Abstract
Macroinvertebrate community taxonomic and trait structure showed consistent differences between riffles and pools across 12 headwater streams in the Sierra Nevada (California) even as flows varied from wet to dry years and between seasons. Densities of Ephemeroptera, Plecoptera, Trichoptera, Elmidae, Orthocladiinae and Diamesinae midges, and mites were greater in riffles, whereas Tanypodinae, Chironominae, Sialis, and Pisidium were more abundant in pools. Pools had higher densities but estimated biomass was greater in riffles. Collector-gatherer and micropredator abundances were greater in pools whereas grazers, collector-filterers, and macropredators were more abundant in riffles. Stonefly shredders were most abundant in riffles but some caddis shredders were more abundant in pools. Trait state patterns were related to food resource and physical habitat differences between riffles and pools. Of the distinct pool–riffle differences we found among taxa, only about half conformed to expectations from the literature. Pool and riffle assemblages were most dissimilar at intermediate discharge and converged at low and high flows when one or the other habitat dominated. Bioassessment sampling will need to account for these flow-related differences. Benthic invertebrate communities in these mountain streams clearly differ between pools and riffles, but the relative extent of habitats and biological similarity shift with flow regime.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depositional and erosional areas in streams are ubiquitous, ranging from step-pools in steep headwater streams to meander-pools in streams with moderate slopes to point bar-pools associated with low-gradient rivers, all interspersed with faster-flowing erosional riffle segments (Montgomery & Buffington, 1997). These geomorphic forms play a key role in the movement of water, sediment, solutes, and particulate matter down streams; form discrete local flow, storage, productivity, and resource turnover process domains (sensu Montgomery, 1999); and provide a habitat template that serves to both organize, and be influenced by, stream communities (Townsend, 1989; Winemiller et al., 2010). Despite the importance of this habitat template, few studies have examined how the taxonomic and trait structure of benthic communities and indices of environmental tolerance differ between pools and riffles and vary with flow, or have compared data to classification schemes assigning taxa to depositional versus erosional habitat categories.
The most comprehensive studies on differences in stream invertebrate communities between riffles and pools (Logan & Brooker, 1983; Brown & Brussock 1991) have reported that total densities were often greater in riffles than pools, that pool–riffle differences in species richness were inconsistent across streams, and that habitat differences can be greater than longitudinal continuum transitions in invertebrate community structure (Brussock & Brown, 1991). Community differences between pools and riffles, however, may depend on encompassing environmental conditions, such as habitat-specific responses to varied hydraulic conditions (Statzner & Higler, 1986), as well as to the impacts or legacies of watershed disturbance and shifting channel form (Frissell et al., 1986; Stanley et al., 1997). Our goal was to evaluate the spatial coverage, physical, and invertebrate community differences of pool and riffle habitats over changing discharge conditions (Poff & Ward, 1990). An associated objective was to compare our results to published schemes that assign stream invertebrate taxa to erosional versus depositional habitats, including those presented in Poff et al. (2006) and Merritt et al. (2008) (Online Resource 1).
Owing to greater depths and slower currents, pools can be more difficult to sample than riffles, so many sampling designs have concentrated on riffles. As a consequence, few papers include separate pool and riffle data or comprehensively contrast invertebrate assemblage structure in these two habitats or their responses to perturbations (Carter & Fend, 2001). Although many bioassessment programs have developed standard sampling protocols that amalgamate pool and riffle samples, proportional to the relative lengths of these habitats through reaches (USEPA, 2013; Mazor et al., 2016), such approaches can confound changes in invertebrate community structure within pool and riffle habitats with changes in the relative contribution of pool and riffle habitat lengths or areas to reaches. It is therefore important to distinguish the roles of changes in relative habitat area from changes in invertebrate communities within habitats in determining reachwide alterations in invertebrate community structure. In addition, another role of biomonitoring is to determine the biodiversity and species composition of a given stream system, but patterns in species composition and diversity cannot be established without sampling the biota of these major habitats. How changes in flow and pool–riffle geomorphology affect the structure and function of benthic communities also will contribute to an improved understanding of the patch dynamics of streams (Townsend & Hildrew, 1994). Finally, pool and riffle habitats are linked, with production or organic matter processing in one habitat affecting consumers in the other habitat (Finlay et al., 2002).
Our hypotheses were derived from considerations of the expected effects of differing physical and food resource conditions in pools versus riffles on invertebrate traits (Online Resource 2), including responses of functional feeding groups to food resource distributions, of behavioral habit groups and inter-related voltinism, development, and body size groups to substratum type, of rheophily groups to current velocity, and of thermal and stress tolerance indices to variables affecting water volume turnover rates (e.g., depth and current velocity). We expected that slower current velocities and greater depths in pools than riffles would result in higher temperatures, lower dissolved oxygen, and the accumulation of organic matter and fine sediment in pools, and in cooler temperatures, higher water quality, and greater amounts of coarse substrata and algae in riffles. We predicted that taxa associated with pools would be deposit-feeding collector-gatherers with small body sizes, short life cycles, burrowing habits, and a tolerance for lower environmental quality. We hypothesized that riffles would be dominated by grazers and filterers that inhabit rock surfaces with higher current velocities for feeding and attachment, as well as by taxa with larger body sizes, clinging habits, mixed life histories, and a high sensitivity to degraded environmental conditions (see list of specific hypotheses in Online Resource 2).
We further used the depositional, erosional, or mixed habitat use designations for specific taxa in Poff et al. (2006) and Merritt et al. (2008) as predictions of habitat associations for the taxa we collected, then tested these predictions by statistically comparing the relative and absolute abundances of commonly collected taxa in pools versus riffles (see designations and tests in Online Resource 1). Given our expectations that erosional processes would dominate at high flows and depositional processes at low flows (cf. Hjulstrom diagram, Gordon et al., 2004), we further hypothesized that pool and riffle communities would become more similar during times of high and low discharge when scour and deposition, respectively, dominated the physical environments of both subhabitats, making them less distinct, with greatest pool–riffle community differences at intermediate discharges, when environmental conditions should show the greatest pool–riffle discontinuities. Finally, we predicted that the relative areas of pools versus riffles would increase with decreasing discharge, owing to the contraction of shallow riffle zones at low flows, resulting in dominance by pool taxa at low flows and by riffle taxa at high flows.
Materials and methods
Study area
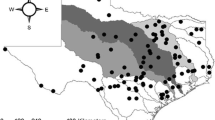
The study area encompasses the drainages of 3 sub-basins (Bull, Teakettle, Providence) within the Kings River drainage basin on the western slopes of the Sierra Nevada of California, spanning the rain–snow transition zone with elevations varying from 1400 to 2200 m (Table 1). Ten of the 12 stream study sites are within the Kings River Experimental Watersheds (KREW) area, a long-term watershed research area established and run by the Pacific Southwest Research Station, US Forest Service (USFS), and the other two (Teakettle T002 and T02A) are in the USFS’s Teakettle Experimental Forest (Fig. 1). These watersheds have little development and few roads, so their drainage streams conform to reference quality conditions for California (Ode et al., 2016). Annual precipitation is approximately 50 cm, and air temperatures range from sub-freezing in winter to averages of 15–20°C in the summer (Hunsaker et al., 2012). Landforms are dominated by granite and soils are coarse (Johnson et al., 2011). Study sites encompassed ten first- and second-order streams and two additional 3rd order study sites, downstream of the confluences of the headwater tributaries draining the Bull and Providence sub-basins (Fig. 1). Study reaches of the 10 headwater streams were located at the lower ends of their catchments, and the confluence reaches were located within 500 m below where contributing tributaries merged. Sampled reach lengths were all 100 m and gradients ranged from 6 to 13%, being representative of alluvial gravel-bed streams with some pools formed by woody debris. During the study period, hydrological conditions varied from bankfull spring floods in wet years to spatially intermittent flows for several smaller streams during drought. The study was conducted across spring runoff periods that covered a range of average to heavy snowmelt flows to low flows during drought (high flows: June 2005 and 2006; average flows: June 2002–2004; drought flows: June 2013 and 2015), as well as in falls during base flow (September 2002 and 2005). Following the conventions of USGS WaterWatch (https://waterwatch.usgs.gov), 2002–2004 were defined as average years (25th–75th percentiles of long-term flow), 2005–2006 as wet years (> 75th percentile), and 2013 and 2015 as dry years (< 25th percentile) for Sierra Nevada streams.
Map showing the location of study sites in Sierra National Forest, California, USA. Tributary drainage networks are shown only for the study watersheds, whereas only larger streams are shown outside the study basins. Inset shows the location of the Kings River Experimental Watersheds (KREW) in Sierra National Forest within California
Habitat surveys and macroinvertebrate sampling
Stream physical habitat
We conducted surveys of stream channel and bank features at each study site on each sampling date. The 100-m reach at each site was first delineated into riffle, pool, and transitional zone segments, with riffles defined as swift flows over relatively shallow, steeper segments with turbulent surfaces, pools defined as deeper, wider segments with slower currents, flat surfaces, and fine sediment deposits, and transitional zones as segments with intermediate characteristics (Table 2). These subhabitat types were delineated in the field using observations of width:depth ratio, current velocity, breaks in channel slope, and the occurrence of bends, obstructions, emergent rocks, and point bars. Steps also occurred in these streams as abrupt short falls flowing into or out of pools, usually at the end or start of a riffle. At ten cross-stream transects at 10-m intervals along each study reach, starting at the bottom and moving upstream, we measured wetted width, bank angle, and percent canopy cover, the latter with a concave densiometer (after US EPA habitat protocols, Kaufman et al., 1999). At five equal-spaced points along each cross-stream transect within the wetted channel, we also measured depth, current velocity at mid-depth (Global Water flow probe FP1111), substrata size classes (fines < 0.1 mm, sand 0.1–2 mm, gravel 2–65 mm, cobble 65–254 mm, boulder > 254 mm), and cover, where present at the intercept point, of macroalgae, detritus (fine particulate organic matter), leaves, wood, or moss. We also measured conductivity, pH, and water temperature at the upstream boundary of each study reach during each sample event using an Oakton pH Con10 meter, and slopes along the reach using a clinometer at 25-m intervals. We calculated discharge as the sum of the product of average depth x current velocity x width/5 over all points for each transect and averaged these products over the 10 reach transects.
Benthic macroinvertebrates
We sampled invertebrates at the 12 sites on 9 occasions, during or just after spring runoff (June) in 2002–2006, 2013, and 2015 and during fall base flows in 2002 and 2005 (late September). We collected samples of benthic stream invertebrates using a D-frame net (30 cm wide, 250 µm mesh), because this method allowed flexibility in where samples with variable substratum characteristics in pools and riffles could be taken and because it is a standard, replicable technique widely used by researchers and agencies (Carter & Resh, 2001; US EPA, 2013). Riffle samples consisted of the combined net contents from nine riffle locations (total sampled area = 0.84 m2) and the pool samples consisted of the net collections from three separate pool locations (0.28 m2), both distributed over the study reach. We took samples by placing the net against bottom substrata, then turning and brushing all substrata by hand in the 30 cm × 30 cm area immediately above the net for ca. 60 s, with dislodged invertebrates being carried by currents into the net. In a few cases, pool currents were weak, so we swept dislodged material into the net by hand. After large rocks and wood were cleaned and removed from D-net samples, we elutriated invertebrates from substrata by repeatedly mixing samples in a bucket, then pouring lighter sample fractions through a small 100-µm mesh net, and then hand-picked heavier organisms (e.g., shelled mollusks and cased caddisflies) from sand and gravel in shallow inspection pans. Invertebrate samples were then placed in 500-mL containers and preserved in denatured 90% ethanol.
We subsampled each composite sample in the laboratory using a rotating-drum sample splitter to successively divide the sample into equal fractions to achieve target counts of 500 individuals (in practice averaging over 600, minimum 404), which were sorted, identified, and counted. Inspection of sample remnants sometimes added single-counts of large or rare taxa that were not otherwise represented in the sample split. We identified most invertebrates to genus or species with the exceptions of Capniidae (except Eucapnopsis), oligochaetes (segmented worms), Turbellaria (flatworms), and ostracods (seed shrimp). Ambiguous taxa in early development stages were associated with mature specimens of the closest genus in the same sample. We converted invertebrate densities to biomass using median sizes for each taxon (Online Resource 1 size trait) and Benke et al. (1999) length–weight regression equations to obtain an individual biomass estimate for each taxon, which was multiplied by the density of that taxon at a site and time, then summed over all taxa to obtain a total biomass estimate for each site and time.
Data analysis
We examined substrata, cover, velocity, depth, and size measures between pool and riffle habitats over all sites and times using the data associated with cross-stream transects that intersected these habitats (Table 2). We compared average riffle and pool habitat variable values over time among the 12 stream reaches using one or two-tailed paired t tests, depending on initial predictions (pool > riffle or riffle > pool examined with one-tailed tests, pool = riffle with two-tailed tests), with Benjamini–Hochberg-corrections (BH) for comparisonwise error (Benjamini & Hochberg, 1995; false discovery rate, FDR = 0.05 in all uses). We also examined relationships between pool and riffle habitat areas, environmental variables, or community structure and flow (field-measured discharge).
We examined differences in invertebrate taxonomic and trait structure between riffles and pools using non-metric multidimensional scaling (NMS). Invertebrate taxa were assigned to states for 6 different traits for voltinism, development rate, body size, thermal preference (cold stenotherms or eurytherms), behavioral habit, and rheophily, using Vieira et al. (2006), Poff et al. (2006), and Barbour et al. (1999) (see Online Resource 1), resulting in 77 unique combinations of states across the 6 trait groups for the invertebrates that were collected. The basic data consisted of the relative abundances of all taxa or all trait combinations from pools and riffles across all sites and times. Multivariate distances between all pairs of sample sites-times were calculated using the Sørensen distance metric. NMS was used to display the similarity of invertebrate communities or trait structure across sites-times grouped by habitat type along with significant correlations (Pearson’s r, P < 0.00001) between NMS axes and the relative abundances of invertebrate taxa or trait state combinations and transformed (log10 for measurement data, logit for proportions) values for habitat variables. We also performed the multi-response permutation procedure (MRPP) to test the null hypothesis of no difference in inter-habitat multivariate distances. Indicator species analysis (ISA) was used to determine which taxa or trait combinations were associated with habitat type, and contingency table analyses were used to identify the specific traits that best distinguished between pools and riffles. In addition, depositional, erosional, and mixed habitat designations for taxa from Poff et al. (2006) and Merritt et al. (2008) were compared to our tests of riffle versus pool density differences for common taxa (those occurring in at least 5 of the 12 sites).
The log10 (x + 1)-transformed densities and logit-transformed relative abundances of invertebrates belonging to different taxonomic, functional feeding, and trait groups, as well as biotic and thermal indices, also were compared between pools and riffles using one- or two-tailed paired t-tests, depending on initial hypotheses (pools > or < riffles = one-tailed, pool = riffle = two-tailed), with BH corrections (Figs. 3, 4, Online Resource 1 and 2). These tests were conducted on paired pool versus riffle values for each site (n = 12 sites), with values for each site averaged over the 9 sampling times. Biotic and thermal indices for invertebrate communities for each site-time were derived from the summed products of tolerance values and field-based temperature associations for taxa (i.e., CD75 of Yuan, 2006), respectively, weighted by their relative abundances in each sample. The biotic index (0–10) indicates increasing tolerance to degraded water or habitat quality, whereas the thermal index provides a composite temperature tolerance for the community (in °C), scaling from lower to higher temperature tolerances. Relationships between the densities of invertebrates with different traits and postulated environmental drivers were examined using Pearson’s correlational analyses.
We tested the hypothesis that the relative representation of pool versus riffle areas would decrease with increasing discharge, as well as the hypotheses that a key environmental variable (current velocity, see Results) and invertebrate communities would become more similar in pools versus riffles during times of high and low discharge and would diverge at intermediate discharge, using ANCOVAs and regression analyses. In ANCOVAs, pool–riffle differences in areas, log-transformed current velocities, or NMS 1 scores (an index of pool versus riffle community structure, see Results) were the dependent variables, site was an independent class variable, and log-transformed discharge was the continuous covariate, with a site X log discharge interaction effect. ANCOVAs were followed by linear and quadratic regression analyses when interaction effects proved to be non-significant. These analyses were conducted on only spring (June) data to avoid the confounding influences of seasonality.
Results
Comparing pools and riffles
As expected, pools were deeper, wider, and contained more detritus, leaves, woody debris, and fine/sand sediments than riffles, whereas riffles had higher velocities and contained more gravel and cobble substrata, and moss (Table 2). Algae cover and average unit length did not differ between pools and riffles.
We identified 290 invertebrate taxa belonging to 77 unique trait state combinations in the 214 samples collected during this study (Online Resource 1). NMS analyses on the relative abundances of all invertebrate taxa and of all trait state combinations in pools and riffles across all sites and times produced three axes in both cases, accounting for a cumulative 80% (taxa) and 85% (trait combinations) of the variation in the multivariate datasets (stress = 15.2 and 14.5, respectively). NMS axis 1 (NMS 1) from both the taxonomic and trait analyses, accounting for 43% (taxonomic) and 54% (trait) of the variation in the multivariate datasets, clearly partitioned invertebrate assemblages into pool and riffle habitat types (Fig. 2a, b). In the taxonomic analysis, twenty taxa, including 11 EPT taxa (mayflies, stoneflies, caddisflies), 4 Chironomidae (3 Orthocladiinae and one Tanytarsini (Rheotanytarsus)), 2 elmid beetles, 2 water mites, and 1 other dipteran were strongly positively associated (r > 0.40, P < 0.00001, n = 214) with NMS 1, and hence riffles, whereas 4 chironomid taxa (3 Tanytarsini and 1 Tanypodinae) were strongly negatively related (r < − 0.40, P < 0.00001) to NMS 1 and, hence, associated with pools (Fig. 2a). Contingency table analysis on the distribution of states within each trait group for those trait state combinations shown to be indicators of pools versus riffles (ISA) suggested that the erosional-depositional and behavioral habit trait groups showed the greatest differences between pools and riffles, with the relative abundances of erosional, mixed habitat, and clinger trait states being greater in riffles and those of depositional and burrowing states being greater in pools (Χ2, P depositional/erosional = 13.1, 0.0014; habit = 13.8, 0.008). There were very strong multivariate distance differences between pool and riffle habitats across all sites-times (see MRPP results in Fig. 2) and NMS 1 scores from both the taxonomic and trait analyses were positively related to current velocity (r’s = + 0.48 and + 0.45 for taxonomic and trait analyses, respectively, P’s < 0.0001).
Ordination plots of non-metric multidimensional scaling analyses (NMS) on the relative abundances of benthic macroinvertebrate taxa (a) and of trait state combinations (b) in pools (black triangles) and riffles (open circles) across sites and times (n = 214). The % variation in the multivariate data set attributable to each NMS axis is shown next to each axis label. The correlation coefficients (Pearson’s r) of common taxa (occurring in ≥ ½ of samples, a) and trait state combinations (b) significantly related (r > |0.40|, P < 0.00001) to each axis are shown in the margins. Stress for each NMS and multiple response permutation procedure (MRPP) results for multivariate pool versus riffle differences (t, A, and P values) are also shown. Codes for trait state combinations (b) are shown in Table 3
Trait groups also had strong phylogenetic foundations with the majority (ca. 2/3) of observed trait state combinations being represented by single or closely related taxa. Among the 19 trait state combinations that were highly significantly (r > |0.40|, P < 0.0001) correlated with both trait NMS axes (Fig. 2b), 12 were represented by single taxa or closely related taxa within a family (Table 3). The trait state combination (211242) that showed the highest positive correlation (r = + 0.69) with trait NMS 1 scores, and hence riffles, was comprised primarily of orthoclad midges and mites. The remaining trait state combinations positively related to NMS 1 and riffles were represented by single or combinations of EPT, Elmidae, Diptera (specifically Empididae, Tipulidae, Diamesinae), and Turbellaria taxa, which had annual or longer life cycles, mixed development rates and sizes, with both cold and eurythermal temperature preferences, erosional habitat affinities, and clinger and swimmer behaviors. Taxa negatively related to NMS 1, and associated with pools, were represented by the Ceratopogonidae, Tanypodinae, Chironomini, and Tanytarsini, which had short generation times, rapid development, small body sizes, mostly eurythermal temperature tolerances, and burrowing, climbing, and sprawling behaviors. Taxonomic and trait combination NMS 1 scores were correlated with each other (r = + 0.98, P < 0.0000001) and with the same set of taxa, emphasizing the close correspondence between the trait and taxonomic analyses. Trait combination NMS 2 scores were related to pool-dwelling midges, being positively related to Stempellinella and Stempellina, which reached highest relative abundances in high flow years and fall, and negatively related to Micropsectra and Macropelopia, which dominated at low flows.
Pools and riffles also clearly differed in the densities and relative abundances of different taxonomic groups (Fig. 3, Online Resource 1). The overall density of benthic invertebrates was greatest in pools, dominated by chironomids (74% of total invertebrates in pools, 38% in riffles), but also including mollusks (primarily Pisidium), odonates, and Sialis, whereas the densities of EPT taxa, mites, and elmid beetle larvae (comprising most Coleoptera) were significantly greater in riffles (P < 0.05, results of paired t-tests with BH corrections indicated in Fig. 3). Corydalidae were collected exclusively in riffles.
Mean densities (± 1 SE) of (a) major macroinvertebrate taxonomic groups (insect orders or families, higher levels for non-insects) and (b) total invertebrates and chironomids in pools (P, black bars) versus riffles (R, white bars). Asterisks indicate statistically significant differences in time-averaged densities across sites between pools and riffles (paired t tests with Benjamini–Hochberg (BH) corrections, false detection rate = 0.05, n = 12 for each comparison and BH corrections for 16 comparisons (not all shown)): *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001)
Among functional feeding groups, the densities and relative abundances of collector-gatherers were higher in pools than riffles, whereas the densities and relative abundances of filterers, grazers, macropredators, and shredders were higher in riffles (Fig. 4, Online Resource 2). Additionally, micropredator densities were higher in pools than riffles. Although overall shredder densities (e.g., Nemouridae, Peltoperlidae, Leuctridae) were higher in riffles than pools, some cased caddis shredders were restricted to, or more abundant in, pools (e.g., Psychoglypha, Heteroplectron, Yphria and Lepidostoma; Online Resource 1). Among trait groups, the relative abundances and densities of small, depositional, multivoltine, fast seasonal, burrowing, climbing, sprawling, moderate to high tolerance, and eurythermal taxa were significantly greater in pools than riffles, whereas the relative abundances and densities of taxa with medium to large body sizes, semivoltine life histories, erosional habitat affinities, slow seasonal and non-seasonal development cycles, clinger and swimmer behaviors, high sensitivity, and cold stenothermal preferences were significantly more abundant in riffles (P < 0.05, paired t tests with BH corrections, Fig. 4, Online Resource 2). In addition, the relative abundances of univoltine and mixed habitat taxa were greater in riffles than pools, whereas biotic tolerance and thermal index scores were greater in pools than riffles (Fig. 5). Although total invertebrate densities were greater in pools than riffles, invertebrate biomass was greater in riffles owing to the predominance of larger EPT taxa (dry biomass = 31.2 mg/m2 in riffles and 12.9 mg/m2 in pools).
Mean values of the (a) biotic and (b) thermal indices (± 1 SE) in pools (P) and riffles (R), showing stress and temperature tolerance (mean tolerance value and mean °C), respectively. Time-averaged pool and riffle values for both indices across sites were significantly different (paired t tests with Benjamini–Hochberg corrections, P < 0.001)
Our hypotheses regarding the pool versus riffle affinities of different trait groups were based on the probable responses of taxa with different traits to key food resource or habitat conditions in pools versus riffles. As expected, collector-gatherer densities were related to fine detritus levels, filterer densities to current velocities, and predator densities to the densities of their prey (non-predatory chironomids for micropredators, Ephemeroptera for macropredators, which were primarily predatory stoneflies); however, there was no significant relation of grazer densities and coverage by algae (Online Resource 2). Contrary to expectation, shredder densities were negatively related to leaf litter coverage, but this was because the most abundant shredders were stoneflies that preferred riffles, whereas leaf litter coverage was higher in pools (Table 2). There were no significant relationships between shredder densities and leaf litter coverage when pools and riffles were analyzed separately. Voltinism, development, and size traits, which were inter-related, as well as behavioral habit traits, were often correlated, although sometimes weakly, with substrata variables, whereas thermal and tolerance traits, as well as thermal and biotic indices, were correlated with depth and/or current velocity (Online Resource 2). Densities of the depositional trait group were negatively correlated, and densities of the erosional trait group were positively correlated, with current velocity.
Comparing taxa habitat affinities with published designations
About half (48 and 50%) of the associations of taxa with pool, riffle, and mixed habitats conformed to habitat designations indicated by Poff et al. (2006) and Merritt et al. (2008), but patterns in inconsistencies between our results and literature designations differed between these two schemes (Online Resource 1). Approximately 82% of the discrepancies between our results and the Poff et al. (2006) designations were owing to the assignment of taxa for which we found significant habitat differences to the mixed habitat trait state, with another 13% showing the opposite pattern. In comparisons of discrepancies between our results and the Merritt et al. (2008) designations, we found that 42% of taxa were more abundant in pools or riffles when the classification scheme designated them as mixed habitat taxa and that 37% of taxa showed no significant pool–riffle differences although they were listed as erosional or depositional taxa. Our results also indicated the opposite habitat associations for 6 and 20% of taxa listed in Poff et al. (2006) and Merritt et al. (2008), respectively. Four taxa, including two trichopterans (Psychloglypha, Polycentropus) and two chironomids (Boreochlus and Heleniella), were more abundant in pools than riffles even though they were designated as riffle taxa in Poff et al. (2006) and one taxon (Pericoma) showed the opposite pattern. Fifteen taxa, including 2 EPT, 13 chironomid, and 1 other dipteran taxa, showed results contrary to the designations in Merritt et al. (2008), with the 2 EPT taxa (Edmundsius agilis Day 1953, Polycentropus) and 13 chironomid taxa being more abundant in pools than riffles. The dipteran Pericoma was more abundant in riffles than pools but had been assigned to the pool category in Merritt et al. (2008). Water mites have typically been assigned to mixed habitat categories (Vieira et al., 2006), but we found that 12 mite taxa showed preferences as significantly greater abundances in either pools or riffles.
Riffle-pool invertebrate differences with discharge
ANCOVAs showed no significant effects of the site X log discharge interaction effect on pool–riffle differences in habitat area, log current velocity, or taxonomic NMS 1 score, and no significant main site effect on pool–riffle differences in log current velocity or NMS 1 score. There was a significant main effect of site (F11,59 = 2.3, P < 0.05) on pool–riffle area differences, owing to greater differences at confluence than headwater sites, and a highly significant relationship of these differences with log discharge (F1,59 = 14.3, P < 0.0005) (Fig. 6a). Stream reaches were dominated by riffle habitat during high discharge periods, were a more even mixture of pool and riffle habitats at intermediate flows, and were dominated by pools at the lowest discharge (Fig. 6a). Linear regressions of pool–riffle differences in log current velocity and NMS 1 scores versus log discharge were not significant, but the addition of a quadratic term revealed significant curvilinear relationships in both cases (p ca. 0.016 for log current velocity and < 0.0001 for NMS 1 scores, Fig. 6 b, c). Pool minus riffle differences in log current velocity and NMS 1 scores showed a U-shaped relationship with discharge, indicating that current-related environmental conditions and invertebrate assemblage structure in riffles and pools were most similar at the lowest and highest flows and were most different at intermediate flows (Fig. 6b, c, note negative values on y-axis). Although pool–riffle differences in NMS 1 scores were generally large and consistent over time, NMS 1 scores in riffles tended to be lowest in the dry spring of 2015 and in pools highest in the wet springs of 2005–2006. The relative abundances of taxa assigned to the depositional trait by Poff et al. (2006) were significantly higher in riffles during dry springs and fall than wet or average springs and significantly higher in fall than any springs in pools (P < 0.05, paired t tests, BH corrections). Erosional taxa tended to have higher relative abundances in wet springs than other time periods in both pools and riffles, but differences were not significant and erosional taxa were always rare in pools (< 4% relative abundances).
Spring pool—riffle differences in (a) average pool area and average riffle area (in m2), (b) log10 (current velocity), and (c) taxonomic NMS axis 1 scores paired by sites-times versus discharge (in m3/s, log10 scale). The fitted least squares lines for linear (a) and quadratic (b, c) regressions are shown, along with associated regression statistics (equations, coefficients of determination, overall F and P values). The quadratic term in the regression equations was significant at P < 0.01 for b and < 0.0001 for c. Note that x-axis increments are on a log scale and that y-axis values for (b) and (c) are largely negative, indicating degree of dissimilarity
Discussion
Environmental conditions and invertebrate assemblages in riffles versus pools
Riffle and pool differences in width:depth ratios, current velocities, substrata composition, and detritus levels in our mountainous gravel-bed streams conformed to widely reported differences between these subhabitats in streams throughout the world; however, pool–riffle abiotic differences were reduced during low flows when depositional forces dominated and at high flows when erosional processes dominated (Leopold et al., 1964). Riffle-pool sequences can be created by flow reversals, when pool velocities exceed riffle velocities at the highest flows (Keller, 1971) or where upstream constrictions produce converging flows that scour pools (MacWilliams et al., 2006). In the context of the river continuum model, riffle and pool habitats should be most distinctive in mid-elevation reaches, the domain encompassed by our study, providing a model for examining how these habitat units modify benthic community structure and function (Brussock et al., 1985).
Regardless of the geomorphic processes creating pool and riffle sequences, we found that pools and riffles differed consistently in their environmental characteristics, with pools being deeper, having lower current velocities, and containing more fine sediment and allochthonous organic matter than riffles. These habitat differences constituted a dominant environmental template for invertebrate communities, accounting for the greatest amount of multivariate variation (43–54%), compared to other sources of temporal or spatial variation (e.g., hydrological regime, season, stream order, subbasin), in the relative abundances of 290 taxa or 77 trait state combinations across 214 samples from 12 sites over 9 times. In general, riffle and pool invertebrate communities showed striking differences in densities, biomass, composition, and traits. Previous studies have shown that total invertebrate density or biomass is often greater in riffles than pools (reviewed by Logan & Brooker, 1983), with Diptera most abundant in pools and Ephemeroptera in riffles. We found, however, that overall invertebrate densities, dominated by Chironomidae (particularly Tanytarsini), were consistently higher in pools than riffles. Our use of a finer mesh net than used in other studies (250 µM vs > 400 µM in most other studies) may account for the higher densities of midges recorded in our study. Congruent with previous studies, we found that total invertebrate biomass was higher in riffles than pools because larger EPT taxa dominated in riffles with coarse substrata.
The community composition, density, diversity, and trait and trophic structure of invertebrate communities in riffles versus pools may be influenced by differences in food resource availability (Huryn & Wallace, 1987), substratum type (Brown & Brussock, 1991), and physical disturbance (Roy et al., 2003) between these habitats. High inputs and the accumulation of coarse and fine benthic organic matter in pools of the small forested streams of the Sierra may account for the high densities of invertebrates, especially small collector-gatherer taxa (Chironomidae), that we observed.
Pool and riffle differences in invertebrate community structure were prominent across all sites and times, despite large changes in environmental conditions over time. Most (85%) of the common invertebrate taxa examined were consistently more abundant in one habitat than another even as flows varied greatly over seasons and from average to wet to dry years (Fig. 2). These distinct pool–riffle differences in stream invertebrate community composition agree with the findings of other studies (Bonada et al., 2006; Bogan & Lytle, 2007; O’Dowd & Chin, 2016), indicating that habitat differences have a great influence on the organization and function of stream benthic communities (Poole, 2002).
The responses of invertebrates with different traits to differences in food resource and physical conditions between pools and riffles mostly matched our predictions (Online Resource 2). Concordant with the results of O’Dowd & Chin’s (2016) study of streams in the Smith River Basin in northern California, we found that the abundances of stoneflies and clinger taxa dominated in step riffles, whereas sprawlers dominated in pools. Similarly, we found that filterers dominated in riffles and gatherers in pools, agreeing with Bogan & Lytle’s (2007) results for Arizona streams. Some of our results, however, contradicted our hypotheses. Although the results for gatherers (pools), filterers (riffles), and grazers (riffles) were consistent with our hypotheses, those for shredders were not. Although leaves and wood were concentrated in pools, different shredder taxa showed different pool versus riffle associations, with higher densities of large caddis shredders in pools and of abundant stonefly shredders (Peltoperlidae, Nemouridae, Leuctridae) in riffles. Some stonefly taxa classified as shredders consume substantial amounts of algae, so their abundances may not depend on allochthonous detritus alone (Rosi-Marshall et al., 2016). Further, grazer and shredder densities were not positively correlated with algal and leaf litter cover, respectively. These results may be owing to resource cover not adequately reflecting resource abundance or availability, or to grazers and shredders both tracking and depressing their resources, which may result in no net relationship between consumers and their resources (Cooper & Dudley, 1988).
Although we were uncertain about our predictions for micropredators and predators, micropredators (primarily Tanypodinae) had higher densities in pools and were positively related to non-predatory chironomid densities, and macropredators (primarily predatory stoneflies) were denser in riffles and were positively correlated with mayfly densities (Cooper et al., 2015). In both cases, however, it was not clear if predators were tracking their presumed prey or if they were responding to abiotic conditions in the same way as their prey.
Comparisons to published pool versus riffle designations
There were substantial discrepancies between our observations and expectations of habitat association based on the literature, with only about half of common taxa conforming to literature designations. As expected, we found that EPT taxa, Corydalidae, and elmid beetles had higher densities in riffles and that most non-insects, Sialis, and odonates were more abundant in pools; however, there was variation in the pool versus riffle affinities across taxa within some of these groups. For example, the baetid mayfly Baetis was consistently, significantly more abundant in riffles than pools, but the baetid mayfly Centroptilum showed the opposite pattern (Online Resource 1). Although we expected many chironomid subfamilies and tribes to be found at similar densities in pools and riffles (Merritt et al., 2008), we found pool–riffle differences in the abundances of different chironomid groups, with Orthocladiinae and Diamesinae being more abundant in riffles and Tanypodinae, Chironomini, and Tanytarsini being more abundant in pools. Among the Tanytarsini, many of our most abundant genera (e.g., Stempellinella, Micropsectra, Tanytarsus) had higher densities in pools, but Rheotanytarsus was more abundant in riffles. Although Merritt et al. (2008) designated the Prodiamesinae (mostly Monodiamesa) as a riffle group, we found that this chironomid subfamily was found almost exclusively in pools. Among non-insects, the dominant mollusk, Pisidium, was more abundant in pools than riffles as expected, the pool versus riffle affinities of ostracods varied over time, there were no pool–riffle differences in oligochaete abundances, and mites, as a group, were more abundant in riffles than pools, although there was considerable variation among mite genera.
Differences between our quantitative comparisons of taxa densities between pools and riffles and published pool–riffle designations may owe, in part, to subjective trait assignments made by experts based on their personal experience (Cummins et al., 2008). The major source of differences between the Poff et al. (2006) scheme versus our results and the Merritt et al. (2008) scheme is that Poff et al. (2006) designated many (71%) of the taxa we collected to the mixed habitat trait state, whereas Merritt et al. (2008) and our results indicated that only 36–37% of these taxa should be assigned to the mixed habitat trait. These considerations emphasize the arbitrary assignment of traits to some taxa, such as the depositional, erosional, and mixed habitat traits, because the criteria used for these assignments are unclear and could range anywhere from the presence versus absence of a taxon in pools or riffles, to statistically significant pool–riffle differences in the densities or relative abundances of that taxon, to average differences in that taxon’s abundance between pools and riffles. In general, then, the criteria for assigning traits should be explicitly described and designations should be based, whenever possible, on quantitative analyses of differences in habitat conditions. In the case of depositional-erosional designations, analyses have been inhibited, as outlined above, by the dearth of pool data, precluding pool–riffle comparisons.
Our conclusions regarding the pool versus riffle affinities of different taxa were based on statistical analyses of pool–riffle differences in their densities or relative abundances; however, statistical results are also arbitrary because they depend on both real abundance differences between pools and riffles and the power of statistical tests used to detect these differences. For our statistical analyses on common taxa (i.e., those with at least 5 pool–riffle pairs of time-averaged non-zero abundance data across the 12 sites), we found that the densities of 27% of the taxa with 10–12 site pairs of data were not significantly different between pools and riffles, whereas 54% of taxa with 5–9 site pairs of data were not significantly different. Because statistical power depends on both the central tendency and associated precision of estimates, and on sample size, variable results across taxa and times could be owing to variation in test power. The prescriptions for controlling for this variability include restricting analyses to abundant and frequently collected taxa and insuring that sampling is adequate to attain reliable test power.
Even beyond considerations of defining the criteria and examining the power of tests to distinguish pool and riffle taxa, it is apparent that our knowledge of the habitat affinities of some groups are limited. For example, although Vieira et al. (2006) listed most mites in mixed habitat associations, we found that 12 mite taxa showed clear pool versus riffle affinities, contributing to our knowledge of the habitat associations of this group. Similarly, 13 of the opposite discrepancies between our results and the Merritt et al. (2008) scheme were for Chironomidae taxa and a perusal of the Merritt et al. (2008) references for chironomid designations indicated that assignments were often based on considerations of general group assignments, rather than on quantitative information on habitat associations for different chironomid genera or species. Among other taxa, Merritt et al. (2008) assigned the siphlonurid mayfly Edmundsius agilis to the riffle designation, but we found that it was almost totally relegated to pools. This large mayfly was poorly known to science after its discovery in the early 1950s, but recent investigations have delineated the distribution of this Sierran endemic, showing its affinity for pool habitats and its inability to co-exist with introduced fish (Silldorff, 2003; Herbst et al., 2009). This exemplifies the need for more quantitative comparisons between pools and riffles for less well-known taxa of conservation concern, and for species-level resolution to improve databases on the habitat associations and trait assignments of many other taxa. A few well-known taxa, such as Polycentropus and Psychoglypha, were assigned to erosional habitats by Poff et al. (2006) and/or Merritt et al. (2008), but we found them to be more abundant in pools. Although the reasons for these discrepancies are unclear, it is possible that different species within each of these genera show different habitat affinities. In addition, Polycentropus caddis weave large, loose nets that would be unstable in riffles and Psychoglypha is a large shredder that builds unwieldy cases of leaves and wood. Although the dipteran Pericoma was designated as a depositional taxon in Poff et al. (2006) and Merritt et al. (2008), we found it to be more abundant in riffles. This genus is commonly found in splash zones at the edges of streams (Hilsenhoff, 1991), which we found were generally associated with fast-flowing riffles rather than pool habitats.
We acknowledge that some of the differences in our data versus literature on invertebrate habitat affinities may arise from the restricted geographic extent of our study, but argue that our results are at least applicable to mountain streams of western North America. In addition, although general traits may be shared by disparate taxa, allowing comparisons of trait composition across different regions and biotic communities, we obtained similar results from analyses of pool–riffle differences based on traits versus taxonomic groups, suggesting that trait and taxonomic analyses, in some contexts, may be redundant. In general and as outlined above, advances in trait analyses would benefit from: clear criteria for assigning traits to taxa; species-level analysis of quantitative data on habitat associations, environmental tolerances, and food habits; concentration on taxa with adequate sample sizes and abundances for statistical analyses; and investigations of trait combination (linked trait) responses to ecological variation (also see Chevenet et al., 1994; Statzner & Bêche, 2010; Boersma et al. 2016).
Flow dependence of invertebrate community similarity between pools and riffles
Although the majority of the collected taxa showed pool or riffle affinities, interannual and seasonal variation in flow can alter the relative extent of riffle and pool habitat with repercussions for invertebrate species composition, food resources, and trophic and trait structure at the reach scale. In our study, high and low flows apparently created more homogeneous environmental conditions throughout stream reaches, with riffle habitat dominating at high and pool habitat at low flows; consequently, the reach-scale representation of both habitats peaked at intermediate flows. Because invertebrate taxa, traits, and communities differed considerably between pools and riffles at all times, the relative areas of pools and riffles will determine reach-scale patterns in community structure.
Further, we found that a key physical factor, i.e., current velocity, showed the greatest pool–riffle differences at intermediate flow, with log-transformed current velocities being more similar in pools and riffles at the lowest and highest flows. These changes in variation in habitat conditions were reflected in pool–riffle differences in invertebrate community structure across flow regimes, as well as in the abundances of trait groups. In the latter case, the relative abundances of depositional taxa in riffles were higher during dry than wet periods. Although erosional taxa tended to have highest relative abundances during wet springs in pools, this pattern was not significant, probably because erosional taxa were always rare in pools. Although we found that invertebrate communities in pools versus riffles became more similar at both the highest and lowest discharges and showed the greatest differences at intermediate flows, Carter & Fend (2001) reported that interannual differences in the relative abundances of collector-gatherers (greater in pools) and grazers (greater in riffles) in pools versus riffles were more pronounced at low than higher flows in the Merced River, California.
Applied implications
These large differences in invertebrate community, trait, and trophic structure between riffles and pools have important implications for ecosystem ecology, conservation, and biomonitoring. Accurate descriptions of biodiversity and the distribution of species of conservation concern will require the sampling of all major habitats. With this information, efforts to preserve or restore native species can be tailored to the specific conditions present in different habitats, to considerations of crucial linkages between habitats, and to the impacts of human activities on different habitats. Understanding ecosystem processes, such as nutrient spiraling and energy flow, also requires information on habitat-specific production and the linked processing of nutrients and organic matter within and across habitats, all of which can be affected by differences in community structure between habitats.
Habitat differences in taxonomic composition also affect our approaches to assessing ecosystem health. Biomonitoring sampling approaches that amalgamate samples across pools and riffles (e.g., the common reachwide benthos method, U.S. Environmental Protection Agency (USEPA, 2013) may produce results that vary among sites or times owing to differing proportions of pool versus riffle habitat, rather than reflect responses to stressors or disturbances that directly alter community structure within habitats. Our data indicate that alterations in stream community structure occur, at least partly, because of altered stream geomorphology (pool/riffle ratios) across a range of stream flows. Further, other studies show that invertebrate community responses to perturbations (sedimentation, drought, fire, land use) differ between pools and riffles (Roy et al., 2003; Cooper et al., 2015; da Silva et al., 2015). Because invertebrate community responses to perturbations and stressors can be habitat-specific, we recommend that monitoring efforts incorporate separate pool and riffle sampling, which can be compared to reachwide results to distinguish responses owing to within-habitat community effects from reach-scale differences in habitat extent. Benthic invertebrate community structure is shaped by the distinctive habitat types formed by riffles and pools, and by how the extent and similarity of these dynamic patches respond to varying stream flow.
References
Barbour, M.T., J. Gerritsen, B.D. Snyder & J.B. Stribling, 1999. Appendix B, of Rapid bioassessment protocols for use in streams and wadeable rivers: periphyton, benthic macroinvertebrates, and fish. 2nd edition. EPA 841-B-99-002, US Environmental Protection Agency, Office of Water, Washington, D.C.
Benjamini, Y. & Y. Hochberg, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B 57: 289–300.
Benke, A. C., A. D. Huryn, L. A. Smock & J. B. Wallace, 1999. Length-mass relationships for freshwater macroinvertebrates in North America with particular reference to the southeastern United States. Journal of the North American Benthological Society 18: 308–343.
Boersma, K. S., L. E. Dee, S. J. Miller, M. T. Bogan, D. A. Lytle & A. I. Gitelman, 2016. Linking multidimensional functional diversity to quantitative methods: a graphical hypothesis-evaluation framework. Ecology 97: 583–593.
Bogan, M. T. & D. A. Lytle, 2007. Seasonal flow variation allows ‘time-sharing’ by disparate aquatic invertebrate communities in montane desert streams. Freshwater Biology 52: 290–304.
Bonada, N., M. Rieradevall, N. Prat & V. H. Resh, 2006. Benthic macroinvertebrate assemblages and macrohabitat connectivity in Mediterranean-climate streams of northern California. Journal of the North American Benthological Society 25: 32–43.
Brown, A. V. & P. P. Brussock, 1991. Comparisons of benthic invertebrates between riffles and pools. Hydrobiologia 220: 99–108.
Brussock, P. P. & A. V. Brown, 1991. Riffle-pool geomorphology disrupts longitudinal patterns of stream benthos. Hydrobiologia 220: 109–117.
Brussock, P. P., A. V. Brown & J. C. Dixon, 1985. Channel form and stream ecosystem models. Water Resources Bulletin 21: 859–866.
Carter, J. L. & S. V. Fend, 2001. Inter-annual changes in the benthic community structure of riffles and pools in reaches of contrasting gradient. Hydrobiologia 459: 187–200.
Carter, J. L. & V. H. Resh, 2001. After site selection and before data analysis: sampling, sorting and laboratory procedures used in benthic macroinvertebrate monitoring programs by USA state agencies. Journal of the North American Benthological Society 20: 658–682.
Chevenet, F., S. Dolédec & D. Chessel, 1994. A fuzzy coding approach for the analysis of long-term ecological data. Freshwater Biology 31: 295–309.
Cooper, S. D. & T. L. Dudley, 1988. The interpretation of “controlled” vs “natural” experiments in streams. Oikos 52: 357–361.
Cooper, S. D., H. M. Page, S. W. Wiseman, K. Klose, D. Bennett, T. Even, S. Sadro, C. E. Nelson & T. L. Dudley, 2015. Physicochemical and biological responses of streams to wildfire severity in riparian zones. Freshwater Biology 60: 2600–2619.
Cummins, K. W., R. W. Merritt & M. B. Berg, 2008. Ecology and distribution of aquatic insects. In Merritt, R. W., K. W. Cummins & M. B. Berg (eds), An Introduction to the Aquatic Insects of North America, 4th ed. Kendall/Hunt Publishing Company, Dubuque: 105–122.
da Silva, M. V. D., B. F. J. V. Rosa & R. G. Alves, 2015. Effect of mesohabitats on responses of invertebrate community structure in streams under different land uses. Environmental Monitoring and Assessment 187: 714.
Finlay, J. C., S. Khandwala & M. E. Power, 2002. Spatial scales of carbon flow in a river food web. Ecology 83: 1845–1859.
Frissell, C. A., W. J. Liss, C. E. Warren & M. D. Hurley, 1986. A hierarchical framework for stream habitat classification: viewing streams in watershed context. Environmental Management 10: 199–214.
Gordon, N. D., T. A. McMahon, B. L. Finlayson, C. J. Gippel & R. J. Nathan, 2004. Stream Hydrology, An Introduction for Ecologists, 2nd ed. Wiley, West Sussex.
Herbst, D. B., E. L. Silldorff & S. D. Cooper, 2009. The influence of introduced trout on the benthic communities of paired headwater streams in the Sierra Nevada of California. Freshwater Biology 54: 1324–1342.
Hilsenhoff, W. L., 1991. Chapter 17. Diversity and classification of insects and Collembola. In Thorp, J. H. & A. P. Covich (eds), Ecology and Classification of North American Freshwater Invertebrates. Academic Press, San Diego: 593–663.
Hunsaker, C. T., T. W. Whitaker & R. C. Bales, 2012. Snowmelt runoff and water yield along elevation and temperature gradients in California’s southern Sierra Nevada. Journal of the American Water Resources Association 48: 667–678.
Huryn, A. D. & J. B. Wallace, 1987. Local geomorphology as a determinant of macrofaunal production in a mountain stream. Ecology 68: 1932–1942.
Johnson, D. W., C. T. Hunsaker, D. W. Glass, B. M. Rau & B. A. Roath, 2011. Carbon and nutrient contents in soils from the Kings River Experimental Watersheds, Sierra Nevada Mountains, California. Geoderma 160: 490–502.
Kaufman, P.R., P. Levine, E.G. Robison, C. Seeliger & D.V. Peck, 1999. Quantifying physical habitat in wadeable streams. EPA 620/R-99/003, US Environmental Protection Agency, Office of Water, Washington, D.C.
Keller, E. A., 1971. Areal sorting of bed-load material: the hypothesis of velocity reversal. Geological Society of America Bulletin 82: 753–756.
Leopold, L. B., M. G. Wolman & J. P. Miller, 1964. Fluvial Processes in Geomorphology. Freeman and Company, San Francisco.
Logan, P. & M. P. Brooker, 1983. The macroinvertebrate faunas of riffles and pools. Water Research 17: 263–270.
MacWilliams, M. L., J. M. Wheaton, G. B. Pasternack, R. L. Street & P. K. Kitanidis, 2006. Flow convergence routing hypothesis for pool-riffle maintenance in alluvial rivers. Water Resources Research 42: W10427.
Mazor, R. D., A. C. Rehn, P. R. Ode, M. Engeln, K. C. Schiff, E. D. Stein, D. J. Gillett, D. B. Herbst & C. P. Hawkins, 2016. Bioassessment in complex environments: designing an index for consistent meaning in different settings. Freshwater Science 35: 249–271.
Merritt, R. W., K. W. Cummins & M. B. Berg (eds), 2008. An Introduction to the Aquatic Insects of North America, 4th ed. Kendall/Hunt Publishing Company, Dubuque.
Montgomery, D. R., 1999. Process domains and the river continuum. Journal of the American Water Resources Association 35: 397–410.
Montgomery, D. R. & J. M. Buffington, 1997. Channel reach morphology in mountain drainage basins. Geological Society of American Bulletin 109: 596–611.
O’Dowd, A. P. & A. Chin, 2016. Do bio-physical attributes of steps and pools differ in high-gradient mountain streams? Hydrobiologia 776: 67–83.
Ode, P. R., A. C. Rehn, R. D. Mazor, K. C. Schiff, E. D. Stein, J. T. May, L. R. Brown, D. B. Herbst, D. Gillett, K. Lunde & C. P. Hawkins, 2016. Evaluating the adequacy of a reference-site pool for ecological assessments in environmentally complex regions. Freshwater Science 35: 237–248.
Poff, N. L. & J. V. Ward, 1990. Physical habitat template of lotic systems: recovery in the context of historical pattern and spatiotemporal heterogeneity. Environmental Management 14: 629–645.
Poff, N. L., J. D. Olden, N. K. M. Vieira, D. S. Finn, M. P. Simmons & B. C. Kondratieff, 2006. Functional trait niches of North American lotic insects: traits-based ecological applications in light of phylogenetic relationships. Journal of the North American Benthological Society 25: 730–755.
Poole, G. C., 2002. Fluvial landscape ecology: addressing uniqueness within the river discontinuum. Freshwater Biology 47: 641–660.
Rosi-Marshall, E. J., K. L. Vallis, C. V. Baxter & J. M. Davis, 2016. Retesting a prediction of the River Coninuum Concept: authochthonous versus allochthonous resources in the diets of invertebrates. Freshwater Science 35: 534–543.
Roy, A. H., A. D. Rosemond, D. S. Leigh, M. J. Paul & J. B. Wallace, 2003. Habitat-specific responses of stream insects to land cover disturbance: biological consequences and monitoring implications. Journal of the North American Benthological Society 22: 292–307.
Silldorff, E., 2003. Stream invertebrate responses to trout introductions: results from large-scale studies in the central Sierra Nevada and Yosemite National Park. Ph.D. Thesis, University of California, Santa Barbara, CA.
Stanley, E. H., S. G. Fisher & N. B. Grimm, 1997. Ecosystem expansion and contraction in streams. BioScience 47: 427–435.
Statzner, B. & L. Bêche, 2010. Can biological invertebrate traits resolve effects of multiple stressors on running water ecosystems? Freshwater Biology 55(s1): 80–119.
Statzner, B. & B. Higler, 1986. Stream hydraulics as a major determinant of benthic invertebrate zonation patterns. Freshwater Biology 16: 127–139.
Townsend, C. R., 1989. The patch dynamics concept of stream community ecology. Journal of the North American Benthological Society 8: 36–50.
Townsend, C. R. & A. G. Hildrew, 1994. Species traits in relation to a habitat templet for river systems. Freshwater Biology 31: 265–275.
U.S. Environmental Protection Agency (USEPA), 2013. National Rivers and Streams Assessment 2013-2014: field Operations Manual—Wadeable. EPA-841-B-12-007. U.S. Environmental Protection Agency, Office of Water, Washington, D.C.
Vieira, N.K.M., N.L. Poff, D.M. Carlisle, S.R. Moulton II, M.L. Koski & B.C. Kondratieff, 2006. A database of lotic invertebrate traits for North America. US Geological Survey Data Series 187, http://pubs.water.usgs.gov/ds187.
Winemiller, K. O., A. S. Flecker & D. J. Hoeinghaus, 2010. Patch dynamics and environmental heterogeneity in lotic ecosystems. Journal of the North American Benthological Society 29: 84–99.
Yuan, L.L. 2006. Estimation and application of macroinvertebrate tolerance values. EPA/600/P-04/116-F, US Environmental Protection Agency, National Center for Environmental Assessment, Washington, D.C.
Acknowledgements
This research was funded through Joint Venture Agreements between the Pacific Southwest Research Station, Forest Service, and the University of California, Santa Barbara (12-JV-11272139-070). We also received support in the early years from California’s State Water Resources Control Board, through Proposition 50 (the Water Security Clean Drinking Water, Coastal, and Beach Protection Act of 2002). We thank Ian Bell, Mike Bogan, Bruce Hammock, Jeff Kane, Sandi Roll, and Matt Wilson for laboratory and field assistance during this study. Helpful reviews from Sherri Johnson and Daren Carlisle improved this paper. We also thank the many Forest Service employees over the years who collected streamflow and stream habitat data for this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Eric Larson
Electronic supplementary material
Below is the link to the electronic supplementary material.
10750_2018_3646_MOESM1_ESM.xlsx
Supplementary material 1 (XLSX 56 kb). Appendix 1. List of collected taxa and their tolerance values (TV, scaled 0 = most sensitive to 10 = most tolerant to stress), thermal preference distributions (TempCD75 is the 75th percentile of the cumulative distribution of temperature (°C) observations for field collections of the taxon specified, Yuan, 2006), and assignment to functional feeding groups (FFG) and trait groups associated with voltinism (Volt), development (Devel), body size, temperature preferences (Temp), behavioral characteristics (Habit), and flow affinity (Rheo), based on Barbour et al. (1999), Vieira et al. (2006), Poff et al. (2006), and Merritt et al. (2008). The right side of this Appendix, after the Rheo column, shows pool (P, depositional), riffle (R, erosional), and mixed habitat (M) designations in Poff et al. (2006) and Merritt et al. (2008), as well as the results of Indicator Species Analysis on taxa relative abundances showing significant associations (P < 0.05, Monte Carlo tests) of common taxa (occurring in > 25% of samples) with pools (P) versus riffles (R) (all sites-times) and statistical results (*P < 0.05, **P < 0.01, ***P < 0.001) of mean time-averaged density differences between pools and riffles (n = 12 for each pool v riffle comparison) using paired t-tests with Benjamini–Hochberg corrections for all comparisons. The next column indicates the number of sites (out of 12) where each taxon was collected. Statistical tests were only performed where n > 5. The last four columns show the mean densities and associated standard errors for individual taxa in pools versus riffles. Abbreviations and codes: For FFGs: p predator, mp micro-predator, cg collector-gatherer, cf collector filterer, g grazer, sh shredder, ph piercer-herbivore. For Traits: Volt (voltinism, 1 = semivoltine < 1 generation/year, 2 = univoltine 1 generation/year, 3 = multivoltine > 1 generation/year); Devel (development rate, 1 = fast, 2 = slow, 3 = nonseasonal); Size (1 = small 2–9 mm, 2 = medium 9–16 mm, 3 = large 16–30 mm); Temp (temperature preference, 1 = cold stenotherm, 2 = cool eurytherm, 3 = warm eurytherm); Habit (behavioral habit, 1 = burrower, 2 = climber, 3 = sprawler, 4 = clinger, 5 = swimmer), and Rheo (rheophily flow/habitat preference, 1 = depositional, 2 = mixed, 3 = erosional). Note that there were insufficient data to code traits of some rare taxa in this data set (blanks), and that Chironomidae are grouped by subfamily or tribe
10750_2018_3646_MOESM2_ESM.xlsx
Supplementary material 2 (XLSX 13 kb). Appendix 2. Predictions and statistical results of comparisons between pools (P) and riffles (R) for the relative and absolute abundances of trait states, and for community indices of stress (biotic index) and temperature (thermal index) tolerance. Hypotheses regarding the pool (P) and riffle (R) affinities of different trait state groups were based on the references listed in the Appendix 1 legend and through considerations of trait state relationships to environmental factors that varied between pools (P) and riffles (R) (Table 2, see text). The results of paired t-tests for time averaged mean values across sites (n = 12 P v R pairs for each comparison) with Benjamini–Hochberg corrections (false detection rate = 0.05) for multiple comparisons across all traits are shown. Significant pool-riffle transformed (logit for proportions, log for densities) abundance differences were designated with asterisks: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Pearson’s correlation coefficients (r) and associated P values are shown, to the right of the trait state pool versus riffle results, for hypothesized relationships between transformed abundances of trait states and transformed environmental variables. This part of the appendix lists specific hypotheses and hypothesized correlations between the abundances of organisms belonging to different traits and environmental factors (Pearson’s correlation coefficient r, P value of test, and sample size n) using the full data set
Rights and permissions
About this article
Cite this article
Herbst, D.B., Cooper, S.D., Medhurst, R.B. et al. A comparison of the taxonomic and trait structure of macroinvertebrate communities between the riffles and pools of montane headwater streams. Hydrobiologia 820, 115–133 (2018). https://doi.org/10.1007/s10750-018-3646-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-018-3646-4