–39% of beta diversity variation (Fig. 5). Environmental filters were the most important for cladocerans and copepods distributions in both seasons, which indicates the effect of environmental gradients on species compositional changes. Spatial and climatic filters were important to the spatial distribution of copepods, thereby corroborating P1 and P2. This suggests a limitation to the dispersion and response to the climatic variation of the subtropical part of the Austral region, especially in the Neotropical region. Our study found no association between Cladocerans and the spatial and the climatic variables we used here.
In relation to the limnological variables, transparency (Fig. 5I) was higher in reservoirs located in upper basins; the conductivity was higher in the Tietê River and in the lower stretch of the La Plata basin (Fig. 5J). In this case, total nitrogen and total phosphorus showed a clear spatial pattern downstream of large cities, such as in Tietê, Iguaçu, and low Paraná rivers (Fig. 5K, L). The total chlorophyll-a showed similar values in the river stretches and a wide range of variation in reservoirs (Fig. 5M).
The total temperature amplitude (Fig. 5N) and mean winter temperature (Fig. 5Q) showed a latitudinal pattern, while mean summer temperature (Fig. 5P) and mean winter temperature (Fig. 5Q) showed a longitudinal pattern (Fig. 5). Mean air temperature (Fig. 5O) showed latitudinal and longitudinal variations.
Discussion
The beta diversity of cladocerans and copepods from La Plata River basin was related to environmental, spatial, and climatic filters according to this study, but the relative importance of each filter varied between these groups and was low in general, similar to found in other recent studies which analyzed large river basins (Xiong et al., 2016; Zhao et al., 2017). These facts suggest that the differential distribution pattern for each group is not driven by the same processes, pointed previously by Cottenie et al. (2003), Pinel-Alloul et al. (2013), Xiong et al. (2016), and Zhao et al. (2017) for zooplankton in large areas in the world. In our study, considering a macroscale view, the results suggests that cladocerans were more governed by local environmental filters and less influenced by regional processes of dispersal and climatic filtering, explaining the wide distribution of many of this group’s species. On the other hand, a difference was that local features governed copepods, as did spatial and climatic variables; this fact might suggest that dispersal limitation and sensitivity to climate variance—in this case, mainly low temperatures—could explain the high rates of endemism in this group, delimited in specific hydrographic basins or regions with different climate.
The importance of these filters was probably due to the large analyzed spatial scale, visible also in other studies in large river basins (Zhao et al., 2017). The watercourses distance was higher than 3,000 km along a latitudinal range of 20° in the south hemisphere. Along this spatial gradient, we found high environmental heterogeneity represented by: (1) the presence of different landscapes inserted in different biomes, altering physical, chemical, and biological conditions; (2) the presence of natural (e.g., water velocity, turbidity, nutrients, geology) and human-made (e.g., reservoirs, drainage, siltation) geographic barriers; (3) wide climatic variations such as 1°C per 100 km over 400 km in the La Plata basin (climatic borders); and (4) anthropic pressures, mainly reservoirs (transparency and water velocity) and eutrophication. Cladocera and Copepoda species responded to these variations distinctly, changing their composition accordingly along the landscapes; all of these factors above mentioned have fomented the insights about assembling processes in continental scales that we discuss below.
The local limnological variables explained significant amounts of community variation, especially: total nitrogen, total suspended material, total phosphorus, depth, electric conductivity, and water transparency. These variables reflect natural characteristics of the surrounding catchment and also anthropic impacts due to multiple land uses along the catchment (Matsumura-Tundisi & Tundisi, 2003; Perbiche-Neves & Nogueira, 2013). Nutrients were also important to zooplankton in a large river basin in China (Zhao et al., 2017). Total suspended material, water transparency, and depth reflect anthropic alterations in the physical environment, mainly due to how governments have constructed reservoirs and produced large lakes. The reservoir cascades along river courses acts as the main cause of small particle retention and decrease in water velocity (Ward & Stanford, 1995), increasing the transparency. This process favors sedimentation, contributing to form distinct zones into a reservoir (Thornton, 1990), influencing zooplankton productivity and diversity (Simões et al., 2015). As the end result here, there is the dominance or/and resilience of some species with seasonally oscillate populations within reservoirs, for example, of the genera Thermocyclops, Mesocyclops, and species Microcyclops anceps (Richard, 1897) among cyclopoids copepods; Notodiaptomus henseni (F. Dahl, 1894) and N. iheringi (S. Wright, 1935) among calanoid copepods; and Bosmina, Bosminopsis, some species of Daphnia, Moina, and Diaphanosoma genera for cladocerans, which almost always occur in the reservoirs of upper Paraná River Basin (Nogueira et al., 2008; Perbiche-Neves & Nogueira, 2013; Perbiche-Neves et al., 2014).
The latitudinal effect of temperature variance and solar incidence act as climatic filters on microcrustaceans, especially in copepods (variation partitioning and pRDA results and difference in beta diversity between summer and winter, see Fig. 5 and Pinel-Alloul et al., 2013). The relationship between species composition, minimum winter temperature, and also of stronger niche selection during winter in comparison to summer (Fig. 3), all coagulate and suggest climatic conditions linked to low temperatures playing a role in controlling copepods’ geographic distribution. Such results raise concerns because they highlight the impacts of climate change on copepods’ spatial distribution in according to recent reviews on continental zooplankton (Vadadi-Fulop et al., 2012; Stoks et al., 2014). Probably, physiological adaptations enable organisms to weather low temperatures in high latitudes and warmer temperatures near the tropic, but the same organisms tend to lack strong plasticity to adapt to climatic variation (Perbiche-Neves et al., 2015). The relationship of copepods to surrounding climatic conditions have practical implications in the context of global change, with a likely alteration in the range of species depending on dispersal capacity and alteration of current temperature (Wrona et al., 2006). While some individual copepod populations have strong potential to colonize places with peculiar conditions, the effects of climatic change for the entire group of copepods remains unknown (Wallace et al., 2014).
In general, oligotrophic reservoirs exhibit low beta diversity contribution, probably reflecting a known pattern where the oligotrophic fauna seems to repeat the composition in several neotropical locations (Matsumura-Tundisi & Tundisi, 2003; Nogueira et al., 2008; Perbiche-Neves et al., 2015). It is worth noting that at least one site from each river basin had high values of local contribution, which suggests each basin contributes to provide elevated levels of beta diversity in the entirety of the La Plata Basin. Specific sites exhibit high values in both sampling periods, for example, at Paraguay River, low Paraná River, and the tributary Paranapanema River. Local contributions indicate the site uniqueness in terms of species composition, indicating sites all over the La Plata Basin as important for endemic species—especially copepods.
Cladocera beta diversity was less related to spatial filters and more related to environmental characteristics, such as recorded by Henriques-Silva et al. (2016) at the north hemisphere. Several studies have acknowledged Cladocera for high resilience (Debastiani-Júnior & Nogueira, 2015; Panarelli et al., 2008) and dispersion capacity (Allen, 2007). In addition, some work has shown that local environmental conditions are prominent factors for the colonization, establishment, and diversity of zooplankton communities in water bodies (Eitam et al., 2004; Hobæk et al., 2002). These would include cladocerans. It is noteworthy that the type of water body investigated in the works regarding Cladocera or zooplankton dispersion/diversity is usually lentic, isolated lakes/pools, which the large rivers analyzed in the present study contrast significantly. Little is known about how zooplankton diversity behaves in riverine conditions and, in this scenario, dispersion through the watercourse and tributaries contributions may be even more powerful for producing homogenization effects. There are increased effects and probably accumulation of the diversity by tributaries downstream in water courses (Portinho et al., 2016). Our results corroborate that, for Cladocera, high dispersal capacity and resilience, as traits of the entire group, led to a wide distribution and beta diversity patterns that considerably disregard spatial distances. The high variation observed to local contribution of beta diversity probably should refer to the not analyzed variables or factors, as biological interactions within aquatic webs or not measured limnological variables, as pollutants, heavy metals, antibiotics, and other substances (Xiong et al., 2016; Zhao et al., 2016).
On the other hand, copepod beta diversity exhibited spatial limitations corroborating their biogeographic patterns. These findings reinforce that watercourse distance is a good proxy for Copepoda dispersal; indeed, some previous biogeographic studies of diaptomid copepods pointed this out earlier (Suárez-Morales, 2003; Suárez-Morales et al., 2005; Boxshall & Defaye, 2008; Perbiche-Neves et al., 2014). Recently in the last 50 years the habitat isolation maximized by dam constructions decreased genetic flux and, together with the response to environmental filtering in short periods and to climatic filters in long periods, can increase the levels of endemism of copepods for the entire basin (Vanschoenwinkel et al., 2011). This affects especially organisms of large body size as microcrustaceans than small ones, as rotifers, that receive more influence of spatial factors (Zhao et al., 2017). Since these organisms respond to climatic filters, it is likely that they will experience local and regional extinctions in the future associated specifically with climatic changes assuming dispersal limitation increases as the number of dams also heightens. Although our data does not specifically support such predictions, one can expect an expansion of tropical cladocerans from the north of the basin to the south. Unfortunately, this will likely not also entail an efficient expansion of copepods due to maximization of their dispersal limitation. In contrast, it is very hard to predict how cladocerans and copepods from the south will respond to decreases in the spatial extension of their optimal climate and by new interspecific interactions with migrating cladocerans from the north.
We found that a high rate of turnover and a low amount of nestedness (or species loss) characterized both Cladocera and Copepoda beta diversity. One can explain these patterns through two main processes: (1) environmental heterogeneity in continental scale is high, and the environmental filters are changing significantly along the La Plata basin, selecting different species and assembling distinct communities (Baselga, 2010). This interpretation supports the results of pRDA, mainly for Cladocera that related exclusively to local variables, but also for copepods that related to local, spatial, and climatic filters. (2) Interspecific competition along different environments can generate high rates of turnover and low nestedness, reflecting competitive exclusion between species pairs that might be the best competitor depending on the conditions (Leprieur et al., 2011). Several studies outline the strong role of competition in shaping local communities of zooplankton (e.g., Von Ruckert & Giani, 2008), but the literature have not yet considered how competition can generate patterns in a continental scale (Pinel-Alloul et al., 2013). It is worth noting that similar to partitioning variance results, the high turnover suggests that endemism in Copepoda results from deterministic processes of environmental and climatic affinities. Moreover, one significant implication is that regions characterized by high rates of turnover require comprehensive strategies of conservation to encompass the whole community composition variation along the basin. Such strategies would be different from those pertaining to nested communities where protection can be focused only in areas with the highest richness and expect substantial success (Baselga, 2010).
Overall, using a metacommunity perspective, this study has determined that the distribution of cladocerans over the continental La Plata River basin is governed mainly by local processes of environmental filtering (strongly related to the trophic state of sites); copepods instead were also influenced by regional (i.e., dispersal limitation) and evolutionary processes (climatic adaptation). Especially copepods will be a complex group to predict its future distribution. This is because local environmental conditions change alongside the increase in eutrophication of South American reservoirs. Accordingly, dispersal routes are changing due to the construction of new dams, while climatic changes are likely happening faster than these species have previously experienced. Given this worsening scenario, we urge ecologists to invest in understanding the magnitude and directions of such changes for the different aquatic communities over large-scale extents.
References
Albert, J. S. & R. E. Reis, 2011. Historical Biogeography of Neotropical Freshwater Fishes. University of California Press, Berkeley, CA.
Allen, M. R., 2007. Measuring and modeling dispersal of adult zooplakton. Oecologia 153: 135–143.
Anderson, M. J., 2006. Multivariate dispersion as a measure of beta diversity. Ecology Letters 9: 683–693.
Baselga, A., 2010. Partitioning the turnover and nestedness components of beta diversity. Global Ecology and Biogeography 19: 134–143.
Baselga, A., 2012. The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Global Ecology and Biogeography 21: 1223–1232.
Blanchet, F. G., P. Legendre & D. Borcard, 2008. Modelling directional spatial processes in ecological data. Ecological Modelling 215: 325–336.
Borcard, D. & P. Legendre, 2002. All-scale spatial analysis of ecological data by means of principal coordinates of neighbor matrices. Ecology Modelling 153: 51–68.
Borcard, D., P. Legendre & P. Drapeau, 1992. Partialling out the spatial component of ecological variation. Ecology 73: 1045–1055.
Boxshall, G. A. & D. Defaye, 2008. Global diversity of copepods (Crustacea: Copepoda) in freshwater. Hydrobiologia 595: 195–207.
Chase, J. M., 2007. Drought mediates the importance of stochastic community assembly. Proceedings of the Natural Academy of Sciences of USA 104: 17430–17434.
Chase, J. M. & J. A. Myers, 2011. Disentangling the importance of ecological niches from stochastic processes across scales. Philosophical Transactions of the Royal Society 366: 2351–2363.
Chesson, P. & N. Huntly, 1997. The roles of harsh and fluctuating conditions in the dynamics of ecological communities. American Naturalist 150: 519–553.
Cole, G. A., 1979. Textbook of limnology, 2nd ed. The C.V. Mosby Company, Saint Louis.
Cottenie, K., N. Nuytten, E. Michels & L. De Meester, 2001. Zooplankton community structure and environmental conditions in a set of interconnected ponds. Hydrobiologia 442: 339–350.
Cottenie, K., E. Michels, N. Nuytten & L. De Meester, 2003. Zooplankton metacommunity structure: regional vs. local processes in highly interconnected ponds. Ecology 84(4): 991–1000.
Debastiani-Júnior, J. R. & M. G. Nogueira, 2015. How water level management affects cladoceran assemblages in lakes lateral to a reservoir. Marine and Freshwater Research. https://doi.org/10.1071/MF14281.
Debastiani-Júnior, J. R., L. M. A. Elmoor-Loureiro & M. G. Nogueira, 2015. High taxonomic resolution as a determinant on finding new species and new records in the Río de La Plata basin: a case on Chydoridae (Crustacea: Branchiopoda: Cladocera). Nauplius 23(1): 21–30.
Dodson, S. I., R. A. Lillie & S. Will-Wolf, 2005. Land use, water chemistry, aquatic vegetation, and zooplankton community structure of shallow lakes. Ecology Applied 15: 1191–1198.
Dray, S., 2013. SpacemakeR: Spatial modelling. R package version 0.0-5/r113. http://R-Forge.R-project.org/projects/sedar.
Eitam, A., L. Blaunstein, K. Van Damme, H. J. Dumont & K. Martens, 2004. Crustacean species richness in temporary pools: relationships with habitat traits. Hydrobiologia 525: 125–130.
Ferdous, Z. & A. K. M. Muktadir, 2009. Potentiality of zooplankton as bioindicator. American Journal of Applied Science 6: 1815–1819.
Forró, L., N. M. Korovchinsky, A. A. Kotov & A. Petrusek, 2008. Global diversity of cladocerans (Cladocera; Crustacea) in freshwater. Hydrobiologia 595: 177–184.
Golterman, H. L., R. S. Clyno & M. A. M. Ohnstad, 1978. Methods for physical and chemical analysis of freshwaters. 2nd ed. Blackwell, Oxford.
Henriques-Silva, R., Z. Lindo & P. R. Peres-Neto, 2013. A community of metacommunities: exploring patterns in species distributions across large geographical areas. Ecology 94: 627–639.
Henriques-Silva, R., B. Pinel-Alloul & P. R. Peres-Neto, 2016. Climate, history and life-history strategies interact in explaining differential macroecological patterns in freshwater zooplankton. Global Ecology and Biogeography 25: 1454–1465.
Hobæk, A., M. Manca & T. Andersen, 2002. Factors influencing species richness in lacustrine zooplankton. Acta Oecologica 23: 155–163.
Kindt, R. & R. Coe, 2005. Tree diversity analysis: a manual and software for common statistical methods for ecological and biodiversity studies. http://www.worldagroforestry.org/output/tree-diversity-analysis.
Lande, R., 1996. Statistics and partitioning of species diversity, and similarity among multiple communities. Oikos 76: 5–13.
Landeiro, V. L., L. M. Bini, R. R. C. Costa, E. Franklin, A. Nogueira, J. L. P. Souza, J. Moraes & W. E. Magnusson, 2012. How far can we go in simplifying biomonitoring assessments? An integrated analysis of taxonomic surrogacy, taxonomic sufficiency and numerical resolution in a megadiverse region. Ecological Indicators 23: 366–373.
Legendre, P., 2008. Studying beta diversity: ecological variation partitioning by multiple regression and canonical analysis. Journal of Plant Ecology 1(1): 3–8.
Legendre, P. & L. Legendre, 1998. Numerical Ecology, 2nd ed. Elsevier, New York.
Legendre, P. & E. D. Gallagher, 2001. Ecologically meaningful transformations for ordination of species data. Oecologia 129: 271–280.
Legendre, P. & M. de Cáceres, 2013. Beta diversity as the variance of community data: dissimilarity coefficients and partitioning. Ecology Letters 16: 951–963.
Legendre, P., D. Borcard & P. R. Peres-Neto, 2005. Analyzing beta diversity: partitioning the spatial variation of community composition data. Ecological Monographs 75: 435–450.
Legendre, P., J. Oksanen & C. J. F. Ter Braak, 2011. Testing the significance of canonical axes in redundancy analysis. Methods in Ecology and Evolution 2: 269–277.
Leibold, M. A., M. Holyoak, N. Mouquet, P. Amarasekare, J. M. Chase & M. E. Hoopes, 2004. The metacommunity concept: a framework for multi-scale community ecology. Ecology Letters 7: 601–613.
Leibold, M. A., E. P. Economo & P. Peres-Neto, 2010. Metacommunity phylogenetics: separating the roles of environmental filters and historical biogeography. Ecology Letters 13: 1290–1299.
Leprieur, F., P. A. Tedesco, B. Hugueny, O. Beauchard, H. H. Dürr, S. Brosse & T. Oberdorff, 2011. Partitioning global patterns of freshwater fish beta diversity reveals contrasting signatures of past climate changes. Ecology Letters 14(4): 325–334.
Marckereth, F. I. H., J. Heron & J. F. Talling, 1978. Water Analysis: Some Revised Methods for Limnologists. Freshwater Biological Association, London.
Marrone, F., G. Alfonso, L. Naselli-Flores & F. Stoch, 2017. Diversity patterns and biogeography of Diaptomidae (Copepoda, Calanoida) in the Western Palearctic. Hydrobiologia 800: 45–60.
Matsumura-Tundisi, T. & J. G. Tundisi, 2003. Calanoida (Copepoda) species composition changes in the reservoirs of São Paulo State (Brazil in the last twenty years). Hydrobiologia 504: 215–222.
Moss, B., S. Kosten, M. Meerhoff, R. W. Battarbee, E. Jeppesen, N. Mazzeo, K. Havens, G. Lacerot, Z. Liu, et al., 2001. Allied attack: climate change and eutrophication. Inland Waters 1: 101–105.
Nogueira, M. G., P. C. Reis-Oliveira & Y. T. Britto, 2008. Zooplankton assemblages (Copepoda and Cladocera) in a cascade of reservoirs of a large tropical river (SE Brazil). Limnetica 27(1): 151–170.
Oksanen, J., 2018. Multivariate analysis of ecological communities in R: vegan tutorial. https://cran.r-project.org/web/packages/vegan/vegan.pdf. Accessed 26 June 2018.
Panarelli, E. A., S. M. C. Casanova & R. Henry, 2008. The role of resting eggs in the recovery of zooplankton community in a marginal lake of the Paranapanema River (São Paulo, Brazil), after a long drought period. Acta Limnologica Brasiliensia 20: 73–88.
Perbiche-Neves, G. & M. G. Nogueira, 2013. Reservoir design and operation: effects on aquatic biota – a case study of planktonic copepods. Hydrobiologia 707: 187–198.
Perbiche-Neves, G., D. Previattelli, M. Pie, A. Duran, E. Suárez-Morales, G. A. Boxshall, M. G. Nogueira & C. E. F. Rocha, 2014. Historical biogeography of the neotropical Diaptomidae (Crustacea: Copepoda). Frontiers in Zoology 11: 36.
Perbiche-Neves, G., G. A. Boxshall, D. Previattelli, D. A. O. Naliato, M. Pie, C. E. F. Rocha & M. G. Nogueira, 2015. Regulation of the abundance and turnover of copepod species by temperature, turbidity and habitat type in a large river basin. Austral Ecology 40(6): 718–725.
Peres-Neto, P. R., P. Legendre, S. Dray & D. Borcard, 2006. Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87: 2614–2625.
Pinel-Alloul, B., A. André, P. Legendre, J. A. Cardille, K. Patalas & A. Salki, 2013. Large-scale geographic patterns of diversity and community structure of pelagic crustacean zooplankton in Canadian lakes. Global Ecology and Biogeography 22: 784–795.
Portinho, J. L., G. Perbiche-Neves & M. G. Nogueira, 2016. Zooplankton community and tributary effects in free-flowing section downstream a large tropical reservoir. International Review of Hydrobiology 100: 1–9.
R Development Core Team, 2012. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria.
Simões, N. R., A. H. Nunes, J. D. Dias, F. A. Lansac-Tôha, L. F. M. Velho & C. C. Bonecker, 2015. Impact of reservoirs on zooplankton diversity and implications for the conservation of natural aquatic environments. Hydrobiologia 758: 3–17.
Siqueira, T., C. G. L. T. Lacerda & V. S. Saito, 2015. How does landscape modification induce biological homogenization in tropical stream metacommunities? Biotropica 47(4): 509–516.
Stegen, J. C., X. Lin, J. K. Fredrickson, X. Chen, D. W. Kennedy, C. J. Murray, M. L. Rockhold & A. Konopka, 2013. Quantifying community assembly processes and identifying features that impose them. ISME Journal 7(11): 2069–2079.
Stoks, R., A. N. Geerts & L. De Meester, 2014. Evolutionary and plastic responses of freshwater invertebrates to climate change: realized patterns and future potential. Evolutionary Applications 7(1): 42–55.
Strickland, J. D. & T. R. Parsons, 1960. A manual of sea water analysis. Bulletin of Fisheries Research Board of Canada 125: 1–185.
Suárez-Morales, E., 2003. Historical biogeography and distribution of the freshwater calanoid copepods (Crustacea: Copepoda) of the Yucatan Peninsula, Mexico. Journal of Biogeography 30: 1851–1859.
Suárez-Morales, E., J. W. Reid & M. Elías-Gutiérrez, 2005. Diversity and distributional patterns of neotropical freshwater copepods (Calanoida: Diaptomidae). International Review of Hydrobiology 90(1): 71–83.
Talling, J. F. & D. Driver, 1963. Some problems in the estimation of chlorophyll a in phytoplankton. Proceedings of the Conference of Primary Productivity Measurements in Marine and Freshwater. USAEE: 142–146.
Thornton, K. W., 1990. Sedimentary processes. In Thornton, K. W., B. L. Kimmel & F. E. Payne (eds), Reservoir Limnology: Ecological Perspectives. Wiley, New York: 43–70.
Vadadi-Fulop, C., C. Sipkay, G. Meszaros & L. Hufnagel, 2012. Climate change and freshwater zooplankton: what does it boil down to? Aquatic Ecology 46(4): 501–519.
Vanschoenwinkel, B., J. Mergeay, T. Pinceel, A. Waterkeyn, H. Vandewaerde, M. Seaman & L. Brendonck, 2011. Long distance dispersal of zooplankton endemic to isolated mountaintops – an example of an ecological process operating on an evolutionary time scale. PLoS One 6: 1–10.
Vellend, M., 2010. Conceptual synthesis in community ecology. Quaternary Revision Biology 85: 183–206.
Viana, D. S., J. Figuerola, K. Schwenk, M. Manca, A. Hobæk, M. Mjelde, C. D. Preston, R. J. Gornall, J. M. Croft, et al., 2015. Assembly mechanisms determining high species turnover in aquatic communities over regional and continental scales. Ecography 39: 281–288.
Von Ruckert, G. & A. Giani, 2008. Biological interactions in the plankton community of a tropical eutrophic reservoir: is the phytoplankton controlled by zooplankton? Journal of Plankton Research 30(10): 1157–1168.
Wallace, G. T., T. L. Kim & C. J. Neufeld, 2014. Interpopulational variation in the cold tolerance of a broadly distributed marine copepod. Conservation Physiology 2: 1.
Ward, J. V. & J. A. Stanford, 1995. Ecological connectivity in alluvial river ecosystems and its disruption by flow regulation. Regulated Rivers 11: 105–119.
Whittaker, R. H., 1967. Gradient analysis of vegetation. Biological Reviews 42: 207–264.
Wrona, F. J., T. D. Prowse, J. D. Reist, J. E. Hobbie, L. M. Lévesque & W. F. Vincent, 2006. Climate change effects on aquatic biota, ecosystem structure and function. Ambio 35(7): 359–369.
Xiong, W., J. Li, Y. Chen, B. Shan, W. Wang & A. Zhan, 2016. Determinants of community structure of zooplankton in heavily polluted river ecosystems. Scientific Reports 6: 22043.
Zhao, K., K. Song, Q. Wang, L. Da & Q. Wang, 2017. Metacommunity structure of zooplankton in river networks: roles of environmental and spatial factors. Ecological Indicators 73(2017): 96–104.
Acknowledgements
The authors wish to thank the FAPESP for financial support (process numbers 2008/02015-7 and 2011/18358-3 for GPN; 2011/23444-6 for JRDJ; 2009/06149-0 for DAON; and 2009/00014-6 for MGN); J.L. Portinho and S. Casanova for help in fieldwork; and the anonymous referees for their valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Karl E. Havens
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Perbiche-Neves, G., Saito, V.S., Simões, N.R. et al. Distinct responses of Copepoda and Cladocera diversity to climatic, environmental, and geographic filters in the La Plata River basin. Hydrobiologia 826, 113–127 (2019). https://doi.org/10.1007/s10750-018-3722-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-018-3722-9